Introduction
Sarcoma is a soft-tissue and bone malignancy of
mesenchymal origin, which accounts for ~1% of adult cancers and
15–20% of pediatric cancers in the USA (1,2). In the
USA, ~11,280 soft tissue tumors and 2,650 bone tumors are diagnosed
annually (3). Due to the
heterogeneity of sarcoma, >100 distinct subtypes have been
described to date, with new subtypes frequently reported (4). Synovial sarcoma, a soft-tissue tumor, is
characterized by a reciprocal t(X;18) translocation, in which the
SS18 gene on chromosome 18 fuses with the SSX1, SSX2 or, less
commonly, SSX4 gene on the X chromosome (5,6). Ewing's
sarcoma has a relatively simple genetic signature, consisting of a
t(11;22) translocation (7,8). However, certain other sarcomas,
including osteosarcoma, chondrosarcoma and undifferentiated
sarcoma, are characterized by more complex genetic abnormalities
(9).
The clinical outcomes of sarcoma are dependent upon
the subtype, and current therapies are limited to radiation,
chemotherapy and surgical resection. Although radiation may prevent
local recurrence, and chemotherapy can temporarily delay the
progression of sarcoma, complete surgical resection is the only
curative treatment method (10,11). As
the rate of complication and of chemotherapy resistance are
considerable, a more effective therapy is urgently required
(12). During the last two decades,
many of the molecular mechanisms of sarcoma genesis have been
elucidated; novel insights into such mechanisms, and the
identification of the involved genes may lead to the development of
more effective therapies targeted against the driving events in
sarcomas (13).
In the current review, the structure of Src and its
function as an oncoprotein are described, with a detailed
discussion of the role of Src in sarcoma. In addition, potential
drug therapies for the treatment of sarcoma are also evaluated.
Src
Src structure and regulation of Src
activity
SRC is a proto-oncogene encoding a
non-receptor tyrosine kinase, similar to the v-Src gene of the Rous
sarcoma virus (14), which was
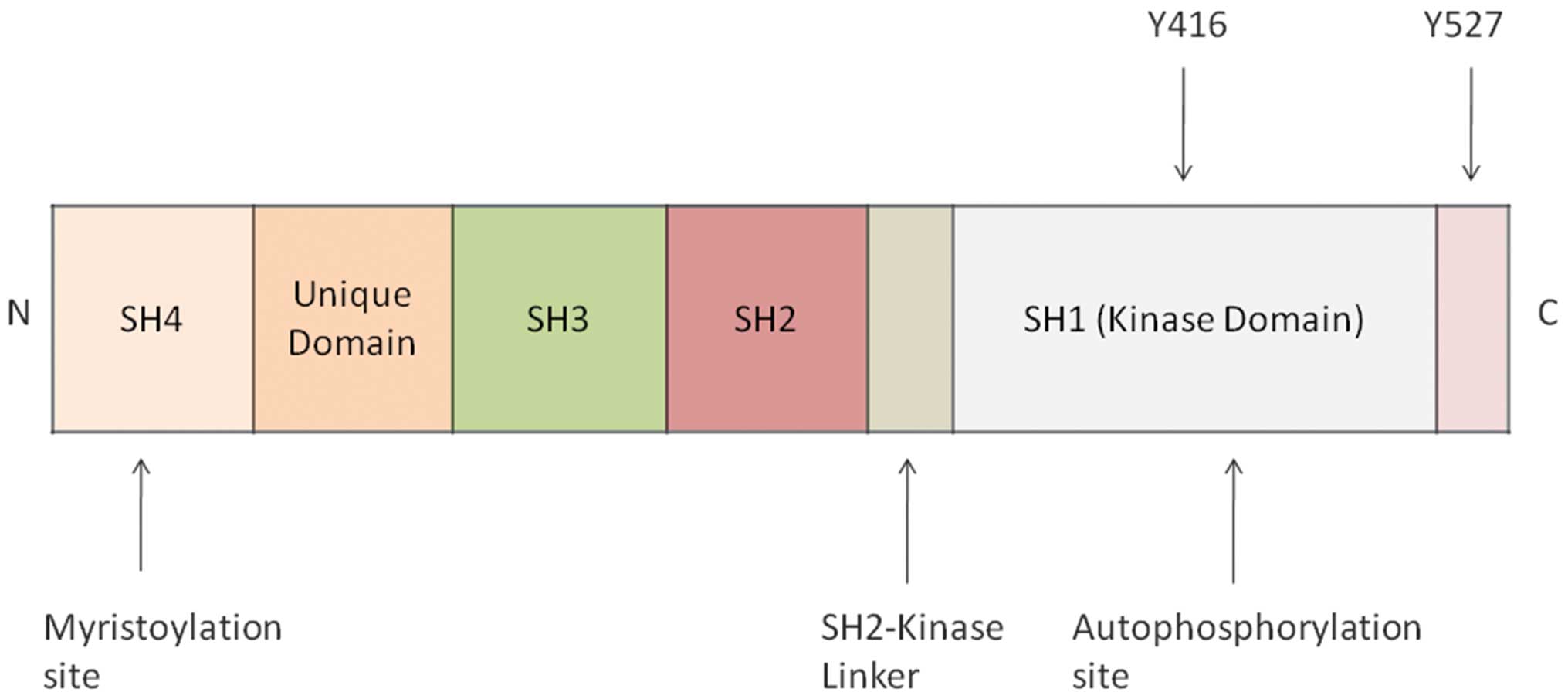
initially discovered by Bishop and Varmus (15). The Src protein is formed of seven
functional regions: i) N-terminal Src homology domain 4 (SH4)
containing a myristic acid moiety, essential for its localization
to the inner surface of the cell membrane; ii) a unique domain
providing functional specificity to each member of the Src family;
iii) SH3 domain, which binds proline-rich sequences to mediate
intra- and intermolecular interactions; iv) SH2 domain, which binds
phosphorylated tyrosine residues on Src and other proteins; v) a
catalytic domain (SH1); and vi) C-terminal tail containing
negative-regulatory Tyr530 (in humans) (16–18)
(Fig. 1).
