Introduction
Pancreatic cancer is one of the most aggressive and
lethal malignant tumors, but despite this, is currently lacking in
efficient treatment options (1).
Among patients with metastatic disease, the median survival time is
~6 months, with a 5-year survival rate of only 2%, and a 1-year
survival rate of 17–23% reported following gemcitabine treatment
(2). First-line chemotherapy drugs
approved for the treatment of pancreatic cancer are gemcitabine and
5-fluorouracil, but gemcitabine in combination with nab-paclitaxel
or erlotinib has also been approved and has been demonstrated to
improve the progression-free survival (PFS) and overall survival
(OS) rates compared with those associated with gemcitabine alone,
together with acceptable side-effects (3). Treatment success in advanced pancreatic
cancer is based on the cancer disappearing or remaining stable, but
importantly, other factors such as the Eastern Cooperative Oncology
Group (ECOG) (4) performance status
and any potential side-effects must also be considered,
particularly for combination agents.
However, the majority of patients with advanced or
metastatic pancreatic cancer are elderly individuals with an ECOG
performance status of 2–3, who cannot tolerate the side-effects of
chemotherapy. Thus, advanced pancreatic cancer is a model illness
to study low toxicity anticancer treatments and supportive
management strategies (5). Palliative
treatment is currently the only beneficial treatment for end-stage,
metastatic pancreatic cancer. At the American Society of Clinical
Oncology (ASCO) annual meeting in 2013, Rodriguez et al and
Burke et al reported that only 4.2% of end-stage metastatic
pancreatic cancer patients received chemotherapy at the MD Anderson
Cancer Center (USA) between 2010 and 2012, and that 98.6% of these
patients only received standard agents (6,7). However,
even if a patient responds to treatment with a gemcitabine-based
agent, this has not been demonstrated to improve the quality of
life (QOL) significantly, and there is no evidence supporting a
favorable outcome associated with chemotherapy in elderly patients
with metastatic pancreatic cancer (8).
In recent years, improved symptom control in
patients with end-stage cancer by palliative care services has been
provided with a lot of attention. On one hand, palliative treatment
is capable of improving body mass index and QOL significantly,
which is associated with the OS time (9,10), but on
the other hand, it is worth questioning what alternatives to
palliative care are available, when chemotherapy or other
treatments associated with severe side-effects are not an option.
Therefore, the development of effective anticancer compounds with
low toxicity is critical for unresectable, advanced pancreatic
cancer that cannot be treated by chemotherapy. As previously
described, targeted therapies aimed at vascular endothelial growth
factor receptor (VEGFR), epidermal growth factor receptor (EGFR),
cyclooxygenase-2, mammalian target of rapamycin, cell cycle
check-points or proteasomes have been studied and are associated
with a relatively low toxicity (3).
The current study presents a novel angle on
understanding cancer and reports on the use of a novel systemic
therapy known as biological intra-control cancer treatment (BICT),
provided by Chengdu Fuxing Hospital (Chengdu, Sichuan, China) and
approved by the State Food and Drug Association (SFDA). This
treatment involves early palliative care and the herbal extract
combinations of ginseng (Panax ginseng C.A. Mey.), and White
Flower Patrinia Herb (Thlaspi arvense Linn.), Herba
Agrimonia (Agrimonia pilosa Ledeb.) and arginine (WHA),
among others, which are associated with low toxicity and have been
approved by the SFDA. BICT is intended to regulate interactive
signals between cells and to improve the QOL of patients. Our group
has recently found that BICT inhibits EGFR and VEGFR expression,
promotes apoptosis (unpublished data) and blocks the cell cycle in
the S phase (11). The present study
describes a novel herbal treatment used to improve survival time
and QOL in a terminal pancreatic cancer patient. Written informed
consent was obtained from the patient's family.
