Introduction
Acute leukemia is a rare comorbidity during
pregnancy, with an incidence of 1 in 100,000 pregnancies (1). Acute promyelocytic leukemia (APL), a
subtype of acute myelogenous leukemia (AML), is a far more rare
disease, accounting for 10–15% of all adult AMLs (2). A diagnosis is confirmed by morphological
analysis, and immunological and cytogenetic studies (3). In total, ~40 cases of APL during
pregnancy or in the postpartum period have so far been reported
(4).
Current therapies, including all-trans
retinoic acid (ATRA) and anthracycline-based induction, and
anthracycline-based consolidation and maintenance, have been proven
to be extremely effective, with 5-year disease-free survival rates
of 70–80% (5). However, ATRA has
certain side-effects, a number of which may be fatal. The present
study describes the case of a pregnant patient with APL and
pregnancy-induced hypertension following the administration of
ATRA. Written informed consent was obtained from the patient.
Case report
A 23-year-old female (gravida 2, para 1) was
admitted to hospital during the sixth month of gestation with
symptoms of fever and diarrhea that had been apparent for 4 days.
The results of a routine blood test were abnormal, with a white
blood cell count of 1.44×109/l (normal range,
4.0–10.0×109/l), a neutrophil value of 0.68% (normal
range, 50–70%), a hemoglobin level of 95 g/l (normal range,
110–150g/l) and a platelet count of 31×109/l (normal
range, 100–300×109/l). The results of the blood cell
count that had been performed during an antenatal examination two
months prior had been normal. The patient was transferred to The
First Affiliated Hospital of Jishou University (Jishou, China) on
August 12, 2008. A physical examination revealed tenderness of the
sternum, a pot belly and mild dropsy in the lower extremities.