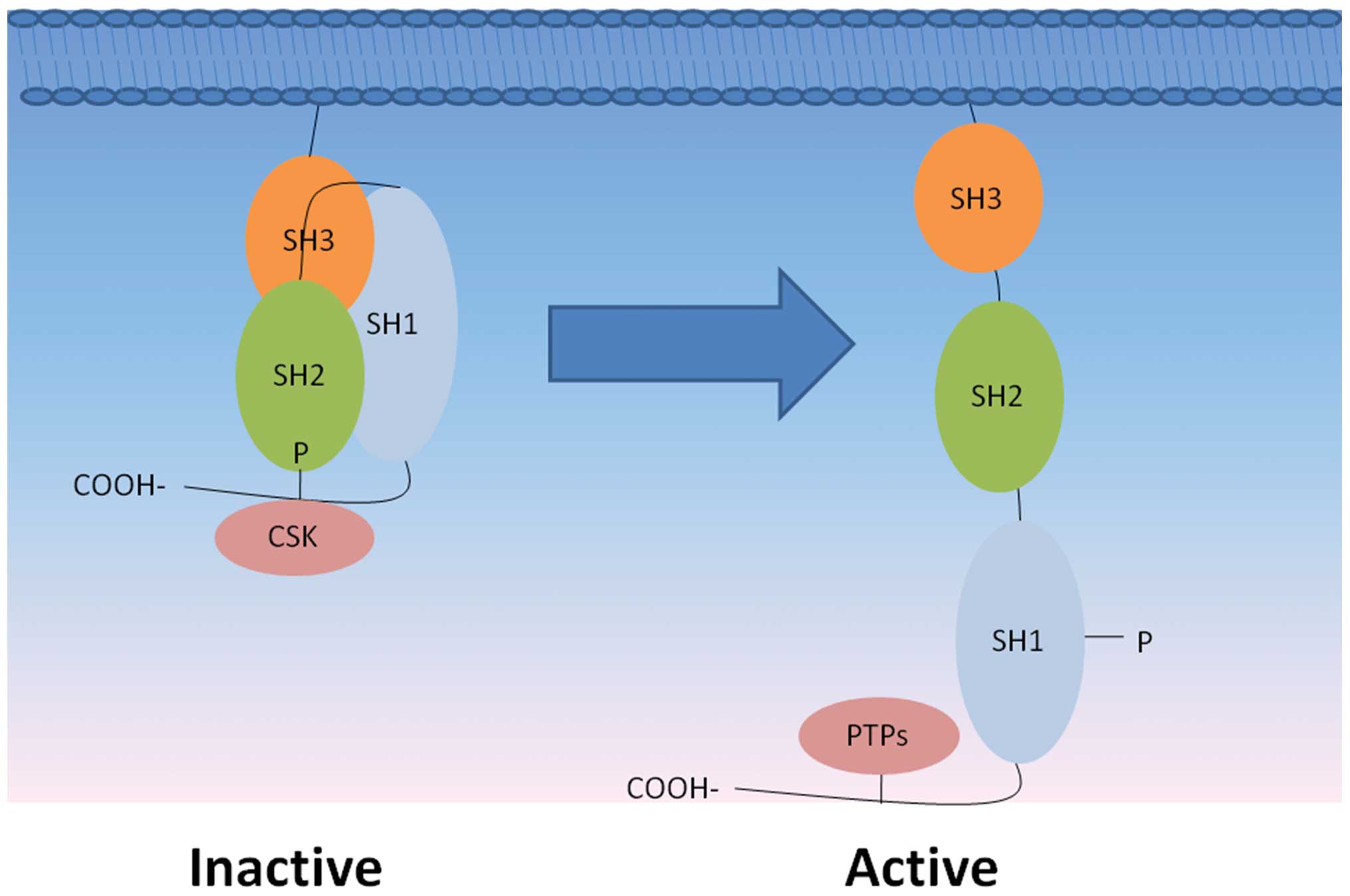
The activity of Src is regulated by the structural
changes that occur following phosphorylation and dephosphorylation
of its tyrosine residues, which is determined by the relative
activities of protein kinases and phosphatases (19). The enzymatic activity of the 60 kDa
human c-Src tyrosine kinase is predominantly regulated at two
phosphorylation sites: Tyr527 and Tyr416. Phosphorylation at Tyr527
reduces the activity of Src, while dephosphorylation of
phosphotyrosine 527 increases activity; autophosphorylation of
Tyr416 also enhances activity (20,21).
