Introduction
Brain-derived neurotrophic factor (BDNF), a member
of the neurotrophin family, is regarded as an oncogenic factor in
tumorigenesis, proliferation and survival, and is associated with a
poor prognosis in nervous system tumors (1–3). Malignant
glioma cells are extremely infiltrative, and patients with
malignant glioma demonstrate a short survival time and poor
prognosis. Neurotrophin genes are widely expressed in 24 cell lines
derived from human malignant gliomas, and the BDNF gene is the most
abundantly expressed (4). There are
two forms of BDNF, consisting of precursor of BDNF (proBDNF) and
mature BDNF, in the central nervous system (CNS) (5). ProBDNF is synthesized and subsequently
cleaved, either intracellularly, by prohormone convertases and
furin, or extracellularly, by plasmin and matrix metalloproteinases
(MMPs), to generate mature BDNF (6,7). Mature
BDNF and proBDNF exert opposing effects through two different
transmembrane receptor signaling systems, consisting of tyrosine
receptor kinase B (TrkB) and p75 neurotrophin receptor (p75NTR) to
modulate cell apoptosis (8–10), long-term depression (11) and synaptic plasticity (12–15).
Specific antibodies have previously been generated
against mature BDNF and proBDNF (5).
In previous studies (16,17), the proBDNF/p75NTR pathway has been
demonstrated to promote cell death and inhibit growth and migration
in C6 glioma cells through p75NTR in vitro, while mature
BDNF demonstrates the opposite effect on C6 glioma cells. It is
hypothesized that mature BDNF plays an essential role in the
development of gliomas towards malignancy. However, histological
data in previous studies were unable distinguish mature BDNF from
proBDNF due to the lack of specific antibodies (1–3). In the
present study, the expression of mature BDNF was examined in human
glioma tissue of various grades using a specific antibody against
mature BDNF.
Materials and methods
Patients
All 42 patients in the present study were enrolled
from the Department of Neurosurgery and the Department of Oncology
at the Second Affiliated Hospital of Kunming Medical University
(Kunming, Yunnan, China). The use of human tissue in the present
study was approved by the Ethics Committee of Kunming Medical
University (Kunming, Yunnan, China), and written informed consent
was obtained from all patients. All tumor specimens were classified
and graded by two independent pathologists, with full diagnostic
agreement, according to the 2007 World Health Organization (WHO)
classification of tumors of the CNS (18). The glioma samples were classified as
follows: 3 samples, all pilocytic astrocytomas, were classified as
grade I; 18 samples, all diffuse astrocytomas, were classified as
grade II; 13 samples, all anaplastic astrocytomas, were classified
as grade III; and 8 samples, all glioblastoma, were classified as
grade IV. For convenience, all gliomas were divided into two groups
for analysis. The low-grade glioma group consisted of tissues from
8 male and 13 female patients with grade I and II glioma (mean age,
35.57±14.95 years), and the high-grade group consisted of tissues
from 13 male and 8 female patients with grade III and IV glioma
(mean age, 47±15.42 years). In total, 19 samples were resected from
the frontal lobe, 12 from the temporal lobe, 5 from the parietal
lobe, 3 from the occipital lobe and 3 from the ventricles. There
were 9 tumors that involved multiple lobes.
In addition, 10 non-neoplastic brain tissues
obtained from 8 males and 2 females (mean age, 41.30±14.49 years)
were used as control tissues. In total, 3 patients underwent lobe
resection for epilepsy, 4 for brain trauma, 2 for hypertensive
cerebral hemorrhage and 1 for internal decompression, the sample
from which consisted of normal tissue located near a tumor. In
addition, 4 control tissues were acquired from the frontal lobe (3
patients with brain trauma, 1 patient with glioma who underwent
internal decompression), 4 from the temporal lobe (3 patients with
epilepsy who underwent lobe resection, 1 patient with brain trauma
who underwent internal decompression), 1 from the parietal lobe (1
patient who underwent internal decompression for hypertensive
cerebral hemorrhage) and 1 from the cerebellum (1 patient who
underwent internal decompression for hypertensive cerebral
hemorrhage).
Immunohistochemistry
Small fragments of tissue were fixed in 10% neutral
buffered formalin and embedded in paraffin for immunohistochemistry
(IHC) of mature BDNF and TrkB. Single-labeling IHC was performed as
described in a previous study (16).