Case report
A 75-year-old female was diagnosed with
pathologically confirmed multi-metastatic pancreatic cancer (liver
metastatic adenocarcinoma, compatible with primary pancreatic
cancer), and presented with discomfort in the liver area and weight
loss (5 kg in 1 month) on November 23, 2012 at St. Anthony Hospital
North (Lakewood, CO, USA). The cancer was diagnosed as American
Joint Committee on Cancer stage IV pancreatic cancer (12), and the estimated survival time was
<3 months. A physical examination revealed upper right quadrant
tenderness without jaundice. A large solid and irregular mass could
be palpated in the upper right quadrant. The laboratory examination
findings were as follows: Serum liver enzyme levels were elevated
[alanine aminotransferase, 11 U/l (normal range, 0–40 U/l);
aspartate aminotransferase, 18 U/l (normal range, 0–49 U/l);
alkaline phosphatase, 92 U/l (normal range, 34–114 U/l); and
γ-glutamyl transferase, 50 U/l (normal range, 11–49 U/l)]; the
total bilirubin level was 7.4 µmol/l (normal range, 3.0–20.0
µmol/l); the international normalized ratio and albumin level were
normal; the serum tumor marker cancer antigen (CA)19-9 level was
81.42 U/ml (normal range, 0–27 U/ml); and the carcinoembryonic
antigen level was normal. Computed tomography (CT) scans showed a
large solid mass, 3.1×4.7×6.1 cm in size, near the pancreatic head
and multiple masses in the liver, with the largest mass measuring
~6.0×7.0 cm and being located in the inferior right hepatic lobe.
The right lobe of the liver appeared to be nearly completely
replaced by the neoplasm. Intra- and extra-hepatic bile duct
dilatations were also observed.
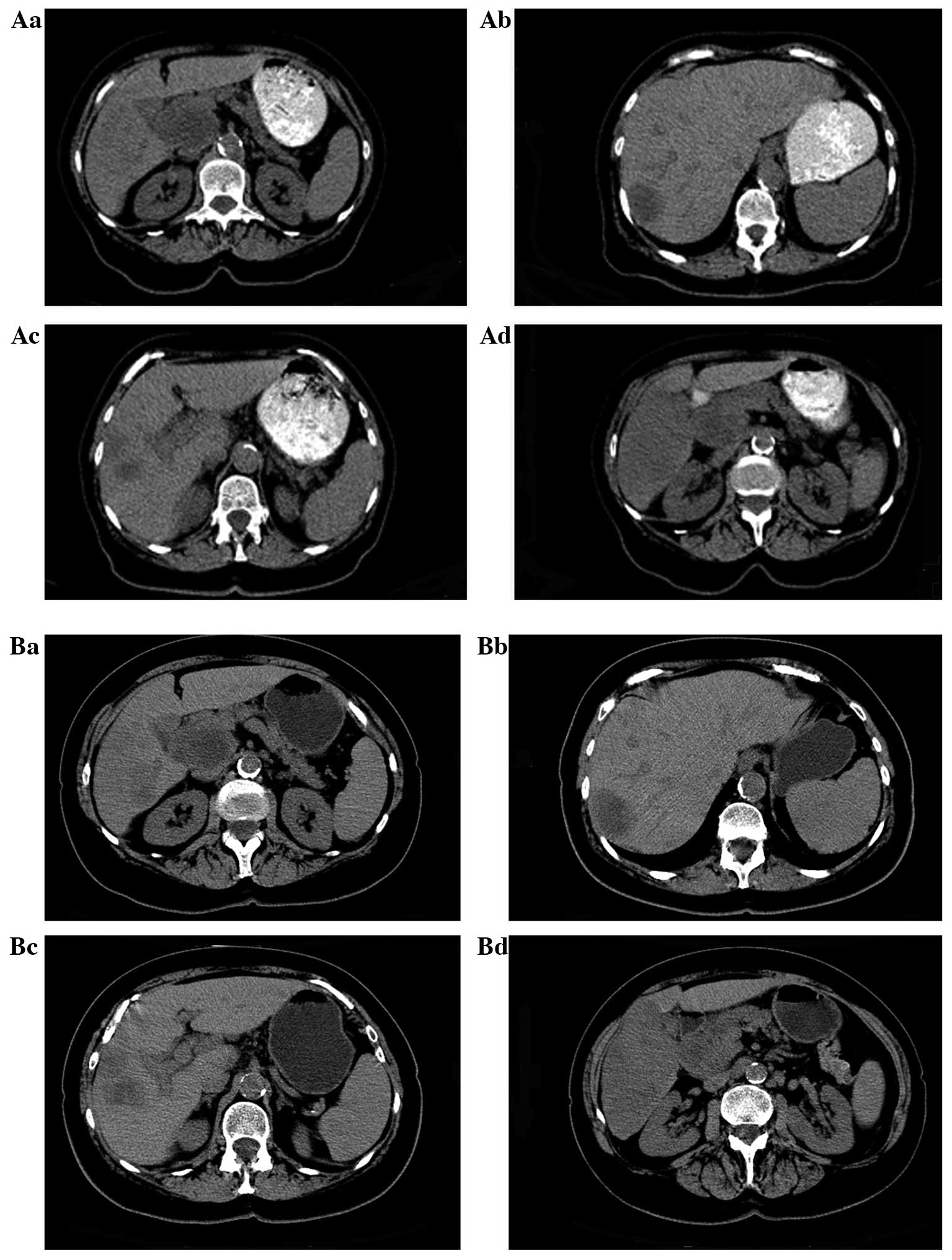
After recovering from a stent insertion on December
16, 2012, a baseline CT scan was performed and BICT was initiated
(Fig. 1A). At this time, the liver
masses were still expanding, since the pancreatic cancer was
diagnosed prior to the initiation of BICT. In comparison to the
results on November 26, 2012, the CT scan revealed that the largest
solid mass in the region of the inferior right hepatic lobe was
~6.0×10.9 cm, as compared to ~6.0×7.0 cm, and the smaller mass in
the liver had increased from 2.4×2.7 to 3.1×3.9 cm in one month.
