Introduction
The B16 melanoma cell exhibits low immunogenicity
and lacks a major histocompatibility complex-I molecule. The
transplanted B16 melanoma mouse model is widely used to study the
immunology and immunological escape of tumor cells (1). In the advanced stage, transplanted B16
melanomas almost always develop lung metastases. Antitumor
immunology has been widely studied, including the use of tumor
vaccines, adoptive lymphocyte treatment and tumor-frozen treatment
(2). These tactics share the same
mechanism of activating the immunological cells to kill the tumor
cells. The immunological cells, including macrophages, γδT
lymphocytes, cytotoxic lymphocytes and the adoptively transferred
tumor-specific lymphocytes may elicit graft-versus-host disease,
although the possibility is extremely low (3). The activated cells achieve their
functions by direct interaction with the tumor cells, such as
through the use of the Fas/FasL killing mechanism, or by indirect
interaction with the tumor cells (4).
The immunological cells usually secrete a number of cytokines,
including tumor necrosis factor-α, interferon and interleukin
(IL)-12, which often induce tumor cell apoptosis or necrosis
(5).
The use of tumor vaccines as tumor therapy has been
explored for numerous decades, and a number of positive results
have been achieved, particularly in combination with other
therapeutics. A number of vaccines can markedly activate the host
immune system to kill the tumor cells, such as the BORIS-based
vaccine, which has been shown to increase effector cluster of
differentiation (CD)4+ and CD8+ T cell
infiltration towards a 4T1 mammary implanted tumor (6). Another common method is the use of
dendritic cells (DCs), the most potent antigen-presenting cells,
which can stimulate the T cells. As DCs can be loaded with a number
of varying types of antigen, DCs can effectively stimulate
cytotoxic T lymphocytes (CTL) (7).
Recently, vaccination techniques have been combined with
nanotechnology to synthesize antitumor vaccines that target certain
tumor cellular antigens. The approach stimulates the body to
generate long-lasting antibodies, and these antibodies can
selectively target the antigens in the tumor cells, resulting in
eventual tumor cell death (8). Whole
tumor cell vaccines have shown great potential with regard to their
antitumor effects; irradiated tumor cells pulsed with an adjuvant
can stimulate the CD4+ T cell-mediated adaptive immune
response (9,10), while another strategy is to fuse the
tumor cell lysate with dendritic cells, which have robust efficacy
in expanding antigen-specific CD8+ T cells (11). Formalin-fixed B16 cells together with
IL-12 show a strong antitumor response, which is mediated by
CD4+ and CD8+ T cells (12). The use of whole tumor cells as
antitumor vaccines is effective in activating tumor infiltrating
lymphocytes (TILs), and as the tumor cells contain various known
and unknown antigens, besides proteins, they can cause a number of
side-effects, including inflammatory reactions (13). When whole tumor cell lysates are fused
with DCs, the whole vaccines can exert much strong antitumor
effects (14). However, the effective
components of whole tumor cell lysates have not been fully
investigated and remain unclear to a certain extent. An ideal
vaccine remains to be discovered or engineered.
TILs have long been considered as the main effector
of antitumor immune responses (15).
The presence of TILs in human cancers shows that the immune system
recognizes the tumor to a certain degree. Studies have shown the
benefit of TILs in human cancers, particularly with regard to the
number of CD8+ T cells (16,17). TILs
contain numerous lymphocytes, including CD4+ T cells,
CD8+ T cells and CD20+ B cells. However, a
number of studies have indicated that the activated CD8+
T cells are the major functioning cells in an antitumor
immunological reaction. CD8+ T cells can induce tumor
cell apoptosis or tumor rejection through direct or indirect
contact with targeted tumor cells. Previous studies have suggested
that high levels of TILs are correlated with a better prognosis
(17). CTLs are crucial in antitumor
immune responses (18,19); CD4+ T cells aid in the
activation of CD8+ T cells and then induce
CD8+ T cells to kill the tumor cells. Although
CD4+ T cells are not the main effectors, they are often
indispensable in this process. Studies concerning
CD4+/CD25+/Foxp3+ Treg cells have
also become a focus of attention. These cells usually have negative
regulatory effects in antitumor responses. Treg can suppress the
antitumor immune responses and maintain the state of immunological
unresponsiveness during the process (20).
In the present study, the B16 melanoma culture
supernatant was isolated and the isolated purified fragments were
used to determine the in vitro and in vivo antitumor
effects, with the aim of identifying potential novel treatment
avenues for melanoma.
Materials and methods
Materials
C57BL/6 mice, 8–10 weeks old and weighing 18–20 g,
were obtained from Xuzhou Medical College Experiment Animal Center
(Xuzhou, Jiangsu, China). All surgical procedures and normal
experimental processes were performed in accordance with the
guidelines of the Xuzhou Medical College Animal Care and Use
Committee. This study was approved by the ethics committee of
Xuzhou Medical College. B16 melanoma cells were purchased from
Shanghai Institutes for Life Science, the Chinese Academy of
Sciences (Shanghai, China). The B16 cells were syngeneic with the
C57BL/6 mice used for the vaccination. The B16 cells were cultured
with RPMI 1640 medium supplemented with 10% heat inactivated fetal
bovine serum (FBS) (Zhejiang Tianhang Biological Technology Co.,
Ltd., Hangzhou, China), 100 U/ml penicillin and 100 µg/ml
streptomycin. Fluorescein isothiocyanate (FITC)-conjugated
anti-mouse CD4 antibody, FITC-conjugated anti-mouse CD8a antibody
and phycoerythrin (PE)-conjugated anti-mouse CD20 antibody were
purchased from BioLegend (San Diego, CA, USA), and PE-conjugated
anti-mouse CD20 was purchased from eBioscience (San Diego, CA,
USA). Cell counting kit-8, used to determine the cytotoxicity of
the TILs, was purchased from Beyotime Institute of Biotechnology
(Haimen, Jiangsu, China), and EZ-SepTM Mouse 1X Lymphocyte
Separation Medium was purchased from Dakewe Biotech Company
(Shenzhen, Guangdong, China).