Sheep monoclonal anti-mature BDNF (2 µg/ml; laboratory of Professor
Xin-Fu Zhou, School of Pharmacy and Medical Sciences, University of
South Australia, Adelaide, Australia) and rabbit anti-TrkB
(dilution, 1:1000; Catalog no. 07-225; EMD Millipore, Billerica,
MA, USA) primary antibodies were used as previously described
(17). IHC staining was assessed
semi-quantitatively by measuring the intensity of the staining and
the number of positive cells, as previously described (16).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of the BDNF and
TrkB genes in the control brain tissues and glioma tissues
of various malignancy grades were determined by RT-qPCR.
Quantitative data were normalized relative to ACTB. The
5′-3′ primer sequences were as follows: BDNF forward,
TACTTTGGTTGCATGAAGGCTGCC and reverse, ACTTGACTACTGAGCATCACCCTG;
TrkB forward, AGGGCAACCCGCCCACGGAA and reverse,
GGATCGGTCTGGGGAAAAG; and ACTB forward, CGGGAAATCGTGCGTGAC
and reverse, TGGAAGGTGGACAGCGAGG. All primers were synthesized by
Invitrogen (Carlsbad, CA, USA). The total RNA extraction from
tissue samples and reverse transcription were performed as
described (16). The data were
analyzed using the 2−ΔΔCt method (19).
ELISA for mature BDNF and TrkB
The tissue samples were homogenized, and levels of
mature BDNF in the homogenate were determined by a highly specific,
mature BDNF ELISA kit, as previously described (20). The procedure is highly specific for