Phosphatases that may interact with phosphotyrosine 527 include
cytoplasmic protein tyrosine phosphatase (PTP) 1B, Shp1 and Shp2,
and transmembrane enzymes including CD45, PTPα, PTPε, and PTPκ
(22,23). Furthermore, PTP-BL and PTP-BAS have
been shown to dephosphorylate phosphotyrosine 416 to decrease Src
kinase activity (24) (Fig. 2).
Functions of Src in cancer
Src has been identified as an important factor in
several human malignancies, and in the promotion of tumor
progression during the multistep process of cancer pathogenesis
(25). Src deregulation primarily
involves protein overexpression and abnormalities in Src kinase
activity. Differences in Src expression have been observed in lung,
breast, pancreatic, colon and prostate cancer cells, compared with
normal adjacent tissue, fibroblasts or normal mucosal cells
(26–31). In the tumor microenvironment, Src
activation has been observed in cancer and inflammatory cells, and
may serve as a critical mechanistic link between inflammation and
cancer. Src propagates a cycle between immune and tissue cells,
ultimately leading to the development and progression of cancer
(32,33). The abnormal activation of Src may
result in the promotion of survival, angiogenesis, proliferation
and invasion pathways observed in tumors cells (34,35).
However, despite the evidence indicating a major role for Src in
the development and progression of cancers, its mechanism of action
is not fully understood.
A number experimental studies have proposed that Src
may be involved in the transmission of signals from extra and
intracellular stimuli. Interactions between the Src pathway and
Signal Transducer and Activator of Transcription (STAT) 5, STAT3,
N-cadherin and basic fibroblast growth factor receptors and
β-catenin have been reported in melanoma cells (36,37). It
may also be of value to understand the effect of Src inhibition on
a number of the environment-sensing and growth-promoting pathways
known to be aberrant in cancer cells, including the
phosphoinositide 3-kinase/protein kinase B/mammalian target of
rapamycin (PI3K/Akt/mTOR), Ras/mitogen-activated protein kinase
(MAPK), platelet-derived growth factor (PDGF), Erb1/Erb2 and
vascular endothelial growth factor (VEGF) pathways (38–40).
Currently, the complex interactions between Src and other pathways
remain to be established. The crosstalk signaling mechanisms that
link inflammatory cells with cancer cells, including
SDF-1-CXCR4-Src and Src-IL-6 signaling axes, result in a cycle
leading to cancer development and progression (41–43). In
leukemia, SDF-1 has been found to induce ‘inside-out’ signaling,
which involves CXCR4 and Lyn, leading to aberrant adhesive
responses. Furthermore, previous studies have shown that Src and
Hck, the Src family members, are involved in the production of IL-6
in osteoblasts and inflammatory macrophages (42,43).
Function of Src in sarcoma
Src aberrant expression in
sarcoma
Src was the first transforming protein and the first
gene product with protein tyrosine kinase activity to be discovered
and isolated (44). With the use of
immunohistochemistry and Western blotting, the total Src and
phosphorylated Src (Y419) were found to be activated in human
sarcoma tissues (leiomyosarcoma, high-grade osteosarcoma and
liposarcoma) and sarcoma cell lines (osteosarcoma, Ewing's sarcoma,
leiomyosarcoma and rhabdomyosarcoma) (45). Furthermore, Src was identified as one
of the most strongly phosphorylated kinases in synovial sarcoma
cells (46). Src activity was
demonstrated to be upregulated in anoikis-resistant human
osteosarcoma cells, SAOS-2, compared with their parental population
(47).
With regard to different subtypes of sarcoma, Src is
thought to be the most reliable discriminator to distinguish
high-grade leiomyosarcoma from undifferentiated pleomorphic
sarcoma, based on gene expression profiling and meta-analysis
(48). Due to its aberrant expression
in sarcoma, Src has been proposed to be important in signal
transduction in human sarcomas, including osteosarcoma,
rhabdomyosarcoma, leiomyosarcoma, fibrosarcoma and Ewing's sarcoma
(49).