Selective isolation of B16 melanoma
supernatant elements and preparation of mouse spleen
lymphocytes
The B16 cells were cultured as previously reported
(21). Briefly, B16 cells were
cultured in RPMI 1640 medium (Gibco Life Technologies, Carlsbad,
CA, USA). After 48 h, the medium was collected and centrifuged at
10,000 × g at 4°C for 10 min. The supernatant was then collected
for analysis of its elements in an ultrafiltration centrifuge tube.
The isolated elements consisted of fragments with sizes of 3–5,
5–10, 10–30, 30–50, 50–100, 100–300 and >300 KDa. A C57BL/6 mice
were euthanized and then the spleen was dissected, minced into
small pieces, passed sequentially through cell strainers (40 µm)
and washed in RPMI 1640 without FBS.
The 50–100 and 100–300-KDa molecular weight
fractions, original supernatant and RPMI 1640 medium were used to
carry out the chemotaxis experiment in a Boyden chamber. The
lymphocytes were placed in the lower compartment and the
aforementioned elements were placed in the upper chamber.
Quantification of the number of lymphocytes in the lower
compartment was performed at the 0.5, 2, 4, 8, 12 and 24-h
time-points.
Immunizations and analyses of tumor
growth or lung metastases
Five groups of C57BL/6 mice (n=8 per group) were
subcutaneously (s.c.) injected into the right flanks twice with the
50–100-KDa molecular weight fraction, the 100–300-KDa molecular
weight fraction, repeatedly freeze-thawed B16 melanoma cells,
original supernatant or RPMI 1640 medium. After two weeks, 200 µl
B16 cells at a concentration of 1×106/ml were injected s.c. at day
14 into the right armpit of the mice. Tumor growth was monitored
daily once the tumor became palpable at day 20, six days after B16
cell implantation. The tumor volume was determined by
two-dimensional measurements and calculation using the formula (a ×
b2) / 2, where a represents the largest diameter and b the smallest
diameter of the tumor. On day 21 post-tumor implantation, all the
mice were sacrificed and the number of lung metastases in the lungs
were counted, and the tumor and lung pathology characteristics were
analyzed using hematoxylin and eosin (HE) staining.
Cytotoxicity of TILs and splenic
lymphocytes (SPLs)