The patient refused to undergo chemotherapy due to an ECOG
performance status of 3, and a survival time of <3 months was
estimated by the oncologist. The WHA herbal extract combination was
orally administered as a 3.0-g capsule plus 15 ml in liquid form
four times daily for a total of 57 days. Furthermore, hyperthermia
was induced in the upper abdominal area to promote drug absorption,
and abdominal palpation was performed every week to evaluate the
size of the liver and to assess the risk of bleeding. Bleeding was
not noted, and after two months of treatment, on February 11, 2013,
an abdominal CT scan revealed a stable pancreatic lesion and no new
metastatic lesions (Fig. 1B).
According to the Response Evaluation Criteria in Solid Tumors
(RECIST), the liver disease was under control (stable disease) with
no discomfort or pain in the abdomen (13). Tumor marker CA19-9 and bilirubin
levels were normal, and the clinical improvement was confirmed by
imaging tests.
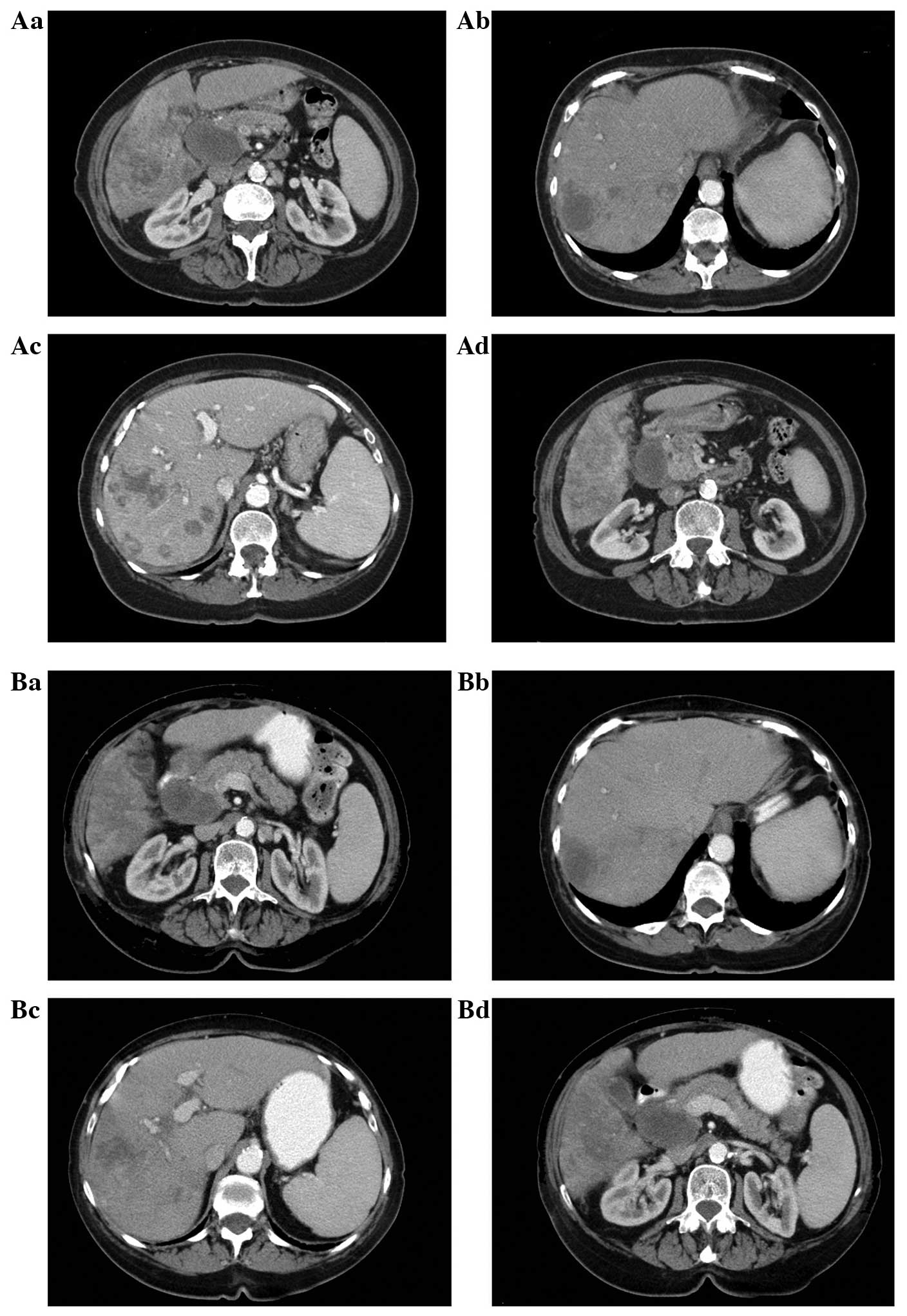
Between March and June 2013, the patient did not
receive BICT due to drug shipment issues between China and the
United States. CT was performed again 4 months after the initiation
of treatment, on May 24, 2013, and revealed that the large lesion
had not changed in size, but that new small metastatic masses had
developed (Fig. 2A). From June 23,
2013, the patient received functional medicinal treatment, a type
of palliative treatment, in Bangkok, and BICT was reinitiated. This
resulted in clinical and QOL improvements. The tumor marker levels
were restored to normal and the tumor masses exhibited a stable
disease state (Fig. 2B). The patient
has remained alive for 11 months since the initial diagnosis.
Discussion
Pancreatic cancer can be classified as resectable or
unresectable. The prognosis is much better for resectable
pancreatic cancer than for unresectable cancer cases. Surgery is
considered as the only potential cure for the disease, and the mean
5-year survival rate increases to >20% in those who have
undergone a resection as part of their multimodal therapy (14–16).