Src in sarcoma proliferation and
apoptosis
A fundamental trait of cancer cells is their ability
to sustain chronic proliferation. The overexpression of Src in U2OS
and MG63 osteosarcoma cells significantly enhances proliferation
and reduces apoptosis of these cells (45,50). In
human osteosarcoma cells SAOS-2, Src was revealed to be activated
in anoikis resistance (47).
Furthermore, Src was identified in 0–20% chondrosarcoma specimens.
However, its expression had no prognostic significance,
particularly in serving as an indicator of cell proliferation
(51). Src and its downstream
signaling via the p38 MAPK-AKT pathway may be activated by the
signaling adaptor protein, Crk, to promote proliferation of human
synovial sarcoma cells (52,53). Inhibition of Src signaling in Ewing's
sarcoma cells was observed to induce apoptosis (45).
These findings indicate that Src may increase
sarcoma proliferation and reduce apoptosis. However, in some
subtypes of sarcoma, there is conflicting evidence with regard to
the expression of Src. For example, high Src expression has been
identified in high-grade leiomyosarcoma, while Src expression has
been found to be variable in chondrosarcoma (48). Additionally, the mechanisms of
proliferation and apoptosis require further investigation.
Src in sarcoma invasion and
metastasis
Despite continual research and increasing knowledge
of the biology of sarcoma, invasion and metastasis remain poorly
understood, and are the predominant cause of sarcoma-related
mortality. The ability of cancer cells to leave their primary site
of growth, move into different tissue compartments, and survive and
proliferate in these foreign environments, defines the biological
program known as ‘invasive growth’ (54). Invasive growth is important for cancer
progression and thus, presents a target for the treatment of
sarcoma. In mouse models of osteosarcoma, depletion of Src
phosphorylation in SaOS-2 cells has been shown to decrease tumor
mass (55). However, other reports
indicate that inhibition of Src phosphorylation in HOS and SaOS-2
cells may only decrease the metastatic potential of osteosarcoma
cells in vitro, and not in vivo (56). The effect of Src on the metastasis of
osteosarcoma cells is therefore controversial. A number of studies
reported that inhibition of c-Src signaling was able to reduce
metastasis of chondrosarcoma (57,58). Other
studies found that Src inhibition could overcome chemoresistance to
induce apoptosis and to inhibit migration (59). In Ewing's sarcoma cells, inhibition of
c-Src was also observed to reduce migration and metastasis
(45).
It has been established that epithelial cells may
acquire migratory capability, a feature typical of the mesenchymal
cells, and gain invasive ability, resistance to apoptosis and the
ability to disseminate (60), in a
process known as the epithelial-mesenchymal transition (EMT). EMT
is a complicated process, whereby cancer cells acquire migratory
and invasive abilities, which are influenced by the tumor
microenvironment and intercellular communication. Src activity
affects metastatic progression, suggesting that Src-induced EMT may
be associated with enhanced metastatic potential (61). However, the effect of Src-related EMT
has yet to be investigated in sarcoma.
Src signaling networks in sarcoma
A number of studies have provided insight into how
Src overexpression and activation may contribute to cancer. CD99, a
transmembrane glycoprotein, may exert anti-oncogenic effects,
reducing the growth and metastatic ability of osteosarcoma cells by
regulating Caveolin-1 (Cav-1) and inhibiting Src kinase activity.
Cav-1 is a caveolar domain associated with the plasma membrane,
which is involved in numerous cellular functions, including
molecular transport, cell adhesion and signal transduction and
thus, the role of Cav-1 in cancer development and progression has
been investigated (62,63). Cav-1 may act as an onco-suppressor and
inhibit Src to reduce osteosarcoma metastasis (64,65).
However, other studies have demonstrated that CD99 isoforms, CD99wt
(full-length CD99 isoform) and CD99sh (short form) have opposing
effects in osteosarcoma malignancy and metastasis, and may activate
or inhibit Src kinase activity (66).
In osteosarcoma, when Src was inhibited, the
downstream components of Src signaling, including focal adhesion
kinase (FAK) and a partnership and Crk-associated substrate
(p130CAS) were also inhibited at the protein level. In
rhabdomyosarcoma, targeting the Src-α-type platelet-derived growth
factor receptor-Raf-MAPK axis has been shown to be effective in
inhibiting mouse and human tumor cell growth (67).
Clinical development of Src inhibitors
Src has recently become an active target for drug
development and a number of Src inhibitors, including dasatinib
(BMS354825), sarcatinib (AZD0530) and bosutinib (SKI-606), are at
various stages in the development process (68). Dasatinib has been approved for the
treatment of chronic myeloid leukemia and Philadelphia-positive
acute lymphoblastic leukemia (69),
saracatinib has been used in a phase II trial for the treatment of
extensive stage small cell lung cancer (70), and bosutinib has been used in a phase
II trial for the treatment of adults with recurrent glioblastoma
(71).