mature BDNF only and does not detect proBDNF or the other
neurotrophins, such as neurotrophins-3 and −4 and nerve growth
factor. Levels of TrkB in the protein samples were determined using
a commercial human TrkB kit (Sino Biological, Inc., Beijing,
China), according to the manufacturer's instructions.
Statistical analysis
The Kruskal-Wallis test, Mann-Whitney U test and
Spearman's rank correlation coefficient were used to compare the
differences between the control brain samples and glioma samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of mature BDNF in human
glioma tissues
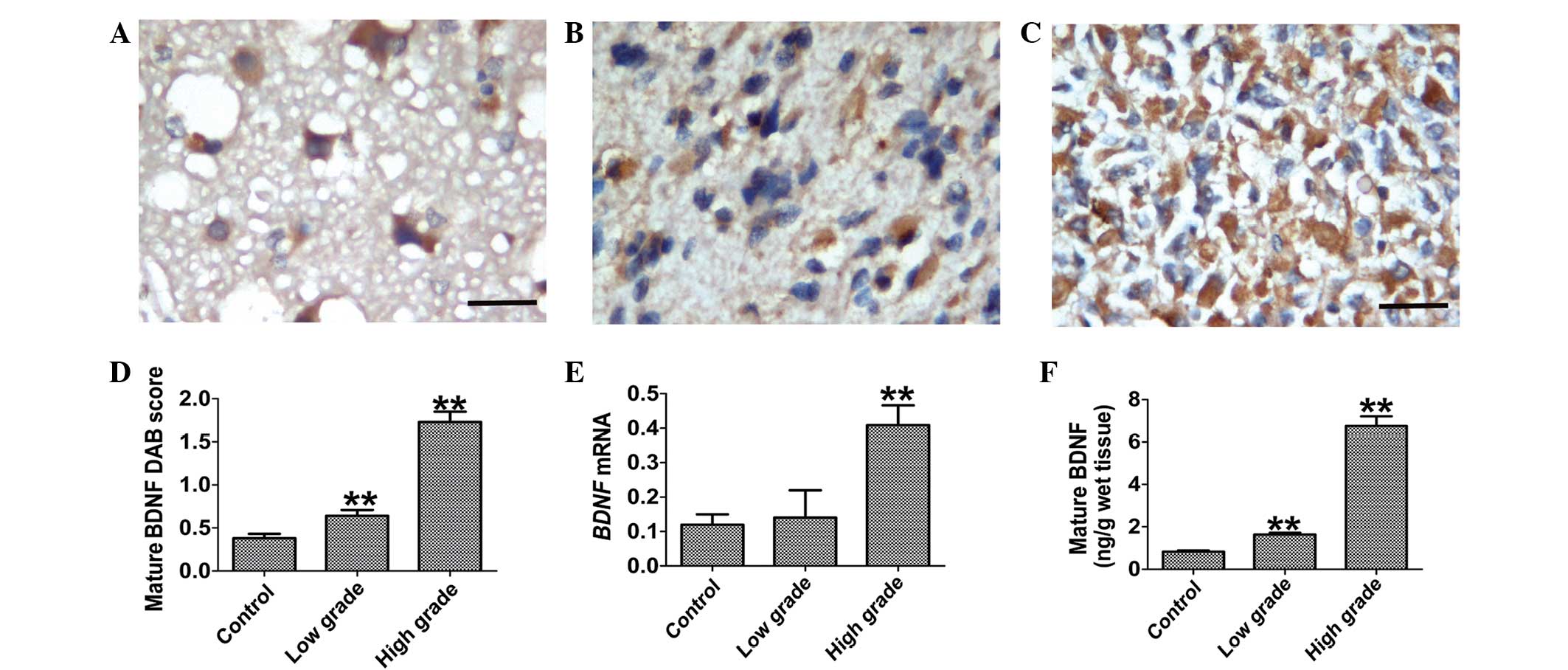
Immunostaining for mature BDNF was detected in the
neuronal cytoplasm of control tissues (Fig. 1A) and cytoplasm of glioma cells
(Fig. 1B and C). Strong staining for
mature BDNF occurred in the cytoplasm of the high-grade glioma
tissues (Fig. 1C). Compared with
low-grade glioma and control tissues, the semi-quantitative
analysis revealed that mature BDNF immunostaining in high-grade
glioma was increased (Fig. 1D;
P<0.001). The RT-qPCR (Fig. 1E)
analysis also revealed the increased BDNF mRNA levels in
high-grade glioma tissues (P=0.003). These results were further
supported by data obtained from ELISA (Fig. 1F). The expression of mature BDNF in
low-grade gliomas was significantly increased 1.98-fold compared
with the normal control tissue specimens (P<0.001). Notably, the
expression of mature BDNF in high-grade gliomas was significantly