Furthermore, complete tumor resection, in addition to
tumor-specific characteristics, including CA19-9 concentration,
tumor size and differentiation, lymph node involvement, and
perineural infiltration or lymphovascular invasion, are likely to
be the prognostic factors that are the most relevant for patients
with resectable pancreatic cancer (15). Adjuvant treatment following surgery
has been has been associated with a reduced risk of relapse and
extended survival, due to the high recurrence risk, as demonstrated
by the European Study Group of Pancreatic Cancer (ESPAC) in the
ESPAC-1 and ESPAC-3 trials (17). The
CONKO-001 and Asian JSAP-02 trials have furthermore confirmed that
surgery in addition to adjuvant chemotherapy is associated with
increased disease-free survival, median survival and 5-year OS
rates compared with surgery alone (16,18,19). The
major adjuvant treatments after resection of pancreatic cancer
traditionally include chemotherapy, fluorouracil-based
chemo-radiation, and chemo-radiation plus chemotherapy. However,
the optimum treatment remains controversial. Evidently, there is a
definite requirement for the development of alternative agents with
novel mechanisms of action to treat this disease. The reality is
that a number of patients, and particularly elderly patients,
suffer from advanced or metastatic pancreatic cancer that is
unresectable. Liver metastasis is an independent adverse predictor
of survival, and for patients with unresectable pancreatic cancer,
liver metastasis is associated with an extremely poor prognosis.
Even if patients can tolerate chemotherapy, the average OS time is
~9 months according to one previous study (20). A recent clinical trial demonstrated
that the median OS time could be extended to 8.5 months in patients
treated with nab-paclitaxel-gemcitabine, as compared with 6.7
months in patients treated with gemcitabine alone (2), suggesting that patients may benefit from
combination regimens. Hence, if the patients have the opportunity
to undergo surgery, or are able to tolerate chemotherapy, this is
an effective strategy.