Dasatinib is a dual Src-AbI kinase inhibitor, which
is already approved by the Food and Drug Administration for the
treatment of chronic myeloid leukemia and Philadelphia chromosome
positive acute lymphoblastic leukemia (72). Several studies have demonstrated the
therapeutic benefit of dasatinib in preventing the growth and
metastasis of sarcomas. In osteosarcoma cell lines, wound-healing,
cell migration and TUNEL assays indicated that dasatinib may block
cell motility and invasion, and induce apoptosis (45,73). In
chondrosarcoma, dasatinib was also capable of decreasing tumor
growth, however, it was unable to reduce invasion (73).
A new pyrazolo[3,4-d] pyrimidine derivative Src-Y416
inhibitor (SI-83) was found to impair osteosarcoma SaOS-2 cell
viability and decrease osteosarcoma tumor mass in vivo, and
exhibited less toxicity in primary human osteoblasts when compared
with osteosarcoma cells. Additionally, SI-83 was shown to induce
apoptosis in SaOS-2 cells (55).
These results indicate that SI-83 may be a novel effective
therapeutic agent, with the advantage of low toxicity in
nonneoplastic cells. A number of tyrosine kinase inhibitors that
target Src tyrosine kinase have also been developed for therapeutic
use (74), such as the pan-RAF
inhibitors, CCT196969 and CCT241161 (75).
Conclusion
Compared with normal tissue, Src expression is
significantly higher in tumor tissue, including gastrointestinal
stromal tumors and renal clear cell carcinomas (76,77). A
number of studies have found that Src signaling is important in
attracting immune cells to tumor cells (32). The activation of Src, mediated by
inflammatory cytokines and chemokines within the tumor
microenvironment, occurs in cancer cells and immune inflammatory
cells (78,79).
However, due to the intra- and inter-tumor
heterogeneity, targeting a single genetic event in sarcoma is
unlikely to produce favorable clinical outcomes. Furthermore,
understanding the role of Src in the initiation and progression of
sarcoma is at an early stage, and the mechanisms by which Src
affects the sarcoma microenvironment and the immune system remain
to be investigated. Optimal treatment may include surgical
resection combined with therapies that target the functional
processes involved in tumor biology and metastasis, including
chemotherapy and immunomodulation (80,81). The
Src protein exhibits high specificity and a positive predictive
value, highlighting its potential as a diagnostic marker for
certain types of sarcoma, such as osetosarcoma and Ewing's sarcoma.
Thus, Src inhibitors may present a novel type of chemotherapeutic
drug for the treatment of sarcoma, however, preclinical studies to
determine the optimal protein sequence for Src-targeted treatments
and methods to monitor the theapeutic effects of such are
required.
Acknowledgments
This work was supported by National Natural Science
Foundation of China (grant no. 81202115), the Key Project of Basic
Research of Shanghai (grant no. 11JC1410101), the Shanghai Pujiang
Program (grant no. 12PJ1407100), and the Excellent Young Talent
Program (grant no. XYQ2013108).
References
|
1
|
Valkov A, Kilvaer TK, Sorbye SW, et al:
The prognostic impact of Akt isoforms, PI3K and PTEN related to
female steroid hormone receptors in soft tissue sarcomas. J Transl
Med. 9:2002011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Helman LJ and Meltzer P: Mechanisms of
sarcoma development. Nat Rev Cancer. 3:685–694. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coindre JM: New WHO classification of
tumours of soft tissue and bone. Ann Pathol. 32:S115–S116. 2012.(In
French). PubMed/NCBI
|
|
4
|
Klein MJ and Siegal GP: Osteosarcoma:
anatomic and histologic variants. Am J Clin Pathol. 125:555–581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Bruijn DR, Allander SV, van Dijk AH, et
al: The synovial-sarcoma-associated SS18-SSX2 fusion protein
induces epigenetic gene (de)regulation. Cancer Res. 66:9474–9482.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Y, Gao D, Liu Y, Huang J, Lessnick S
and Tanaka S: IGF2 is critical for tumorigenesis by synovial
sarcoma oncoprotein SYT-SSX1. Oncogene. 25:1042–1052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kashima TG, Gamage NG, Dirksen U, Gibbons
CL, Ostlere SJ and Athanasou NA: Localized Ewing sarcoma of the
tibia. Clin Sarcoma Res. 3:22013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi EY, Gardner JM, Lucas DR, McHugh JB
and Patel RM: Ewing sarcoma. Semin Diagn Pathol. 31:39–47. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kämmerer PW, Shabazfar N, Vorkhshori
Makoie N, Moergel M and Al-Nawas B: Clinical, therapeutic and
prognostic features of osteosarcoma of the jaws - experience of 36
cases. J Craniomaxillofac Surg. 40:541–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steen S and Stephenson G: Current
treatment of soft tissue sarcoma. In: Proc (Bayl Univ Med Cent).