Blood chemistry on admission revealed 5.5 g/dl total
protein (normal range, 60.0–80.0 g/l) and 28 g/l albumin (normal
range, 40.0–55.0 g/l). Urine protein (++), 75 µmol/l serum
creatinine (normal range, 44–97 µmol/l) and 4.7 mmol/l blood urea
nitrogen levels (normal range, 3.2–7.1 mmol/l) were within the
normal limits. The International Organized Ratio was 0.9, the
activated partial thromboplastin time was normal (32 sec; control,
36 sec), the level of fibrinogen (FIB) was reduced to 0.8 g/l
(normal range, 2.0–4.0 g/l), and the level of fibrin degradation
products (FDP) was elevated to 40±80 mg/ml (normal range, 0±10
mg/ml). In addition, the level of D-Dimers was increased to 988
mg/l (normal range, 0±0.3 mg/l). A bone marrow examination revealed
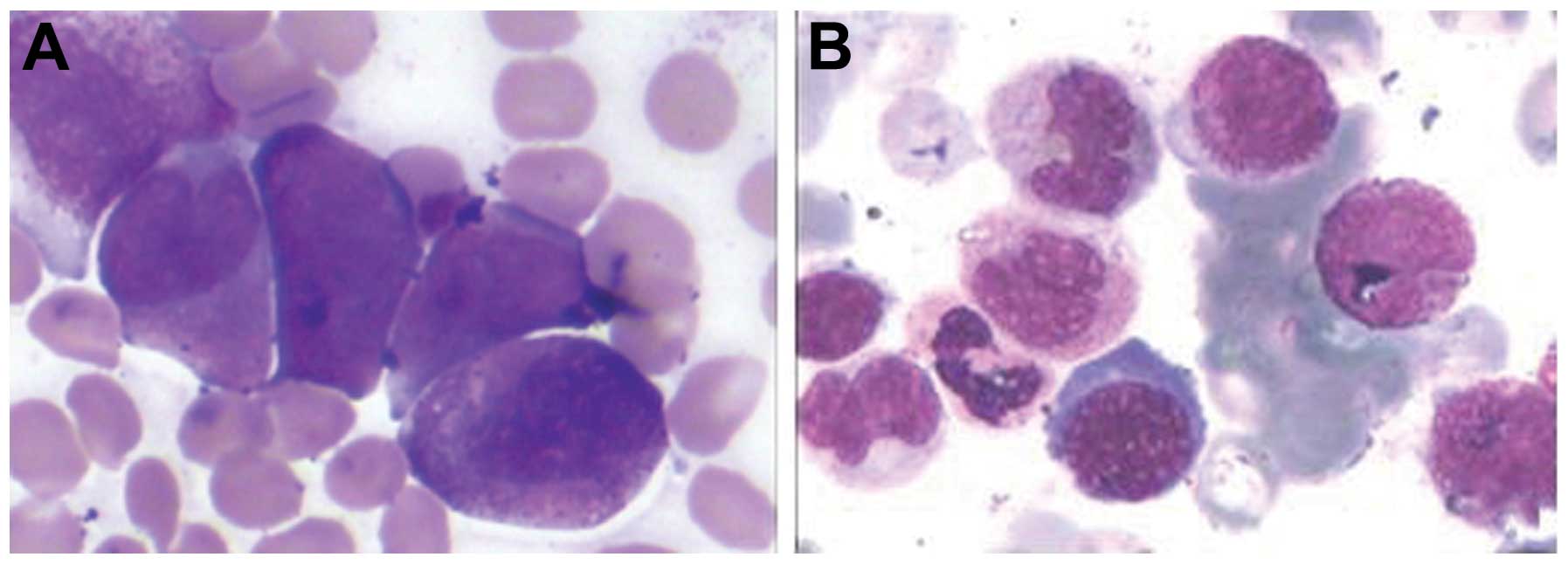
that >70% of the marrow cells were abnormal promyelocytic cells
(Fig. 1). A chromosomal abnormality,
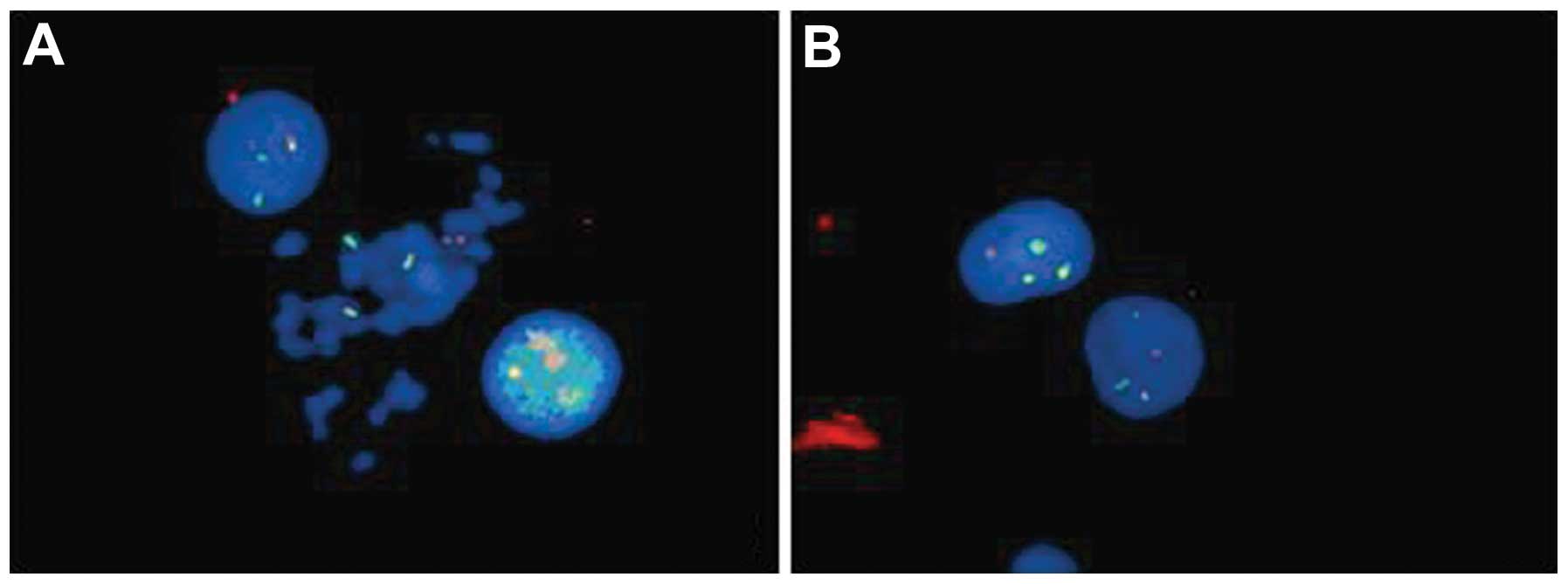
t(15;17), and an abnormal fusion gene product, promyelocytic
leukemia-retinoic acid receptor α (PML-RARα), were detected by
fluorescence in situ hybridization (Fig. 2). A diagnosis of APL, AML
French-American-British classification M3, was established. The
patient was subsequently treated with 45 mg/m2/day ATRA
at 26 weeks gestation. Within 4 days, the white blood cell count
rose to 20×109/l, the hemoglobin level increased to 98.5
g/l, the platelet count reached 46×109/l, the level of
FIB rose to 1.8 g/l and the level of FDP and D-dimers normalized.
However, on day 3 of treatment with ATRA, the patient complained of
chest distress and polypnea. A physical examination revealed dropsy
all over the body and an elevated blood pressure (149/103 mmHg) and
heart rate (110 bpm). Laboratory tests performed on the sixth day
revealed a hemoglobin level of 103 g/l, a platelet count of