On day 21, the tumor and spleen were surgically
removed from each tumor-bearing mouse, and TILs and SPLs were
isolated as aforementioned. The TILs were mixed with the B16 cells
at effector:target (E:T) ratios of 12.5:1, 25:1 and 50:1. The
cytotoxicity of the TILs was determined in a 96-well plate using a
CCK-8 kit, and each experiment was repeated three times. The
cytotoxicity of the SPLs was determined using the same method.
Analysis of TIL and SPL
populations
Once the tumor and spleen had been surgically
removed from each tumor-bearing mouse, the tissues were each minced
and digested in 1 mg/ml collagenase type IV, then subjected to
filtration and washing. The cells were then stained with
anti-CD4-FITC, anti-CD28-PE, anti-CD8-FITC, anti-CD28-PE and
anti-CD20-PE antibodies. The obtained TILs and SPLs were each
adjusted to 1×106/ml. Following incubation with fluorescence
antibodies for 30 min in the dark on ice, the cell suspensions were
analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose,
CA, USA).
Statistical analysis
One-way repeated measures analysis of variance was
used for the statistical analysis, and P<0.05 was considered to
indicate a statistically significant difference. The data were
expressed as the means ± standard error of the mean and were
representative of three different experiments.
Results
Characterization of B16 cell culture
isolated purified fragments
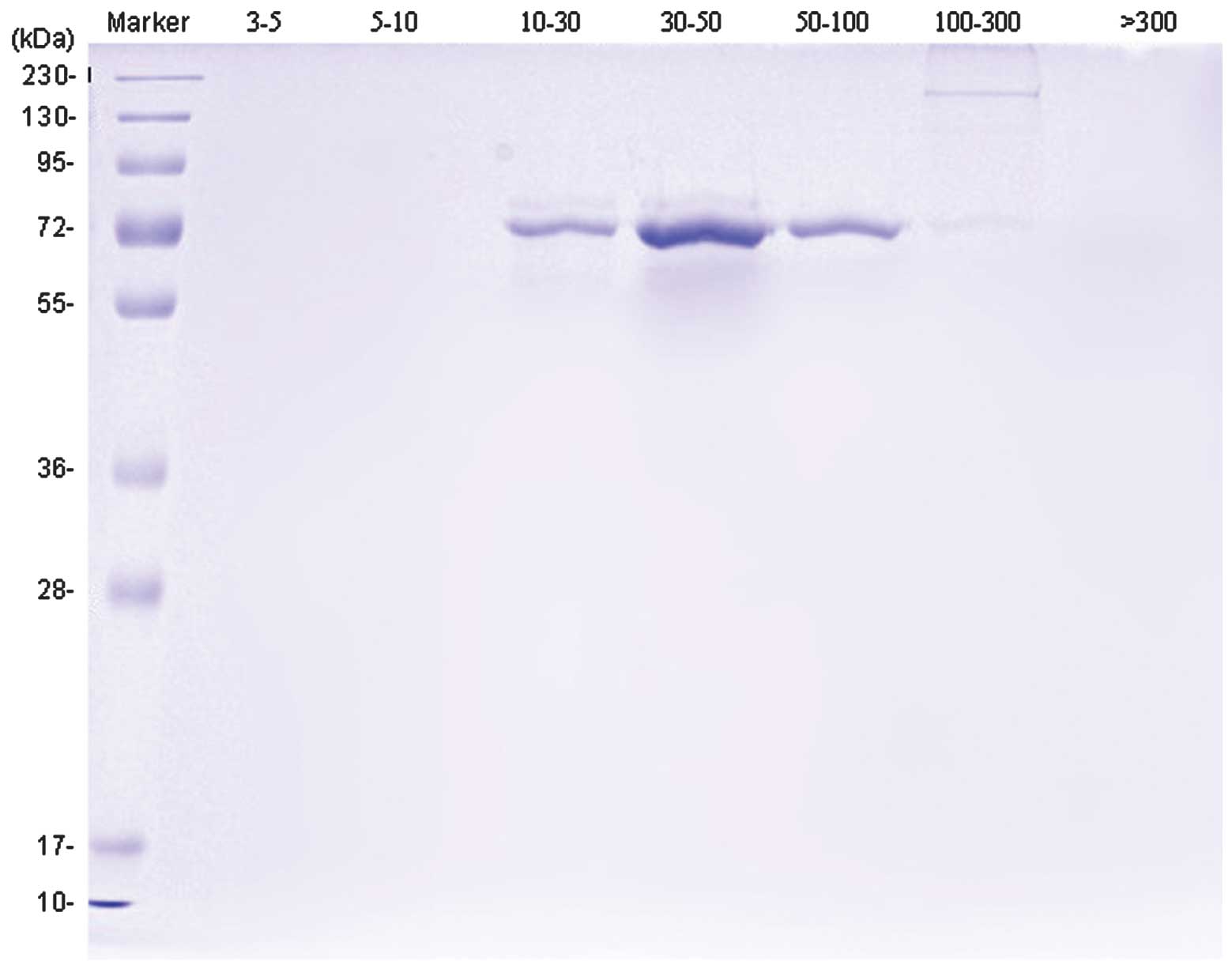
Subsequent to separation and purification, SDS-PAGE
gel electrophoresis showed that the B16 cell culture supernatant
was comprised of proteins with molecular weights of ~70 or 130–250
KDa, although much more of the 70-KDa protein was present in
comparison. No obvious bands were apparent in other lanes (Fig. 1).
Evaluation of chemotaxis of isolated
purified fragments
With regard to the 50–100 and 100–300-KDa molecular
weight fractions, the original supernatant and the RPMI 1640
medium, the chemotaxis of the four groups was enhanced with
increasing chemotaxis time intervals. The quantity of lymphocytes
attracted by the chemotaxis reached a summit at the 8-h time-point.
At the 8-h time-point, the level of chemotaxis lymphocytes in the
50–100 KDa group was the highest at 103.33±5.86×104/ml, while the