21. pp. 392–396. 2008; PubMed/NCBI
|
|
11
|
von Mehren M, Randall RL, Benjamin RS, et
al: National Comprehensive Cancer Network: Soft tissue sarcoma,
version 2.2014. J Natl Compr Canc Netw. 12:473–483. 2014.PubMed/NCBI
|
|
12
|
Clark MA, Fisher C, Judson I and Thomas
JM: Soft-tissue sarcomas in adults. N Engl J Med. 353:701–711.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demicco EG, Maki RG, Lev DC and Lazar AJ:
New therapeutic targets in soft tissue sarcoma. Adv Anat Pathol.
19:170–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Byeon SE, Yi YS, Oh J, Yoo BC, Hong S and
Cho JY: The role of Src kinase in macrophage-mediated inflammatory
responses. Mediators Inflamm. 2012:5129262012.PubMed/NCBI
|
|
15
|
Levinson WE, Varmus HE, Garapin AC and
Bishop JM: DNA of Rous sarcoma virus: its nature and significance.
Science. 175:76–78. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gojis O, Rudraraju B, Gudi M, et al: The
role of SRC-3 in human breast cancer. Nat Rev Clin Oncol. 7:83–89.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boggon TJ and Eck MJ: Structure and
regulation of Src family kinases. Oncogene. 23:7918–7927. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guarino M: Src signaling in cancer
invasion. J Cell Physiol. 223:14–26. 2010.PubMed/NCBI
|
|
19
|
Hunter T and Sefton BM: Transforming gene
product of Rous sarcoma virus phosphorylates tyrosine. In: Proc
Natl Acad Sci USA. 77. pp. 1311–1315. 1980; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roskoski R Jr: Src kinase regulation by
phosphorylation and dephosphorylation. Biochem Biophys Res Commun.
331:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bjorge JD, Jakymiw A and Fujita DJ:
Selected glimpses into the activation and function of Src kinase.
Oncogene. 19:5620–5635. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng XM, Resnick RJ and Shalloway D: A
phosphotyrosine displacement mechanism for activation of Src by
PTPalpha. EMBO J. 19:964–978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cooper JA, Gould KL, Cartwright CA and
Hunter T: Tyr527 is phosphorylated in pp60c-src: implications for
regulation. Science. 231:1431–1434. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levin VA: Basis and importance of Src as a
target in cancer. Cancer Treat Res. 119:89–119. 2004.PubMed/NCBI
|
|
25
|
Sirvent A, Benistant C and Roche S:
Oncogenic signaling by tyrosine kinases of the SRC family in
advanced colorectal cancer. Am J Cancer Res. 2:357–371.
2012.PubMed/NCBI
|
|
26
|
Cao M, Hou D, Liang H, et al: miR-150
promotes the proliferation and migration of lung cancer cells by
targeting SRC kinase signalling inhibitor 1. Eur J Cancer.
50:1013–1024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roskoski R Jr: Src protein-tyrosine kinase
structure and regulation. Biochem Biophys Res Commun.
324:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Huang WC, Zhang L, et al: SRC
family kinases as novel therapeutic targets to treat breast cancer
brain metastases. Cancer Res. 73:5764–5774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gargalionis AN, Karamouzis MV and
Papavassiliou AG: The molecular rationale of Src inhibition in
colorectal carcinomas. Int J Cancer. 134:2019–2029. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Je DW, O YM, Ji YG, Cho Y and Lee DH: The
inhibition of SRC family kinase suppresses pancreatic cancer cell
proliferation, migration, and invasion. Pancreas. 43:768–776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saini S, Majid S, Shahryari V, et al:
Regulation of SRC Kinases by microRNA-3607 located in a frequently
deleted locus in prostate cancer. Mol Cancer Ther. 13:1952–1963.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kulbe H, Levinson NR, Balkwill F and
Wilson JL: The chemokine network in cancer - much more than
directing cell movement. Int J Dev Biol. 48:489–496. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bjorge JD, Pang A and Fujita DJ:
Identification of protein-tyrosine phosphatase 1B as the major
tyrosine phosphatase activity capable of dephosphorylating and
activating c-Src in several human breast cancer cell lines. J Biol
Chem. 275:41439–41446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dehm SM and Bonham K: SRC gene expression
in human cancer: the role of transcriptional activation. Biochem
Cell Biol. 82:263–274. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mirmohammadsadegh A, Hassan M, Bardenheuer
W, et al: STAT5 phosphorylation in malignant melanoma is important
for survival and is mediated through SRC and JAK1 kinases. J Invest
Dermatol. 126:2272–2280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niu G, Bowman T, Huang M, et al: Roles of
activated Src and Stat3 signaling in melanoma tumor cell growth.