increased 4.14-fold compared with low-grade gliomas
(P<0.001).
Expression of TrkB in human glioma
tissues
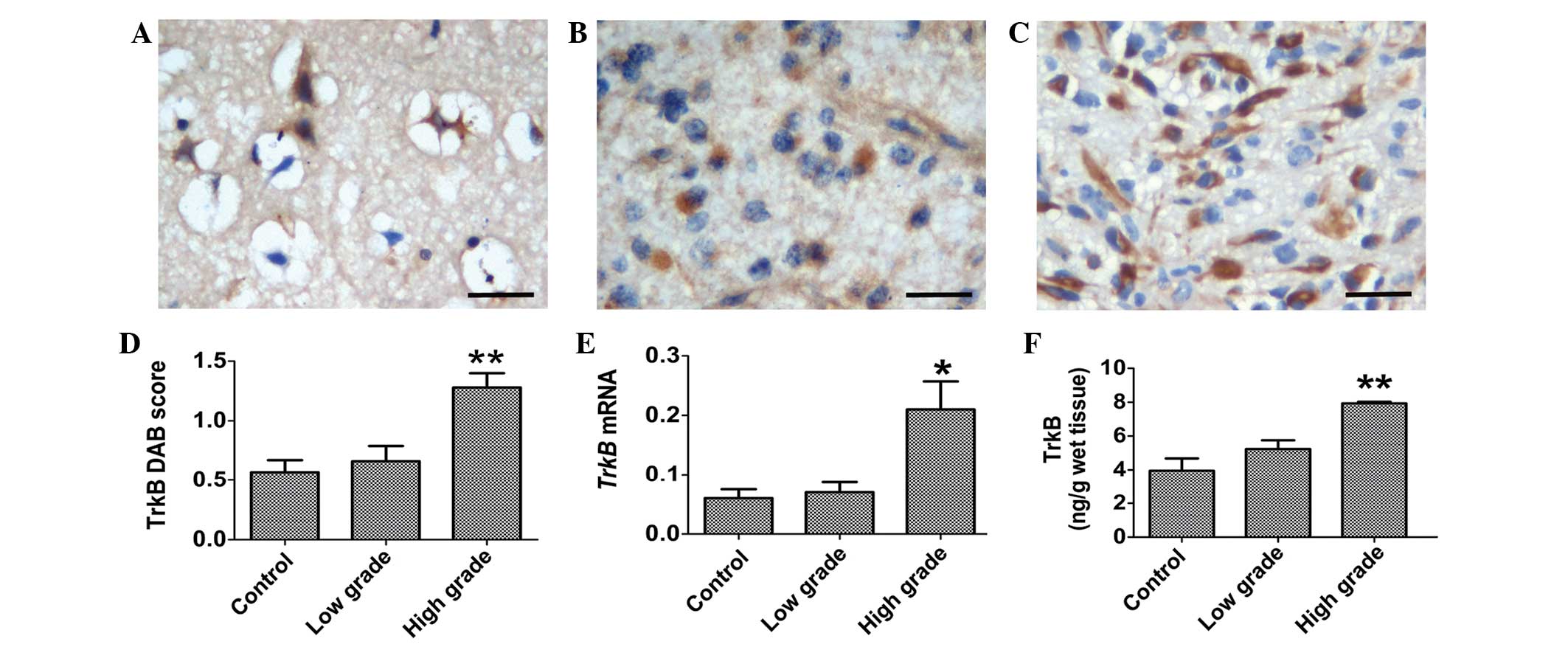
TrkB immunostaining was evidently present in the
cytoplasm of neurons in the control tissues (Fig. 2A). There was a weak immunostaining for
TrkB in the cytoplasm of the tumor cells of low-grade gliomas
(Fig. 2B). By contrast, strong TrkB
immunostaining was observed in the cytoplasm of the high-grade
glioma cells (Fig. 2C). The
semi-quantitative analysis revealed that TrkB immunostaining in
high-grade gliomas was significantly increased compared with
low-grade glioma and control tissues (Fig. 2D; P<0.001). The RT-qPCR analysis
revealed that the expression of TrkB mRNA was increased in
high-grade glioma tissues, which was consistent with the results of
immunostaining (Fig. 2E; P=0.032). An
ELISA assay for TrkB revealed that the concentration of TrkB was
significantly increased in high-grade gliomas (Fig. 2F; P<0.001).
Correlation between the expression of
mature BDNF and TrkB and the glioma malignancy grade
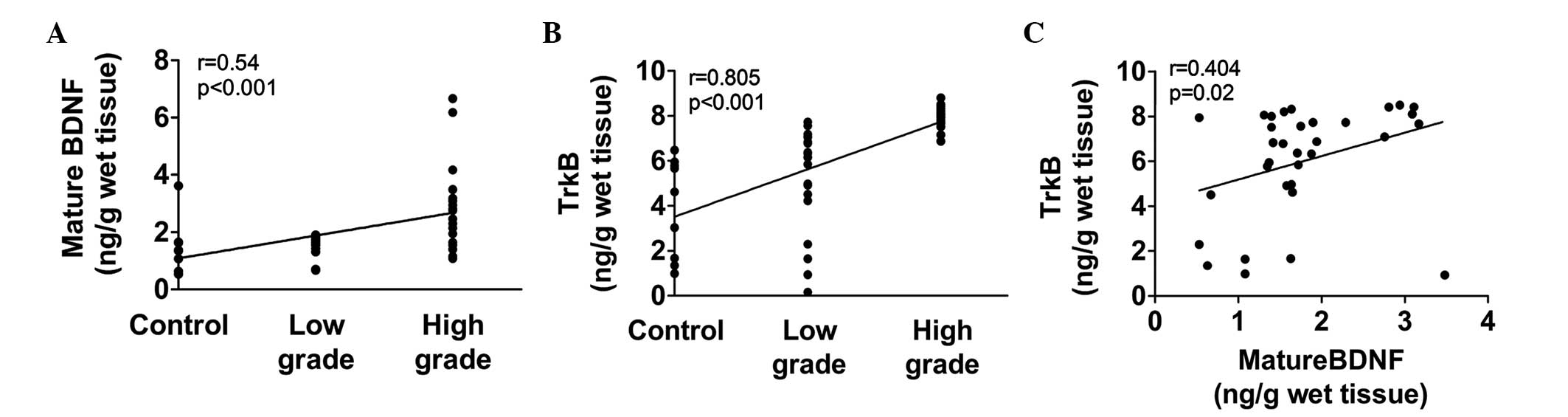
Spearman's rank correlation analysis revealed that
mature BDNF (r=0.54, P<0.001) and TrkB (r=0.805, P<0.001)
were positively associated with the grade of malignancy in glioma
(Fig. 3A and B). Spearman's rank
correlation analysis also revealed that there was a positive
correlation between the expression levels of mature BDNF and TrkB
(r=0.404, P=0.02; Fig. 3C).
