Introduction
Gastrin-releasing peptide (GRP) is a 27-amino acid
peptide, which was first identified as a cytokine secreted from P
cells in the alimentary tract. The local secretion of GRP regulates
gastrointestinal hormones and gallbladder contraction (1). Previous studies revealed that GRP and
the GRP receptor (GRPR) were overexpressed not only in the
gastrointestinal tract, but also in a number of other malignant
tumors and cell lines (2–5). It has been established that epithelial
cells from regions other than the parenteral tract can also
excessively secret GRP under the regulation of carcinogenic
factors. GRP can then bind to the GRPR and promote the mitosis of
cells.
We previously screened samples of serum, ascites and
cyst fluid from 30 ovarian carcinoma patients by matrix-assisted
laser desorption/ionization time-of-flight mass spectrometer
technology combined with WCX magnetic beads. The results indicated
that the elevated peptide peaks at 2881 and 2897 Da may correspond
to the same GRP polypeptide, and confirmed that GRP may be involved
in the carcinogenesis of ovarian cancer (6). The biological effects of GRP in
colorectal, gastric and prostate cancer have been previously
reported (7–9). However, studies concerning the
biological effects of GRP in ovarian cancer have yet to be
conducted.
In the present study, a small interfering RNA
(siRNA) against GRP was constructed (10) and transfected into human ovarian
cancer ES2 cells, which are known to highly express GRP. The
biological changes in the ES2 cells were then investigated.
Materials and methods
Materials
The Escherichia coli (E. coli; DH5α)
and pGenesil-1.1dBm2 vector were purchased from the Wuhan JinSai
Biological Technology Engineering Company (Wuhan, China).
Monoclonal mouse anti-human GRP (dilution, 1:1,000) and mouse
anti-human β-actin (dilution: 1:1,000) antibodies were obtained
from R&D Systems, Inc., (Minneapolis, MN, USA). The human
ovarian cancer ES2 cell line was supplied by the China Center for
Type Culture Collection at Wuhan University (Wuhan, China).
Cell culture
The ES2 cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum, 100 µg/ml streptomycin
and 100 U/ml penicillin. The cell cultures were maintained in a
humidified atmosphere of 5% CO2 at 37°C in an
incubator.
Design of short hairpin (sh)RNA
construction for GRP
According to the GRP complementary (c)DNA sequence
obtained from Genbank, the GRP-targeted shRNA construct was
designed using the RNAi software from Ambion Life Technologies
(Carlsbad, CA, USA). The cDNA target sequence was 192–210 bp in
length. The oligonucleotide was synthesized by Wuhan JinSai
Biological Technology Engineering Company, and the sequence of the
GRP-shRNA was as follows (the guide peptide is in bold): Sense,
5′-CACCACCGTGCTGACCAAGATGTTTCA
AGACGACATCTTGGTCAGCACGGTTTTTTTG-3′; and antisense,
5′-AGCTCAAAAAAACCGTGCTGACCAAGA
TGTCGTCTTGAAACATCTTGGTCAGCACGGT-3′.
Construction and cloning of
pGenesil-GRP-shRNA
The GRP-shRNA was inserted into the SacI site
of the expression vector, pGenesil-1.1dBm2. The product of the
recombination reactions, pGenesil-GRP-shRNA, was used to transform
the competent E. coli strain, DH5α, during the
transformation assay. Briefly, the bacteria were washed with a
0–100 mM CaCl2 solution, resuspended in 200 µl of the
same solution, and then placed on ice for 1 h. Next, the
pGenesil-GRP-shRNA plasmid DNA was added for 30 min. The sample was
then incubated at 42°C for 90 sec. Following the addition of 1 ml
Luria-Bertani broth, the sample was incubated for 1 h at 37°C. In
total, 100 µl of the transformation mixture was transferred onto a
Luria-Bertani agar plate containing 100 µg/ml kanamycin and 100
µg/ml neomycin. The transformation frequency was determined by
calculating the ratio of the number of transformants per viable
cell per milliliter.
The plate was then cultured at 37°C for 16–24 h, the
positive colonies were amplified in Luria-Bertani broth and the
plasmid was extracted using a plasmid extraction kit (Qiagen GmbH,
Hilden, Germany) The plasmid was identified by electrophoresis and
restriction enzyme digestion.
pGenesil-GRP-shRNA transfection
An equal number of ES2 cells (0.8×106 per
well) were seeded into 35-mm dishes. The cells were left for 24 h
to attach and reach 70–80% confluency. The transfection assay was
performed using Lipofectamine 2000 transfection reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. Untransfected cells and cells
transfected with an empty vector were used as the controls.