second highest level of 78.33±5.69×104/ml was found in the 100–300
KDa group. These levels were significant compared with the other
three groups, respectively, (P<0.05) (Table I).
 | Table I.B16 cell culture supernatant isolated
purified fragment chemotaxis (mean ± standard error of the mean;
n=4). |
Table I.
B16 cell culture supernatant isolated
purified fragment chemotaxis (mean ± standard error of the mean;
n=4).
|
| Time-points |
|---|
|
|
|
|---|
| Group | 0.5 h | 2 h | 4 h | 8 h | 12 h | 24 h |
|---|
| 50–100 KDa |
2.13±0.31 |
5.50±0.20 |
64.00±3.61 |
103.33±5.86a,b |
91.33±3.21a,b |
95.00±2.00a,b |
| 100–300 KDa |
2.00±0.25 |
5.07±0.40 |
49.67±3.51 |
78.33±5.69a |
77.33±2.52a |
79.00±2.65a |
| Original
supernatant |
3.40±1.06 |
9.67±2.08 |
18.00±2.00 |
47.67±2.08a |
47.33±1.53a |
48.33±1.53a |
| RPMI 1640 |
2.10±0.36 |
3.50±0.50 |
10.00±0.00 |
17.00±2.00 |
17.00±1.00 |
18.00±1.00 |
Evaluation of therapeutic potency of
isolated purified fragments
The separated and purified protein, repeatedly
freeze-thawed B16 cells, original supernatant and RPMI 1640 medium
were used to vaccinate five groups of C57BL/6 mice. All mice
manifested no changes in living conditions. The tumors of all five
groups of mice became palpable on day 6 post-transplantation; the
tumor growth of the 50–100 KDa group was the slowest, while the
repeatedly freeze-thawed B16 cell group was the second slowest
among the five groups. The tumors of the RPMI 1640 group grew the
fastest compared with the 50–100 KDa group on the same day, and the
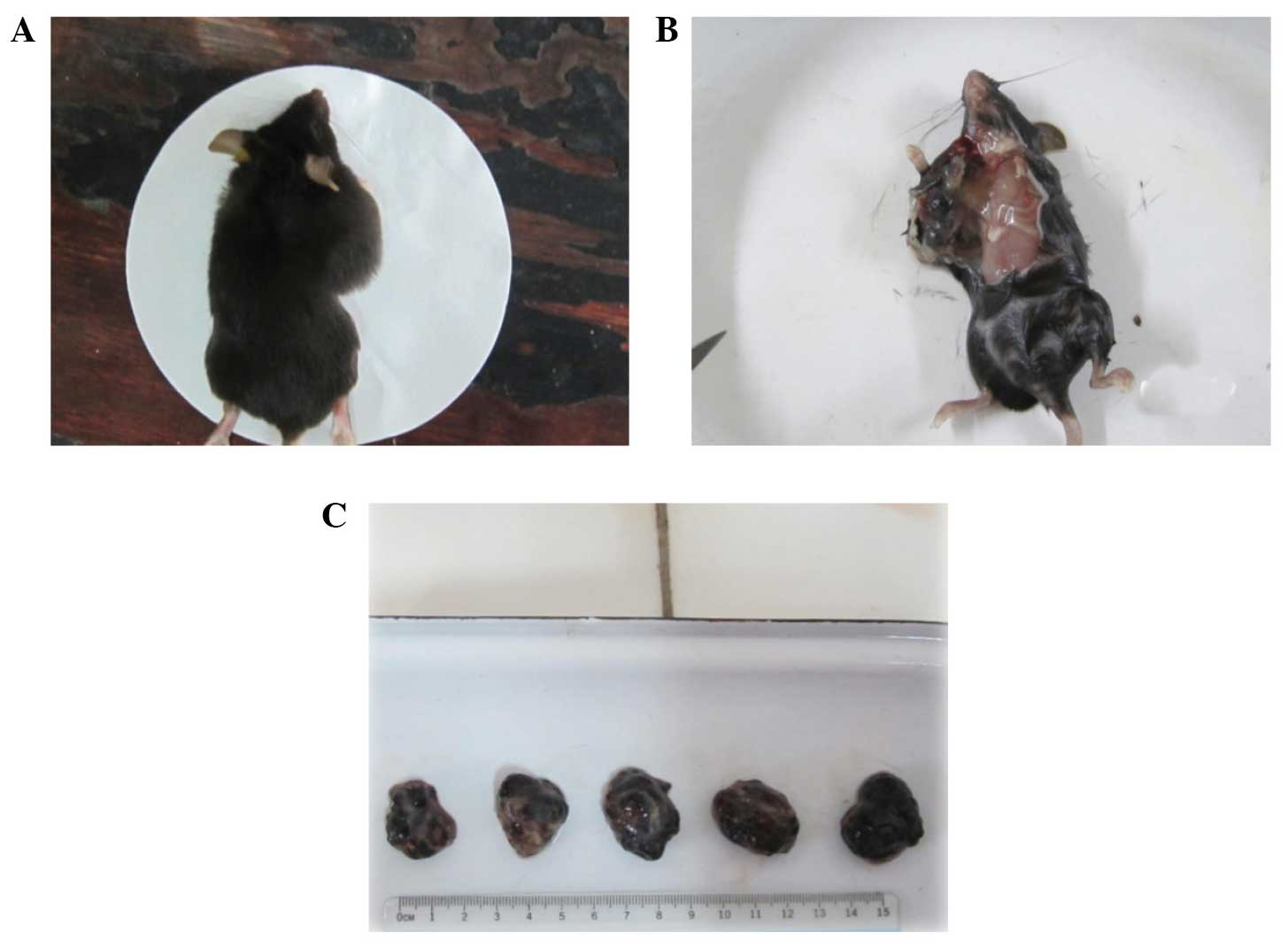
difference was statistically different (P<0.05) (Fig. 2). On day 14, when the tumors were
surgically removed from the tumor-bearing mice, the volume and
weight of the tumors from the 50–100 KDa group was 520.15±36.69 mm3
and 1323.75±27.54 mg, respectively, which were the smallest
measurements when compared with the other groups. The next smallest
measurements were those of the repeatedly freeze-thawed B16 cell
group, while the volume and weight of the tumors in the RPMI 1640
group was 2363.50±43.05 mm3 and 2593.75±95.65 mg, which were the
largest measurements among all the groups (P<0.05). No
accidental mortality occurred during this period (Fig. 3).