One of the most difficult and challenging barriers
to overcome is how to deal with elderly, end-stage pancreatic
cancer patients, particularly those who cannot tolerate
chemotherapy and have a life expectancy of <3 months. Few
studies have addressed this, partly since chemotherapy will
severely affect the QOL of patients in this group (21,22). For
this reason, and since cancer is not just a mass, but rather a
syndrome associated with systemic comorbidities that negatively
impact the QOL and overall health of the patient, palliative
treatment has become a focus of attention (23). Palliative care focuses on managing
symptoms and on psychosocial support. Temel et al (24) demonstrated that early palliative care
can improve QOL and prolong survival in patients with metastatic
cancer compared with standard care alone. In recent years, cancer
has been considered a systemic disease. According to the RECIST,
evaluation criteria only focus on the tumor mass size and not on
the entire body, and consider factors such as OS or PFS that are
only indicative of the tumor size and not QOL. For advanced
pancreatic cancer, not only is it difficult to control the
pancreatic cancer mass itself, but the QOL and OS generally cannot
be improved significantly as well. As aforementioned, in the 2013
ASCO meeting, the current methods of treating advanced pancreatic
cancer were questioned and it was concluded that chemotherapy may
not always be the only answer. For patients classified as having an
ECOG performance status of 3–4, no standard chemotherapy regimen
has been established owing to the associated high toxicity and low
effectiveness of these drugs. For these patients, individual care
is commonly employed (18).
Thus, if possible, in addition to supportive and
palliative treatments, patients should receive low toxicity
anticancer treatments to control the cancer, as a novel strategy to
prolong life. Novel treatment strategies based on this idea are
currently being developed in laboratories and clinics worldwide.
One of these novel treatments is BICT; a systemic therapy
comprising palliative care with herbal extract combinations of
P. ginseng and WHA, among others, which could inhibit cancer
growth and improve symptoms. Although the cell toxicity of BICT is
not as strong as that of gemcitabine-based combinations, it remains
effective, while being much less toxic. Herbal extract combinations
such as WHA have previously been demonstrated to be effective
against colon and liver cancer in vitro and in vivo,
and have been demonstrated to possess anti-angiogenic
characteristics. We have recently shown that EGFR and VEGFR
expression can be inhibited and that apoptosis can occur in
vivo in breast cancer and rectal cancer models (unpublished
data).
In summary, the patient described in the present
case study was a 75-year-old female with end-stage pancreatic
cancer and multiple liver metastases, with a predicted survival
time of only 3 months. Due to the patient's advanced age and the
late stage of the disease, surgery and chemotherapy were not
suitable treatment options. For these types of patients, the goals
of treatment are to decrease or control tumor burden, in order to
reduce the risk of jaundice and bleeding in the liver, which are
independent prognostic factors. The use of BICT enabled the
achievement of this goal, and the patient has thus far survived for
11 months post-diagnosis. Furthermore, according to the Common
Terminology Criteria for Adverse Events, version 3.0, no vomiting
or hematological toxicity of more than grade 1 severity was
observed (25). During the entire
treatment period, only ibuprofen was used as an analgesic. Oral
BICT is administered daily and can be administered at home. Hence,
this treatment is not limited by the requirement for travel or
ambulation, resulting in high levels of compliance. In the present
case, BICT was successful in improving the QOL and prolonged the
patient's survival time.
This study describes a novel approach for treating
advanced metastatic pancreatic cancer, and supports the use of BICT
in patients with an ECOG performance status of 2/3, thereby
enabling the prolongation of survival in patients who cannot
tolerate chemotherapy and resulting in an improvement in their QOL.
Further research should be performed prior to conclusions being
formed with regard to the mechanisms underlying BICT and the
associated benefits.
References
|
1
|
Breuer S, Maimon O, Appelbaum L, et al:
TL-118-anti-angiogenic treatment in pancreatic cancer: a case
report. Med Oncol. 30:5852013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Von Hoff DD, Ervin T, Arena FP, et al:
Increased survival in pancreatic cancer with nab-paclitaxel plus
gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zagouri F, Sergentanis TN, Chrysikos D, et
al: Molecularly targeted therapies in metastatic pancreatic cancer:
a systematic review. Pancreas. 42:760–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shahrokni A and Saif MW: Metastatic
pancreatic cancer: The dilemma of quality vs. quantity of life.
JOP. 14:391–394. 2013.PubMed/NCBI
|
|
6
|
Rodriguez MA, DeJesus YA, Cheng L, Buzdar
A and Burke TW: Factors related to end-of-life (EOL) chemotherapy
in solid tumor (ST) patients. J Clin Oncol. 31:abstract 9538.