Oncogene. 21:7001–7010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song L, Morris M, Bagui T, Lee FY, Jove R
and Haura EB: Dasatinib (BMS-354825) selectively induces apoptosis
in lung cancer cells dependent on epidermal growth factor receptor
signaling for survival. Cancer Res. 66:5542–5548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Z, Lee FY, Bhalla KN and Wu J: Potent
inhibition of platelet-derived growth factor-induced responses in
vascular smooth muscle cells by BMS-354825 (dasatinib). Mol
Pharmacol. 69:1527–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schittenhelm MM, Shiraga S, Schroeder A,
et al: Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor,
inhibits the kinase activity of wild-type, juxtamembrane, and
activation loop mutant KIT isoforms associated with human
malignancies. Cancer Res. 66:473–481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakata Y, Tomkowicz B, Gewirtz AM and
Ptasznik A: Integrin inhibition through Lyn-dependent cross talk
from CXCR4 chemokine receptors in normal human CD34+ marrow cells.
Blood. 107:4234–4239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen YY, Malik M, Tomkowicz BE, Collman RG
and Ptasznik A: BCR-ABL1 alters SDF-1alpha-mediated adhesive
responses through the beta2 integrin LFA-1 in leukemia cells.
Blood. 111:5182–5186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Smolinska MJ, Page TH, Urbaniak AM, Mutch
BE and Horwood NJ: Hck tyrosine kinase regulates TLR4-induced TNF
and IL-6 production via AP-1. J Immunol. 187:6043–6051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lutz MP, Esser IB, Flossmann-Kast BB, et
al: Overexpression and activation of the tyrosine kinase Src in
human pancreatic carcinoma. Biochem Biophys Res Commun.
243:503–508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shor AC, Keschman EA, Lee FY, et al:
Dasatinib inhibits migration and invasion in diverse human sarcoma
cell lines and induces apoptosis in bone sarcoma cells dependent on
SRC kinase for survival. Cancer Res. 67:2800–2808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Michels S, Trautmann M, Sievers E, et al:
SRC signaling is crucial in the growth of synovial sarcoma cells.
Cancer Res. 73:2518–2528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Díaz-Montero CM, Wygant JN and McIntyre
BW: PI3-K/Akt-mediated anoikis resistance of human osteosarcoma
cells requires Src activation. Eur J Cancer. 42:1491–1500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Villacis RA, Silveira SM, Barros-Filho MC,
et al: Gene expression profiling in leiomyosarcomas and
undifferentiated pleomorphic sarcomas: SRC as a new diagnostic
marker. PLoS One. 9:e1022812014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bai Y, Li J, Fang B, et al:
Phosphoproteomics identifies driver tyrosine kinases in sarcoma
cell lines and tumors. Cancer Res. 72:2501–2511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Geng S, Wang X, Xu X, et al: Steroid
receptor co-activator-3 promotes osteosarcoma progression through
up-regulation of FoxM1. Tumour Biol. 35:3087–3094. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Scully SP, Layfield LJ and Harrelson JM:
Prognostic markers in chondrosarcoma: evaluation of cell
proliferation and of regulators of the cell cycle. Sarcoma.
1:79–87. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Watanabe T, Tsuda M, Tanaka S, et al:
Adaptor protein Crk induces Src-dependent activation of p38 MAPK in
regulation of synovial sarcoma cell proliferation. Mol Cancer Res.
7:1582–1592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Watanabe T, Tsuda M, Makino Y, et al:
Adaptor molecule Crk is required for sustained phosphorylation of
Grb2-associated binder 1 and hepatocyte growth factor-induced cell
motility of human synovial sarcoma cell lines. Mol Cancer Res.
4:499–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mazzone M and Comoglio PM: The Met
pathway: master switch and drug target in cancer progression. FASEB
J. 20:1611–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Spreafico A, Schenone S, Serchi T, et al:
Antiproliferative and proapoptotic activities of new
pyrazolo[3,4-d]pyrimidine derivative Src kinase inhibitors in human
osteosarcoma cells. FASEB J. 22:1560–1571. 2008.PubMed/NCBI
|
|
56
|
Hingorani P, Zhang W, Gorlick R and Kolb
EA: Inhibition of Src phosphorylation alters metastatic potential
of osteosarcoma in vitro but not in vivo. Clin Cancer Res.
15:3416–3422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Horng CT, Shieh PC, Tan TW, Yang WH and
Tang CH: Paeonol suppresses chondrosarcoma metastasis through
up-regulation of miR-141 by modulating PKCδ and c-Src signaling
pathway. Int J Mol Sci. 15:11760–11772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu CM, Li TM, Tan TW, Fong YC and Tang CH:
Berberine Reduces the Metastasis of Chondrosarcoma by Modulating
the α v β 3 Integrin and the PKC δ, c-Src, and AP-1 Signaling
Pathways. Evid Based Complement Alternat Med.