Discussion
The important role of the BDNF/TrkB signaling system
in tumor cell proliferation and survival have been demonstrated in
previous studies (1–3). The expression of BDNF is correlated with
a poor prognosis in various TrkB-expressing human cancers,
including tumors in the nervous system (1–3). Previous
studies have revealed that BDNF mRNA is a prevalent transcript in
24 cell lines derived from human malignant gliomas (4).
ProBDNF and mature BDNF coexist in the CNS and
perform opposite roles in neuronal functions through two
transmembrane receptor signaling systems, consisting of p75NTR and
TrkB (5–7). ProBDNF is a high-affinity functional
ligand for the pro-apoptotic p75NTR, whereas the proteolytically
cleaved mature BDNF is the preferred ligand for TrkB (6–7). A
previous study has demonstrated that proBDNF negatively regulates
the growth and migration of C6 glioma cells through p75NTR
(16). It has also been demonstrated
that mature BDNF induces C6 glioma cell proliferation and invasion
in vitro (17). Considering
the opposite effects on the biological behavior of gliomas cells
in vitro, measurement of the level of mature BDNF alone in
normal human brain tissue and glioma tissue samples in vivo
may be informative.
The present study has revealed that mature BDNF and
its receptor TrkB are highly expressed in human glioma cells using
various approaches, consisting of IHC, ELISA and RT-qPCR assays. In
the present study, the expression of TrkB was found to be
upregulated in human high-grade gliomas, and the receptor is
positively correlated with the grade of the gliomas. The present
results are consistent with previous studies that reported the
frequent robust expression of TrkB in highly invasive tumors
(21) and the activity of TrkB as a
key regulator of tumor malignancy. Notably, the expression profiles
of mature BDNF and TrkB are similar, demonstrating increased
expression parallel to the increase in gliomas grades. In addition,
the co-localization of mature BDNF and TrkB in human gliomas was
increased in high-grade samples, suggesting that the mature
BDNF/TrkB signaling pathway plays a role in modulating the
malignancy and prognosis of glioma. These results are consistent
with the results of a previous study that reported the promotion of
the proliferation, infiltration and migration of C6 glioma cells
demonstrated by mature BDNF in vitro (17). The promotion of the proliferation,
infiltration and migration of glioma cells by mature BDNF may be
critical in the promotion of low-grade gliomas to over-aggressive
high-grade gliomas. Suppression of the mature BDNF/TrkB signaling
pathway by either specific mature BDNF neutralizing antibodies or
TrkB-receptor antibodies may demonstrate therapeutic significance
in high-grade glioma.
These results are consistent with previous findings
that suggested the possibility of the proBDNF-p75-sortilin pathway
being a balancing signal for tumor growth through the mature
BDNF-TrkB pathway (7,16). The balance between tumor cell death
and survival may depend upon the proportions of mature and proBDNF
available to cells expressing TrkB and p75NTR. ProBDNF-converting
enzymes, such as MMPs or tissue plasminogen activator are produced
and released by glioma cells (22,23). In
particular, these proteases have been reported to be closely
associated with invasion and angiogenesis in malignant gliomas
(22,23). It would be of considerable interest to
study the precise mechanisms controlling the relative expression
levels of proBDNF and mature BDNF to limit or amplify distinct
neurotrophin activities in the development of glioma.
Overall, the present data have indicated that
increased levels of mature BDNF contribute notably to the
development of malignancy in human gliomas through the primary BDNF
receptor TrkB. The present study suggests that the suppression of
the mature BDNF signaling pathway, but not the proBDNF signaling
pathway, may be a novel therapeutic strategy for high-grade
gliomas.
Acknowledgements
This study was supported by the National Key Basic
Research Program of China (grant no., 2011CB944200).
References
|
1
|
Nakagawara A, Azar CG, Scavarda NJ and
Brodeur GM: Expression and function of TRk-B and BDNF in human
neuroblastomas. Mol Cell Biol. 14:759–767. 1994.PubMed/NCBI
|
|
2
|
Stephan H, Zakrzewski JL, Bölöni R,
Grasemann C, Lohmann DR and Eggert A: Neurotrophin receptor
expression in human primary retinoblastomas and retinoblastoma cell
lines. Pediatr Blood Cancer. 50:218–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Artico M, Bronzetti E, Pompili E, Ionta B,
Alicino V, D'Ambrosio A, Santoro A, Pastore FS, Elenkov I and
Fumagalli L: Immunohistochemical profile of neurotrophins in human
cranial dura mater and meningiomas. Oncol Rep. 21:1373–1380.