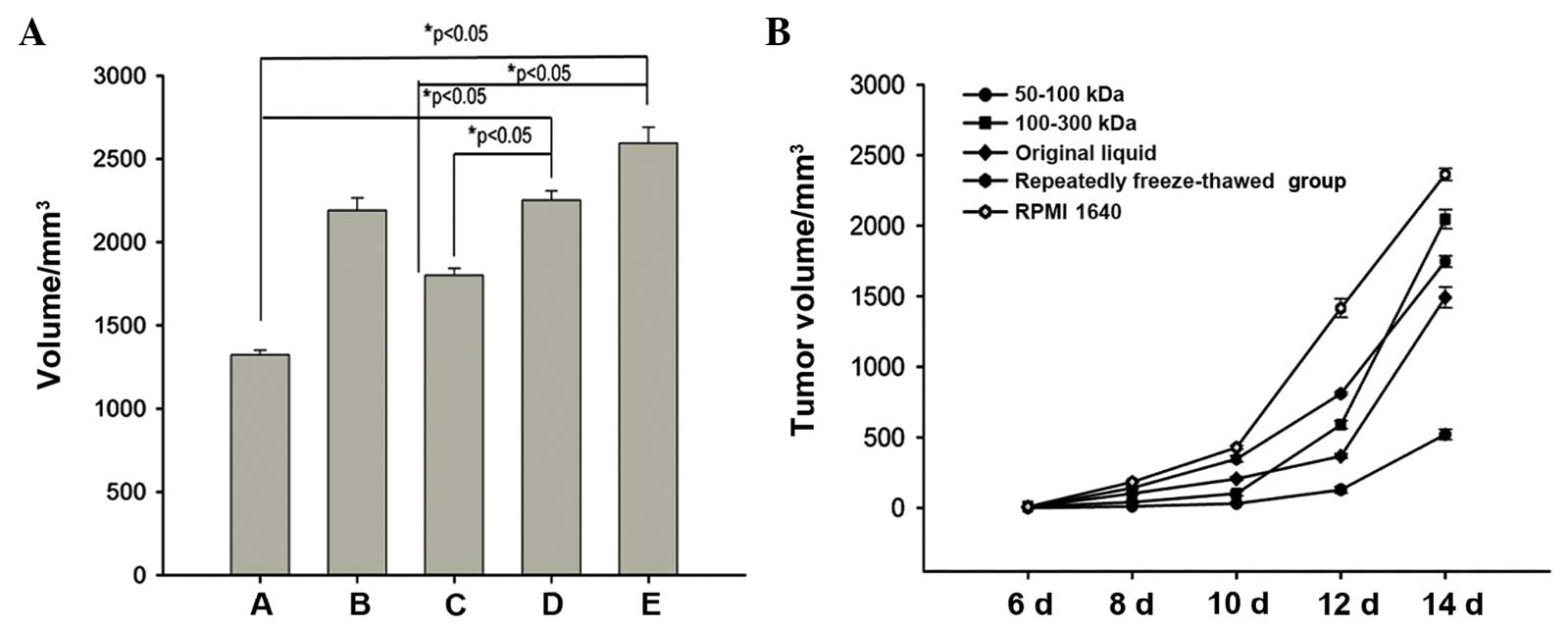 | Figure 3.Growth curves of (A) B16 melanomas
vaccinated with 50–100 KDa molecular weight fractions, 100–300 KDa
molecular weight fractions, repeatedly freeze-thawed B16 melanoma
cells, original supernatant and RPMI 1640 medium. The 50–100 KDa
group exhibited the lowest tumor volume, while the RPMI 1640 medium
group exhibited the highest. A, 50–100 KDa group; B, 100–300 KDa
group; C, repeatedly freeze-thawed B16 cell group; D, original
supernatant group; E, RPMI 1640 medium group. (B) Histogram showing
the average weight of the dissected tumors from the five different
groups (n=8). Data in (A) and (B) are representative of results
from two separate experiments with eight mice/group in each
experiment (bars represent the standard deviation: *P<0.05
relative to the same group on day 6, *P<0.05 relative to RPMI
1640 medium group and *P<0.05 relative to original supernatant
group). |
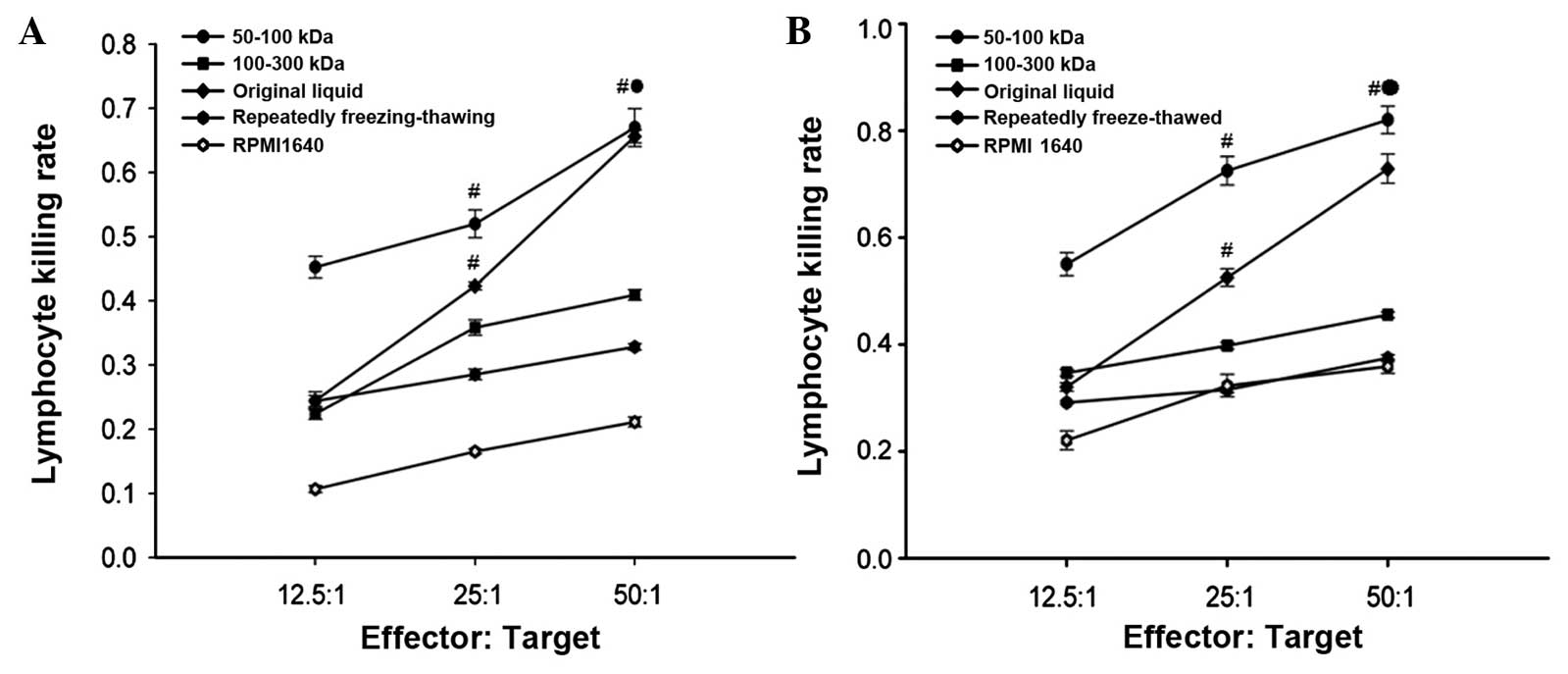
Cytotoxicity of TILs and SPLs
CCK-8 analysis determined that the cytotoxicity of
the TILs was much higher than that in the corresponding SPLs within
the same group, and the cytotoxicity increased as the E:T ratio
increased. Among the five different groups, the cytotoxicity of the
TILs and SPLs of the 50–100 KDa group was the highest at 0.82±0.026
and 0.67±0.029, respectively, which was statistically different
compared with the four other groups (P<0.05) (Fig. 4).
Lymphocyte subsets in transplanted B16
melanomas and mouse spleens
The tumors and spleens were surgically removed from
the mice, then subjected to analysis of their activated CD4+ T
cells, activated CD8+ T cells and CD20+ B cells using flow
cytometry. In each respective group, the percentage of activated
CD8+ T cells from the TILs was much higher than the percentage from
the SPLs, with the exception of the RPMI 1640 group. Among the five
different groups, the percentage of activated CD8+ T cells from the
corresponding TILs was markedly higher in the 50–100 KDa group
(39.61%) compared with the other four groups. The second highest
percentage was found in the 100–300 KDa group, while the RPMI 1640
group exhibited the lowest percentage of activated CD8+ T cells.