46×109/l, a white blood cell count of
1.6×109/l with 76.9% neutrophils, 28 g/l albumin, 246
µmol/l blood creatinine and urine protein (++). The patient was
diagnosed with pregnancy-induced hypertension syndrome, rather than
retinoic acid syndrome (RAS), following the presentation of
pancytopenia. Fetal mortality in utero was established by
ultrasonic inspection on the eighth day. A cesarean delivery was
performed in order to terminate the pregnancy. Post-surgery, the
patient's blood pressure and heart rate returned to normal. The
dose of ATRA was reduced to 25 mg/m2/day from day 14.
Although the recovery of peripheral blood was slow, bone marrow
tests revealed complete remission morphologically and
cytogenetically, with no PML-RARa fusion transcript expression on
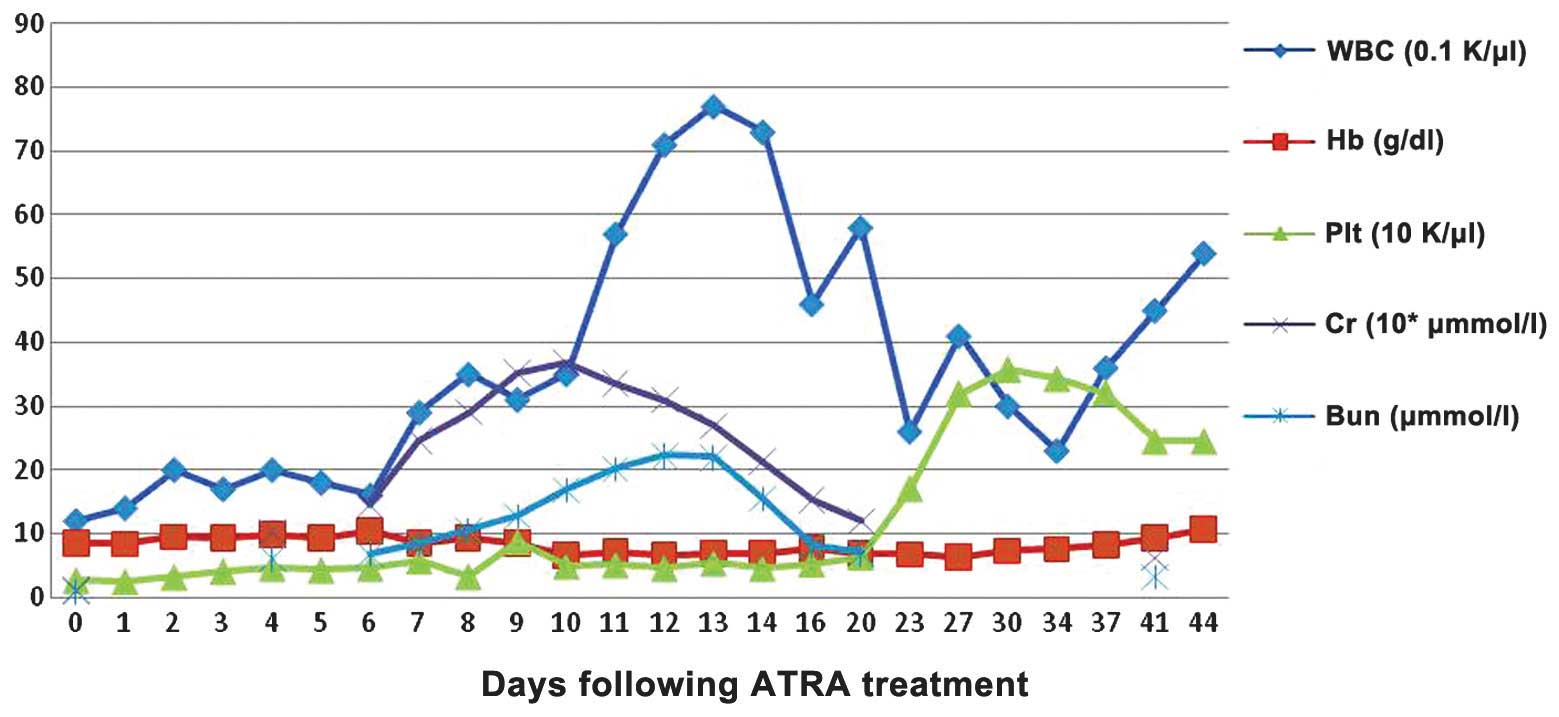
day 33. Hemogram remission was achieved on day 44 (Fig. 3).
Discussion
APL management is dependent upon the onset of
pregnancy-induced hypertension. If a diagnosis is established
during the first trimester, termination of the pregnancy followed
by the induction of leukemia-targeted therapies should be discussed
with the patient. As the pregnancy progresses into the second and
third trimester, the decision becomes problematic (6). Surgery appears to be the optimal
approach and the easiest to be accepted. When a decision has been
reached, the importance of normalized clotting function should be
emphasized to the patient. As disseminated intravascular
coagulation (DIC) is the leading cause of mortality in M3 patients,
correcting clotting function prior to abortion effectively
decreases the risk of bleeding (7).
In the present study, induced abortion under a condition of reduced
FIB posed the risk of DIC and increased bleeding. Therefore,
clotting function was corrected prior to the abortion. The clotting