The efficiency was assessed according to the
concentration of GFP at 24 h post-transfection. The cells were
harvested 2–3 days after transfection, and then cultured in
complete medium containing 800 µg/ml G418 for 7–8 days. Fresh
medium was added every 3 days until the untransfected control cells
were all dead. Next, the cells were cultured in complete medium
containing 200 µg/ml G418. The positive clones appeared ~20 days
later. These positive cells were recultured in fresh bottles and
designated pGenesil-GRP-shRNA cells or pGenesil-1.1dBm2 cells.
Reverse transcription polymerase chain
reaction (RT-PCR)
The total RNA was extracted from the positive ES2
cell clones using TRIzol (Invitrogen Life Technologies), and then
reversed into cDNA by the Super SCRIPTM cDNA kit from Takara
Biotechnology Co., Ltd. (Dalian, China). In order to examine the
expression of endogenous GRP mRNA, the RT-PCR was performed using
the following primers: GRP forward, 5′-AGAGTACATCAGGTGGGA-3′ and
reverse, 5′-CAGAAG ATGCTGCTTTAAAA-3′ (product size, 375 bp); and
β-actin forward, 5′-ACTCTTCCAGCCTTCCTTC-3′ and reverse,
5′-AATCCTGAGTCAAGCCAAA-3′ (product size, 490 bp). β-actin was used
as the internal reference. The amplification conditions were as
follows: 94°C for 5 min, followed by 30 cycles at 94°C for 40 sec,
60°C for 30 sec and 72°C for 30 sec, and finally 72°C for 5 min.
The PCR products were electrophoresed in 1.5% agarose gel, stained
with ethidium bromide and then analyzed using the Q550IW image
analysis system (Leica Imaging Systems, Cambridge, UK). The
integral optical density of each band was calculated according to
the following equation: Integral absorbance = average absorbance ×
area.
Western blotting
The positive ES2 cell clones were resuspended in SDS
loading buffer, heated for 10 min at 100°C, and then centrifuged at
13,000 × g for 15 min at 4°C. The supernatants were collected and
separated by SDS-PAGE electrophoresis, and then transferred to a
polyvinylidene difluoride membrane. The membrane was dipped in 5%
skimmed milk powder for 2 h in order to block non-specific binding
sites, and then incubated with GRP or β-actin primary antibodies at
4°C overnight. Subsequent to rinsing three times in Tris-buffered
saline and Tween 20 (TBST), the membrane was incubated with the
corresponding secondary antibody labeled with horseradish
peroxidase for 2 h, and then rinsed in TBST a further three times.
Next, the membrane was incubated with SuperSignal West Dura
Chemiluminescent Substrate (Thermo Fisher Scientific) for 5 min.
Finally the images were obtained by X-ray film exposure.
Cell proliferation detection
The untransfected cells and the ES2 cells
transfected with pGenesil-GRP-shRNA or pGenesil-1.1dBm2 vector were
cultured. The percentage of dead cells was determined by staining
with Trypan blue each day for 7 days.
Flow cytometric analysis of
apoptosis
The ES2 cells were collected and washed once with
phosphate-buffered saline and then resuspended in
1×106/ml annexin-binding buffer. In total, 100 µl of the
cell solution was stained with 5 µl Annexin V and 5 µl propidium
iodide for 15 min. The stained cells were analyzed by FACScan flow
cytometry (BD Biosciences, Franklin Lakes, NJ).
Cell invasion
The Transwell assay was performed using a commercial
kit purchased from Corning Inc. (Corning, New York, NY, USA), as
previously described (11). Briefly,
collagen-coated membranes were seeded with 1×105 cells
in 500 µl DMEM. In all instances, cell migration was assessed 5 h
after plating, as recommended by the manufacturer.
Statistical analysis
The data are expressed as the mean ± standard
deviation and were analyzed using SPSS 13.0 (SPSS, Inc., Chicago,
IL, USA). Student's t-test was performed for comparison between two
groups. P<0.05 was used to indicate a statistically significant
difference.