The percentage of activated CD4+ T cells from the corresponding
TILs was markedly higher in the 50–100 KDa group (47.76%) compared
with the other four groups, while the RPMI 1640 group exhibited the
lowest percentage. With regard to the SPL subsets, the percentage
of activated CD8+ T cells was the highest in the freeze-thawed
group (18.7%), while the 50–100 KDa group exhibited the lowest
percentage. The percentage of activated CD4+ T cells was the
highest in the original supernatant group (19.8%) and the lowest in
the 50–100 KDa group (Table II).
 | Table II.Lymphocytes subsets percents in TIL
and SP. |
Table II.
Lymphocytes subsets percents in TIL
and SP.
|
| TIL, % | SPL, % |
|---|
|
|
|
|
|---|
| Group |
CD28+/CD4+ T
cells |
CD28+/CD8+ T
cells | CD20+ B
cells |
CD28+/CD4+ T
cells |
CD28+/CD8+ T
cells |
|---|
| A | 47.76 | 39.61 | 35.60 | 10.19 |
6.64 |
| B | 25.10 | 32.30 | 34.80 | 15.7 | 17.9 |
| C | 13.80 | 26.60 | 38.40 | 17.7 | 18.7 |
| D | 15.00 | 17.90 | 34.3 | 19.8 | 15.6 |
| E | 8.94 | 10.20 | 36.1 | 16.0 | 12.2 |
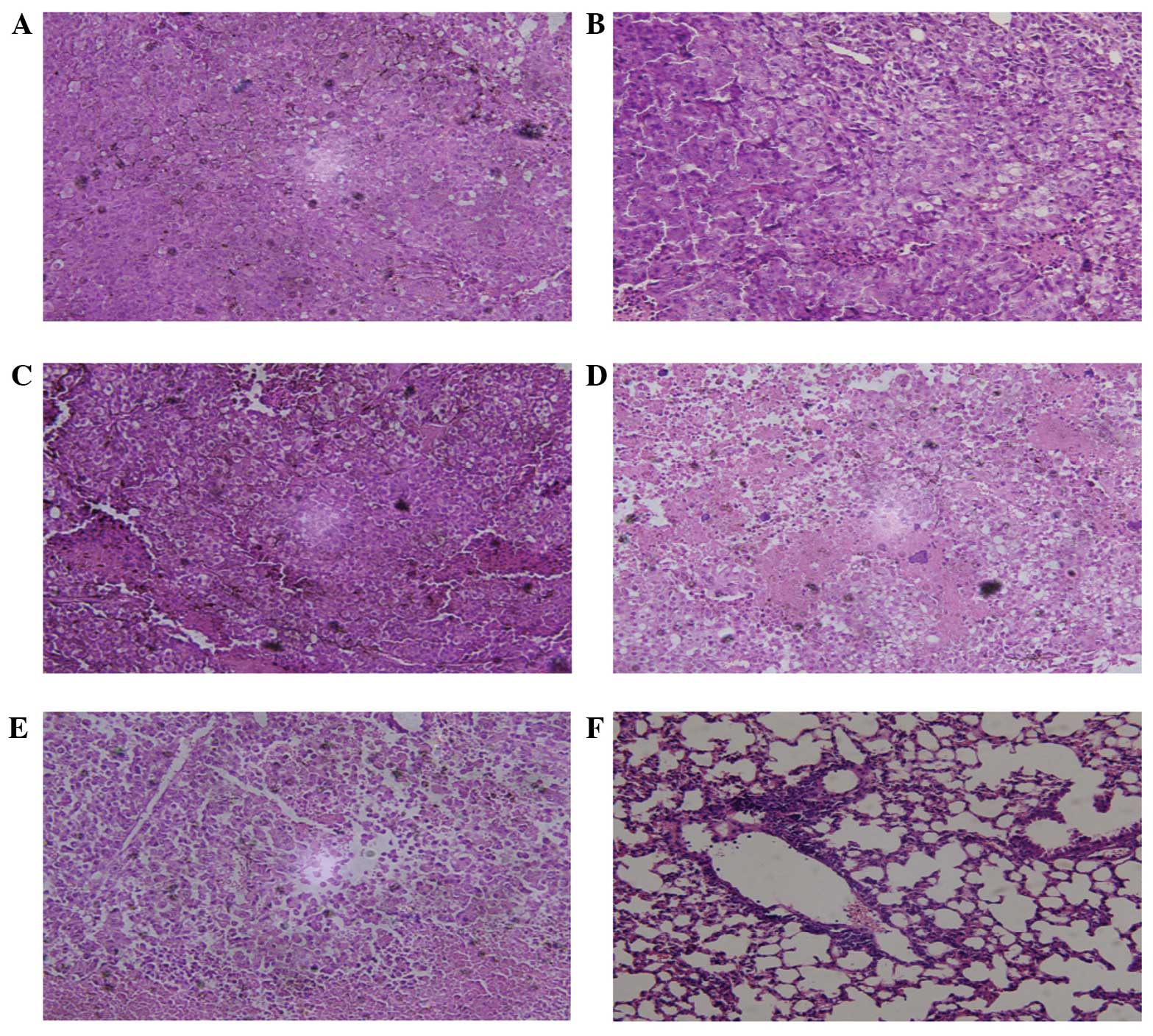
Histological characteristics of the
transplanted tumor and lung
Histological characteristics were assayed using HE
staining, which revealed no lung metastasis in any group. However,
local axillary lymph node metastasis was observed in each group.
The number of metastatic lymph nodes was the highest in the RPMI
1640 group and the lowest in the 50–100 KDa group. Hemorrhage and
necrosis of the transplanted tumor only occurred in the RPMI 1640
group. Using microscopy, a large degree of cytologic atypia was
apparent in several of the groups, particularly the RPMI 1640
group. The tumor cells were also observed to have matured and
differentiated dysfunctionally; this was particularly evident in
the RPMI 1640 group, while the 50–100 KDa group was much less
affected (Fig. 5).