function had almost returned to normal within 4 days of ATRA
treatment. However, the symptoms became progressively worse and
intrauterine fetal mortality occurred. This was believed to be
associated with severe anemia. Fortunately, the patient survived
the abortion and achieved bone marrow complete remission on day 26
of continuous ATRA treatment.
Although ATRA is an effective drug for the treatment
of APL, a number of side-effects, including hypercalcemia, male
infertility, bone marrow necrosis and fibrosis, acute pancreatitis,
acute damage of the liver and kidneys, erythema nodosum,
hyperhistaminemia, granulomatous proliferation, and certain
pulmonary complications may occur. In addition, ATRA confers the
risk of severe teratogenicity to the fetus during pregnancy
(8). However, data concerning the use
of ATRA in pregnancy is limited. Previous studies demonstrated that
ATRA was reasonably safe and well tolerated if not administered
during the first trimester of pregnancy. However, the most common
maternal side-effect of ATRA is the potentially lethal RAS, which
is characterized by fever, dyspnea, pulmonary infiltrates, pleural
or pericardial effusion and episodic hypotension (9). In the present study, similar symptoms
presented during ATRA treatment. However, due to the presentation
of pancytopenia, a diagnosis of RAS was not made. Instead, due to
no previous history of chronic hypertension, urinary tract
infection or renal disease, or additional complaints of polypnea or
palpitation, the symptoms were more likely to have resulted from
hypertension in pregnancy induced by treatment with ATRA. An APL
patient with ATRA-induced hypertension during pregnancy has not
been previously reported. Therefore, data concerning diagnoses and
therapeutic policies were limited.