2009.PubMed/NCBI
|
|
4
|
Hamel W, Westphal M, Szönyi E, Escandón E
and Nikolics K: Neurotrophin gene expression by cell lines derived
from human gliomas. J Neurosci Res. 34:147–157. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou XF, Song XY, Zhong JH, Barati S, Zhou
FH and Johnson SM: Distribution and localization of
pro-brain-derived neurotrophic factor-like immunoreactivity in the
peripheral and central nervous system of the adult rat. J
Neurochem. 91:704–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seidah NG and Chretien M: Proprotein and
prohormone convertases: A family of subtilases generating diverse
bioactive polypeptides. Brain Res. 848:45–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker PA: Whither proBDNF? Nat Neurosci.
12:105–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teng HK, Teng KK, Lee R, Wright S, Tevar
S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, et
al: ProBDNF induces neuronal apoptosis via activation of a receptor
complex of p75NTR and sortilin. J Neurosci. 25:5455–5463. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kenchappa RS, Zampieri N, Chao MV, et al:
Ligand-dependent cleavage of the P75 neurotrophin receptor is
necessary for NRIF nuclear translocation and apoptosis in
sympathetic neurons. Neuron. 50:219–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan YJ, Wu LL, Li HY, Wang YJ and Zhou XF:
Differential effects of pro-BDNF on sensory neurons after sciatic
nerve transection in neonatal rats. Eur J Neurosci. 27:2380–2390.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woo NH, Teng HK, Siao CJ, Chiaruttini C,
Pang PT, Milner TA, Hempstead BL and Lu B: Activation of p75NTR by
proBDNF facilitates hippocampal long-term depression. Nat Neurosci.
8:1069–1077. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu B: Pro-region of neurotrophins: Role in
synaptic modulation. Neuron. 39:735–738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu B, Pang PT and Woo NH: The yin and yang
of neurotrophin action. Nat Rev Neurosci. 6:603–614. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koshimizu H, Hazama S, Hara T, et al:
Distinct signaling pathways of precursor BDNF and mature BDNF in
cultured cerebellar granule neurons. Neurosci Lett. 473:229–232.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Lim Y, Li F, Liu S, Lu JJ,
Haberberger R, Zhong JH and Zhou XF: ProBDNF collapses neurite
outgrowth of primary neurons by activating RhoA. PLoS One.
7:e358832012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong J, Zhou L, Yang M, Lim Y, Zhu YH, Fu
DL, Li ZW, Zhong JH, Xiao ZC and Zhou XF: ProBDNF and its receptors
are upregulated in glioma and inhibit the growth of glioma cells in
vitro. Neuro Oncol. 15:990–1007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong J, Zhou L, Lim Y, Yang M, Zhu YH, Li
ZW, Zhou FH, Xiao ZC and Zhou XF: Mature BDNF promotes the growth
of glioma cells in vitro. Oncol Rep. 30:2719–2724. 2013.PubMed/NCBI
|
|
18
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou L, Xiong J, Lim Y, Ruan Y, Huang C,
Zhu Y, Zhong JH, Xiao Z and Zhou XF: Upregulation of blood proBDNF
and its receptors in major depression. J Affect Disord.
150:776–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Assimakopoulou M, Kondyli M, Gatzounis G,
Maraziotis T and Varakis J: Neurotrophin receptors expression and
JNK pathway activation in human astrocytomas. BMC Cancer.
7:2022007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagemann C, Anacker J, Ernestus RI and
Vince GH: A complete compilation of matrix metalloproteinase
expression in human malignant gliomas. World J Clin Oncol. 3:67–79.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salmaggi A, Croci D, Prina P, Cajola L,
Pollo B, Marras CE, Ciusani E, Silvani A, Boiardi A and Sciacca FL:
Production and post-surgical modification of VEGF, tPA and PAI-1 in
patients with glioma. Cancer Biol Ther. 5:204–209. 2006. View Article : Google Scholar : PubMed/NCBI
|