Results
Successful construction and
transfection of pGenesil-GRP-shRNA
A SacI restriction site was designed at each
end of the GRP-shRNA fragment. The fragment was inserted into the
vector, pGenesil-1.1dBm2, which was digested by SacI. As
shown in Fig. 1, when the
pGenesil-GRP-shRNA plasmid was digested by SacI, a 916-bp
band appeared, which corresponded to GRP-shRNA. The
pGenesil-GRP-shRNA plasmid was sequenced, and it was confirmed that
pGenesil-GRP-shRNA had been successfully constructed.
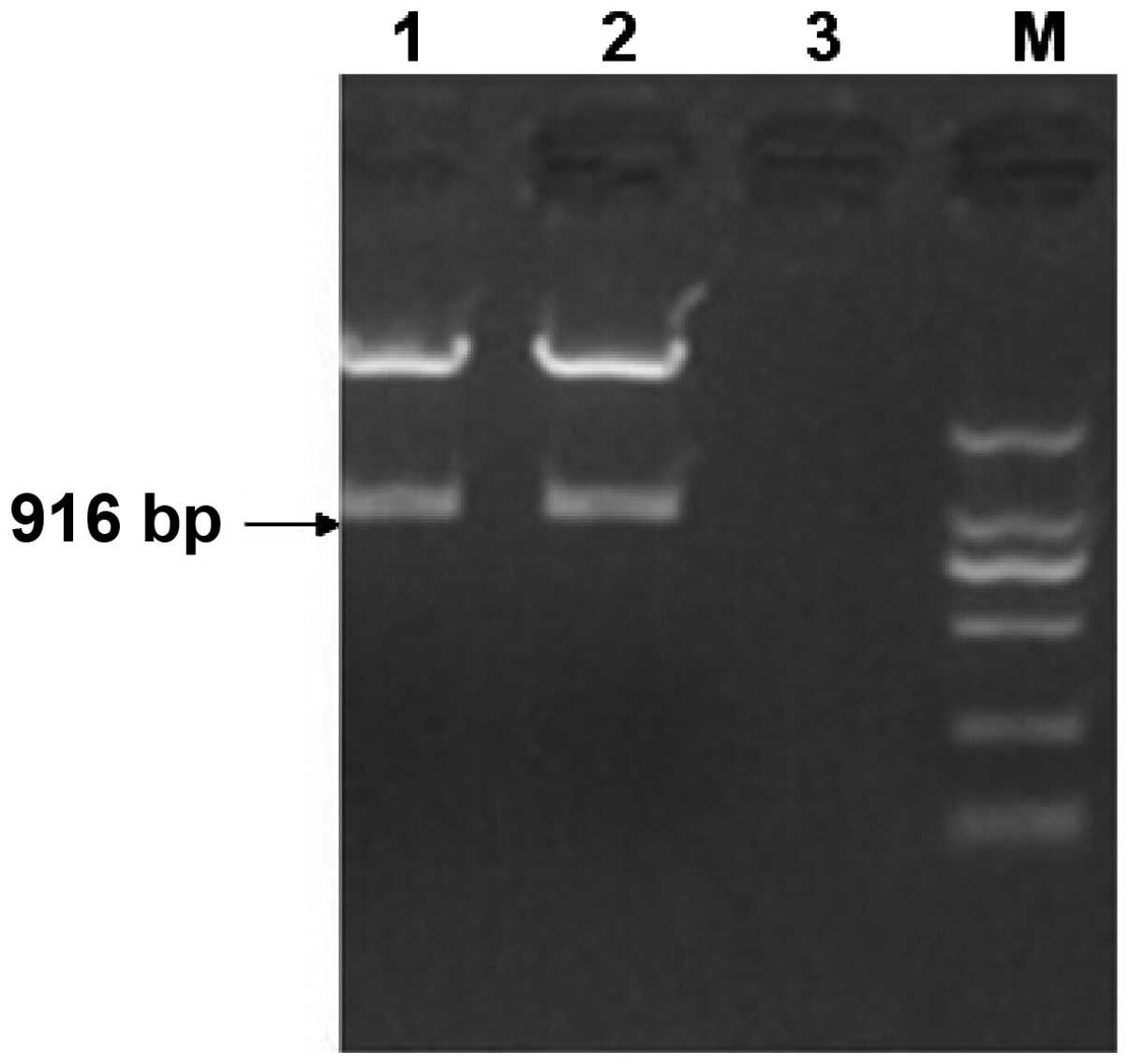 | Figure 1.Digestion of pGenesil-GRP-shRNA by