Pregnancy-induced hypertension is a maternal
hypertension syndrome diagnosed during the later part of pregnancy,
usually after the 20th week of gestation (10). Due to the prompt aggravation of the
function of the kidneys and heart, it was suggested that the
pregnancy-induced hypertension in the present study may have
resulted from ATRA management. This is because pregnancy-induced
hypertension is less frequently observed in the second trimester of
pregnancy, and heart failure is rare in clinical course (11). In addition, the ATRA treatment may
have initiated or aggravated the pregnancy-induced hypertension
during the administration process. Therefore, it was concluded that
the pregnancy-induced hypertension in the present study resulted
from ATRA treatment.
To the best of our knowledge, limited data exists
with regard to the treatment of such patients. In the present
study, the patient demonstrated a good response to the treatment.
It is important for clinicians to carefully consider the
administration of ATRA, particularly in APL patients who are
pregnant.
References
|
1
|
Murrin RJ, Adjetey V, Harrison P and
Warwick A: Acute promyelocytic leukaemia presenting as postpartum
haemorrhage. Clin Lab Haematol. 26:233–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tallman MS: The expanding role of arsenic
in acute promyelocytic leukemia. Semin Hematol. 45(3 Suppl 2):
S25–S29. 2008.
|
|
3
|
Sham RL and Tallman MS: Treatment of acute
promyelocytic leukemia in the very elderly: Case report and review
of the literature. Leuk Res. 28:1347–1350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carradice D, Austin N, Bayston K and Ganly
PS: Successful treatment of acute promyelocytic leukaemia during
pregnancy. Clin Lab Haematol. 24:307–311. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lo-Coco F, Ammatuna E, Montesinos P and
Sanz MA: Acute promyelocytic leukemia: Recent advances in diagnosis
and management. Semin Oncol. 35:401–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fadilah SA, Hatta AZ, Keng CS, Jamil MA
and Singh S: Successful treatment of acute promyelocytic leukemia
in pregnancy with all-trans retinoic acid. Leukemia. 15:1665–1666.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morimatsu Y, Matsubara S, Hirose N,
Ohkuchi A, Izumi A, Ozaki K, Ozawa K and Suzuki M: Acute
promyelocytic leukemia: An unusual cause showing prolonged
disseminated intravascular coagulation after placental abruption.
Arch Gynecol Obstet. 277:267–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giagounidis AA, Beckmann MW, Giagounidis
AS, Aivado M, Emde T, Germing U, Riehs T, Heyll A and Aul C: Acute
promyelocytic leukemia and pregnancy. Eur J Haematol. 64:267–271.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tallman MS, Andersen JW, Schiffer CA,
Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Rowe JM, François C,
Larson RS and Wiernik PH: Clinical description of 44 patients with
acute promyelocytic leukemia who developed the retinoic acid
syndrome. Blood. 95:90–95. 2000.PubMed/NCBI
|
|
10
|
Satpathy HK, Fleming A, Frey D, Barsoom M,
Satpathy C and Khandalavala J: Maternal obesity and pregnancy.
Postgrad Med. 120:E01–E09. 2008.
|
|
11
|
Alwan S, Polifka JE and Friedman JM:
Angiotensin II receptor antagonist treatment during pregnancy.
Birth Defects Res A Clin Mol Teratol. 73:123–130. 2005. View Article : Google Scholar : PubMed/NCBI
|