2013:4231642013.PubMed/NCBI
|
|
59
|
van Oosterwijk JG, van Ruler MA,
Briaire-de Bruijn IH, et al: Src kinases in chondrosarcoma
chemoresistance and migration: dasatinib sensitises to doxorubicin
in TP53 mutant cells. Br J Cancer. 109:1214–1222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Boyer B, Bourgeois Y and Poupon MF: Src
kinase contributes to the metastatic spread of carcinoma cells.
Oncogene. 21:2347–2356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang WS, Wang RJ, Ding JL, et al:
Caveolin-1: a novel biomarker for prostate cancer. Zhonghua Nan Ke
Xue. 18:635–638. 2012.(In Chinese). PubMed/NCBI
|
|
63
|
Mercier I and Lisanti MP: Caveolin-1 and
breast cancer: a new clinical perspective. Adv Exp Med Biol.
729:83–94. 2012.PubMed/NCBI
|
|
64
|
Cantiani L, Manara MC, Zucchini C, et al:
Caveolin-1 reduces osteosarcoma metastases by inhibiting c-Src
activity and met signaling. Cancer Res. 67:7675–7685. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Manara MC, Bernard G, Lollini PL, et al:
CD99 acts as an oncosuppressor in osteosarcoma. Mol Biol Cell.
17:1910–1921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Scotlandi K, Zuntini M, Manara MC, et al:
CD99 isoforms dictate opposite functions in tumour malignancy and
metastases by activating or repressing c-Src kinase activity.
Oncogene. 26:6604–6618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Abraham J, Chua YX, Glover JM, et al: An
adaptive Src-PDGFRA-Raf axis in rhabdomyosarcoma. Biochem Biophys
Res Commun. 426:363–368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Homsi J, Cubitt C and Daud A: The Src
signaling pathway: a potential target in melanoma and other
malignancies. Expert Opin Ther Targets. 11:91–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Creedon H and Brunton VG: Src kinase
inhibitors: promising cancer therapeutics? Crit Rev Oncog.
17:145–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Molina JR, Foster NR, Reungwetwattana T,
et al: A phase II trial of the Src-kinase inhibitor saracatinib
after four cycles of chemotherapy for patients with extensive stage
small cell lung cancer: NCCTG trial N-0621. Lung Cancer.
85:245–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Taylor JW, Dietrich J, Gerstner ER, et al:
Phase 2 study of bosutinib, a Src inhibitor, in adults with
recurrent glioblastoma. J Neurooncol. Nov 20–2014.(Epub ahead of
print).
|
|
72
|
Montero JC, Seoane S, Ocaña A and
Pandiella A: Inhibition of SRC family kinases and receptor tyrosine
kinases by dasatinib: possible combinations in solid tumors. Clin
Cancer Res. 17:5546–5552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Schrage YM, Briaire-de Bruijn IH, de
Miranda NF, et al: Kinome profiling of chondrosarcoma reveals
SRC-pathway activity and dasatinib as option for treatment. Cancer
Res. 69:6216–6222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Musumeci F, Schenone S, Brullo C and Botta
M: An update on dual Src/Abl inhibitors. Future Med Chem.
4:799–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Girotti MR, Lopes F, Preece N, et al:
Paradox-Breaking RAF Inhibitors that Also Target SRC Are Effective
in Drug-Resistant BRAF Mutant Melanoma. Cancer Cell. 27:85–96.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rotert JV, Leupold J, Hohenberger P, Nowak
K and Allgayer H: Src activity is increased in gastrointestinal
stromal tumors - analysis of associations with clinical and other
molecular tumor characteristics. J Surg Oncol. 109:597–605. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qayyum T, McArdle PA, Lamb GW, et al:
Expression and prognostic significance of Src family members in
renal clear cell carcinoma. Br J Cancer. 107:856–863. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sell H, Habich C and Eckel J: Adaptive
immunity in obesity and insulin resistance. Nat Rev Endocrinol.
8:709–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Spaeth E, Klopp A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hemmerle T, Probst P, Giovannoni L, Green
AJ, Meyer T and Neri D: The antibody-based targeted delivery of TNF
in combination with doxorubicin eradicates sarcomas in mice and
confers protective immunity. Br J Cancer. 109:1206–1213. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Spitaleri G, Berardi R, Pierantoni C, et
al: Phase I/II study of the tumour-targeting human monoclonal
antibody-cytokine fusion protein L19-TNF in patients with advanced
solid tumours. J Cancer Res Clin Oncol. 139:447–455. 2013.
View Article : Google Scholar : PubMed/NCBI
|