Discussion
In order to define the functions of TILs, B16 cell
supernatant isolated purified fragments were used to analyze the
hypothesized antitumor immunological reactions. It was found that
the protein molecular weight of the B16 cell culture supernatant
was ~70 KDa, and that the 50–100 KDa group exhibited strong in
vitro chemotaxis. When C57BL/6 mice were vaccinated with this
fragment, it was able to markedly inhibit tumor growth. In
addition, the cytotoxicity of the TILs and SPLs from this group was
higher in comparison to the other examined groups. As T cell
immunity was considered to be much more important than B cell
immunity, the present study mainly analyzed the T cell subsets. The
CD28+/CD4+ T cell percentages of TILs from
the 50–100 KDa group were the highest. The antitumor effects of
50–100 KDa molecular weight fractions may be attributable to the
activation of CD8+ T cells, and the enhanced
cytotoxicity of CD8+ T cells may also relate to the
promotion of differentiation effects.
The results presented in the current study
demonstrate an involvement of isolated purified fragments in
initiating the in vitro chemotaxis and in vivo
antitumor immunological reactions, leading to the marked growth
inhibition of transplanted B16 melanoma. Taken together, these
in vitro and in vivo experiments indicate that the
antitumor effects of B16 cell supernatant isolated purified
fragments are selective, and that the 50–100-KDa molecular weight
fraction has the most potent effects among all the fractions, with
the ability to activate CD8+ T cells and enhance the
cytotoxicity of TILs for B16 cell killing. Tumor vaccines represent
an effective antitumor immunotherapy, with ideal effects being the
elimination of occult micro-metastases (6). However, a number of tumor vaccines are
effective in animal models, but their effects in human tumors are
not as expected (22,23). Whole tumor cell-based vaccinations are
multivalent and can elicit a broad range of responses to
tumor-associated antigens, which are more potent than a single
defined tumor antigen (24). However,
whole cell tumor vaccines consist of numerous cell elements,
including lysosomal enzyme, proteolytic enzyme, cytochrome enzyme
and heat shock protein. These elements, particularly the different
types of enzymes, when administrated into the host can induce
serious inflammation responses (25).
However, the supernatant isolated purified fragments are selective
proteins, and they can exert a strong antitumor immune response
without being detrimental to the host (26). The present results also indicated that
the cytotoxicity of the TILs and SPLs from the mice vaccinated with
the 50–100-KDa molecular weight fraction was much stronger than
that of repeatedly freeze-thawed B16 cells, which is in agreement
with our hypothesis.
The culture supernatant isolated purified fragments
are able to accumulate at a high concentration, which is required
in the vaccination of mice, and most importantly, the fragments are
not one protein, but several proteins with the approximate
molecular weight. The present study is the first to report the
antitumor effects of culture supernatant isolated purified
fragments. The tumor antigens are also variable and tumor cell
killing requires activation of different types of T cells (27). Taken together with the greater
percentage of CD28+/CD8+ T cells in the TILs
of the 50–100 KDa molecular weight fraction vaccinated mice, we
speculate that the supernatant isolated purified fragments,
particularly the 50–100 KDa molecular weight fraction, can mimic
the tumor antigen to activate CD8+ T cells. Furthermore
these CD28+/CD8+ T cells have great in
vitro cytotoxicity towards B16 cells, indicating that
vaccinating mice with a 50–100 KDa molecular weight fraction can
induce a T cell immune response and kill the tumor cells,
eventually making the tumor mass disappear. Notably, the present
study selected two B16 cell culture supernatant isolated purified
fragments, as we have previously shown that the 50–100 and
100–300-KDa molecular weight fractions have potent chemotaxis (Qin
et al unpublished data). The present results demonstrate
that the antitumor immune response is associated with molecular
weight culture supernatant isolated purified fragments.
Although the present study revealed the antitumor
effects of B16 cell culture supernatant isolated purified
fragments, the definitive protein involved and the types of protein
are as yet unknown. Heat shock protein 70 (Hsp70) is a molecular
chaperon, and the intratumoral application of Hsp70 on the surface
of B16F10 melanoma tumors has been shown to reduce the tumor growth
rate and prolong animal survival times (28); we propose that Hsp70 may be part of an
isolated purified fragment, however, further biotechnological
analysis is required to confirm this. Certain membrane proteins
that have a molecular weight of ~70 KDa also require evaluation. A
possible mechanism underlying the antitumor effects is the
activation of CD8+ T cell subsets of TILs and the
enhancement of the cytotoxicity of activated CD8+ T
cells, all without changing the humoral immune response. The
present results are consistent with other studies showing that the
adaptive cellular immune response exerts a more significant role
than the humoral immune response (28). In the present study, there were no
lung metastases in any of the mice, but this is probably due to the
limited growth time of the B16 melanoma. If the growth time had
been extended then the metastases probably would have appeared. HE
staining observations indicated that the tumor cells from the mice
vaccinated with the 50–100-KDa molecular weight fraction exhibited
more evident differentiation than the other four groups, suggesting
that the 50–100-KDa molecular weight fraction can activate the
unknown mechanism to promote B16 cell differentiation.
In conclusion, the present study firstly showed the
chemotaxis and antitumor effects of B16 cell culture supernatant
isolated purified fragments. The possible mechanism of these
effects is also discussed. Mice vaccinated with the 50–100-KDa
molecular weight fraction showed strong antitumor effects and
marked inhibition of tumor growth compared with the repeatedly
freeze-thawed B16 cells and 100–300-KDa molecular weight fraction.