2013.
|
|
7
|
Burke T, DeJesus Y, Cheng L, Buzdar A and
MA R: Pattern of chemotherapy use at end-of-life (EOL) in patients
with solid tumors (ST). J Clin Oncol. 31:abstract 9539. 2013.
|
|
8
|
Romanus D, Kindler HL, Archer L, et al:
Does health-related quality of life improve for advanced pancreatic
cancer patients who respond to gemcitabine? Analysis of a
randomized phase III trial of the cancer and leukemia group B. J
Pain Symptom Manage. 43:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li D, Morris JS, Liu J, Hassan MM, Day RS,
Bondy ML and Abbruzzese JL: Body mass index and risk, age of onset
and survival in patients with pancreatic cancer. JAMA.
301:2553–2562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greer JA, Pirl WF, Jackson VA, et al:
Effect of early palliative care on chemotherapy use and end-of-life
care in patients with metastatic non-small-cell lung cancer. J Clin
Oncol. 30:394–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Zhang L and Li Y: Inhibition of
cancer cell proliferation by color modulation: A pilot study. Ann
Oncol. 24 (Suppl 9):ix832013. View Article : Google Scholar
|
|
12
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging handbook. AJCC Cancer
Staging Manual. 7th. Springer; New York: pp. 63–79. 2010
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, et al: New response evaluation criteria in
solid tumours: Revised RECIST guideline (version 1.1). Eur J
Cancer. 45:228–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neoptolemos JP, Stocken DD, Bassi C, et
al: Adjuvant chemotherapy with fluorouracil plus folinic acid vs
gemcitabine following pancreatic cancer resection: a randomized
controlled trial. JAMA. 304:1073–1081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartwig W, Werner J, Jäger D, et al:
Improvement of surgical results for pancreatic cancer. Lancet
Oncol. 14:e476–e485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Helmut Oettle SP, Peter Neuhaus, Klaus
Gellert, et al: Adjuvant chemotherapy with gemcitabine vs
observation in patients undergoing curative: intent resection of
pancreatic cancer - a randomized controlled trial. JAMA.
297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neoptolemos JP, Stocken DD, Tudur Smith C,
et al: Adjuvant 5-fluorouracil and folinic acid vs observation for
pancreatic cancer: composite data from the ESPAC-1 and -3(v1)
trials. Br J Cancer. 100:246–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oettle H, Neuhaus P, Hochhaus A, et al:
Adjuvant chemotherapy with gemcitabine and long-term outcomes among
patients with resected pancreatic cancer: the CONKO-001 randomized
trial. JAMA. 310:1473–1481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ueno H, Kosuge T, Matsuyama Y, et al: A
randomised phase III trial comparing gemcitabine with surgery-only
in patients with resected pancreatic cancer: Japanese study group
of adjuvant therapy for pancreatic cancer. Br J Cancer.
101:908–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niu L, Chen J, He L, Liao M, Yuan Y, Zeng
J, Li J, Zuo J and Xu K: Combination treatment with comprehensive
cryoablation and immunotherapy in metastatic pancreatic cancer.
Pancreas. 21:1143–1149. 2013. View Article : Google Scholar
|
|
21
|
Epstein AS, Abou-Alfa GK, Shamseddine A,
Al-Olayan A, Ang C, Naghy M, Lowery MA and O'Reilly EM:
Communication and palliative care in a 64-year-old man with
pancreatic adenocarcinoma. Gastrointest Cancer Res. 5:130–134.
2012.PubMed/NCBI
|
|
22
|
Panagiotarakou M, Gupta A, Syrigos K and
Saif MW: Use of supportive care for symptom management in
pancreatic cancer: application of clinical research to patient
care. JOP. 13:342–344. 2012.PubMed/NCBI
|
|
23
|
Paul E and Kenneth P Olive: Pancreatic
cancer: why is it so hard to treat. Ther Adv Gastroenterol.
6:321–337. 2013. View Article : Google Scholar
|
|
24
|
Temel JS, Greer JA, Muzikansky A,
Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD,
Jacobsen J, Pirl WF, Billings JA and Lynch TJ: Early palliative
care for patients with metastatic non-small-cell lung cancer. N
Engl J Med. 363:733–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, et al: CTCAE v3.0: Development of a comprehensive grading
system for the adverse effects of cancer treatment. Semin Radiat
Oncol. 13:176–81. 2003. View Article : Google Scholar : PubMed/NCBI
|