Frequencies of activated CD8+ conventional T cells with
a Th1 profile were increased in the transplanted tumor, and the
cytotoxicity of the activated CD8+ TILs was also
increased. Taken together, these data indicate that the novel
utilization of culture supernatant isolated purified fragments can
effectively retard tumor growth. The present study provides an
excellent platform on which to build effective antitumor
therapeutics.
Acknowledgements
The authors would like to thank Professor Hong Liu
of the Department of Pathology, Xuzhou Medical College, for
providing technical assistance and valuable comments.
References
|
1
|
Gyorki DE, Callahan M, Wolchok JD and
Ariyan CE: The delicate balance of melanoma immunotherapy. Clin
Transl Immunology. 2:e52013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yee C, Thompson J, Byrd D, et al: Adoptive
T cell therapy using antigen-specific CD8+ T cell clones for the
treatment of patients with metastatic melanoma: In vivo
persistence, migration, and antitumor effect of transferred T
cells. Proc Natl Acad Sci USA. 99:16168–16173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui NP, XIE SJ, Han JS, et al: Effective
adoptive transfer of haploidentical tumor-specific T cells in
B16-melanoma bearing mice. Chin Med J (Engl). 125:794–800.
2012.PubMed/NCBI
|
|
4
|
Chiu HY, Sun GH, Chen SY, et al:
Pre-existing Fas ligand (FasL) in cancer cells elicits
tumor-specific protective immunity, but delayed induction of FasL
expression after inoculation facilitates tumor formation. Mol
Carcinog. 52:705–714. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ardolino M, Azimi CS, Iannello A, et al:
Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J
Clin Invest. 124:4781–4794. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mkrtichyan M, Ghochikyan A, Davtyan H, et
al: Cancer-testis antigen, BORIS based vaccine delivered by
dendritic cells is extremely effective against a very aggressive
and highly metastatic mouse mammary carcinoma. Cell Immunol.
270:188–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burdek M, Spranger S, Wilde S, et al:
Three-day dendritic cells for vaccine development: antigen uptake,
processing and presentation. J Transl Med. 8:902010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parry AL, Clemson NA, Ellis J, et al:
‘Multicopy multivalent’ glycopolymer-stabilised gold nanoparticles
as potential synthetic cancer vaccines. J Am Chem Soc.
135:9362–9365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunn MK, Farrand KJ, Broadley KW, et al:
Vaccination with irradiated tumor cells pulsed with an adjuvant
that stimulates NKT cells is an effective treatment for glioma.
Clin Cancer Res. 18:6446–6459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teitz-Tennenbaum S, Li Q, Davis MA and
Chang AE: Dendritic cells pulsed with keyhole limpet hemocyanin and
cryopreserved maintain anti-tumor activity in a murine melanoma
model. Clin Immunol. 129:482–491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiang CL, Kandalaft LE, Tanyi J, et al: A
dendritic cell vaccine pulsed with autologous hypochlorous
acid-oxidized ovarian cancer lysate primes effective broad
antitumor immunity: from bench to bedside. Clin Cancer Res.
19:4801–4815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Obata C, Zhang M, Moroi Y, et al:
Formalin-fixed tumor cells effectively induce antitumor immunity
both in prophylactic and therapeutic conditions. J Dermatol Sci.
34:209–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clay TM, Mosca PJ, Lyerly HK and Morse MA:
Whole-tumor-cell vaccinesHandbook of Cancer Vaccines. Morse MA,
Clay TM and Lyerly HK: Springer; pp. 249–251. 2004
|
|
14
|
Koido S, Homma S, Okamoto M, et al:
Fusions between dendritic cells and whole tumor cells as anticancer
vaccines. Oncoimmunology. 2:e244372013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu P and Fu YX: Tumor-infiltrating T
lymphocytes: friends or foes? Laboratory Invest. 86:231–245. 2006.
View Article : Google Scholar
|
|
16
|
Sharma P, Shen Y, Wen S, et al: CD8
tumor-infiltrating lymphocytes are predictive of survival in
muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA.
104:3967–3972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clemente CG, Mihm MC Jr, Bufalino R, et
al: Prognostic value of tumor infiltrating lymphocytes in the
vertical growth phase of primary cutaneous melanoma. Cancer.
77:1303–1310. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aerts JG and Hegmans JP: Tumor-specific
cytotoxic T cells are crucial for efficacy of immunomodulatory
antibodies in patients with lung cancer. Cancer Res. 73:2381–2388.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dudley ME, Wunderlich JR, Robbins PF, et
al: Cancer regression and autoimmunity in patients after clonal
repopulation with antitumor lymphocytes. Science. 298:850–854.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu WK, Ho JC, Cheung FW, et al: Apoptotic
activity of betulinic acid derivatives on murine melanoma B16 cell
line. Eur J Pharmacol. 498:71–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eggermont AM: Therapeutic vaccines in
solid tumours: can they be harmful? Eur J Cancer. 45:2087–2090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pajtasz-Piasecka E and Indrová M:
Dendritic cell-based vaccines for the therapy of experimental
tumors. Immunotherapy. 2:257–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hira SK, Mondal I and Manna PP: Combined
immunotherapy with whole tumor lysate-pulsed
interleukin-15-activated dendritic cells and cucurbitacin I
promotes strong CD8(+) T-cell responses and cures highly aggressive
lymphoma. Cytotherapy. 17:647–664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manzo T, Michelini RH, Sturmheit T, et al:
Tumor-targeting vaccination instructs graft-vs.-tumor immune
responses. Oncoimmunology. 2:e259962013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu J, Fu W, Luo J and Zhang T: Expression
and purification of human endostatin from Hansenula polymorpha A16.
Protein Expr Purif. 42:12–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kósa JP, Horváth P, Wölfling J, et al:
CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3
on colorectal cancer cells. World J Gastroenterol. 19:2621–2628.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zamarron BF and Chen WJ: Dual roles of
immune cells and their factors in cancer development and
progression. Int J Biol Sci. 7:651–658. 2011. View Article : Google Scholar : PubMed/NCBI
|