SacI. Lanes 1 and 2, pGenesil-GRP-shRNA; lane 3, negative
control; M, DNA marker with 2,000, 1,000, 750, 500, 250 and 100-bp
bands (top to bottom). GRP, gastrin-releasing peptide; shRNA, short
hairpin RNA. |
For the transfection assays, the cells were screened
using the G418 antibiotic; only the successfully transfected cells
were able to survive. The pGenesil-1.1dBm2 vector carried a green
fluorescent protein, which emitted green fluorescence under light
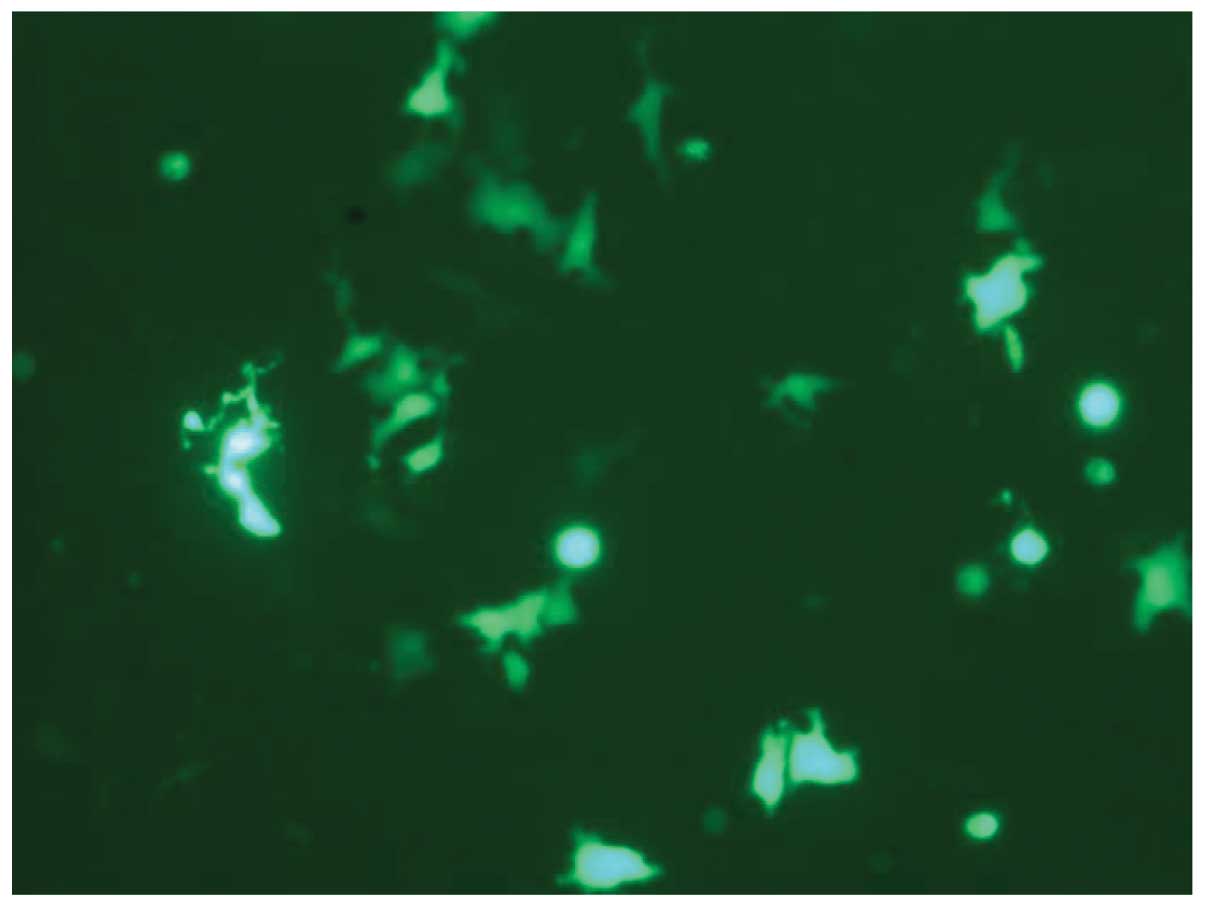
excitation. As shown in Fig. 2, the
positive clones of ES2 appeared green under the laser.
GRP silencing by GRP-shRNA
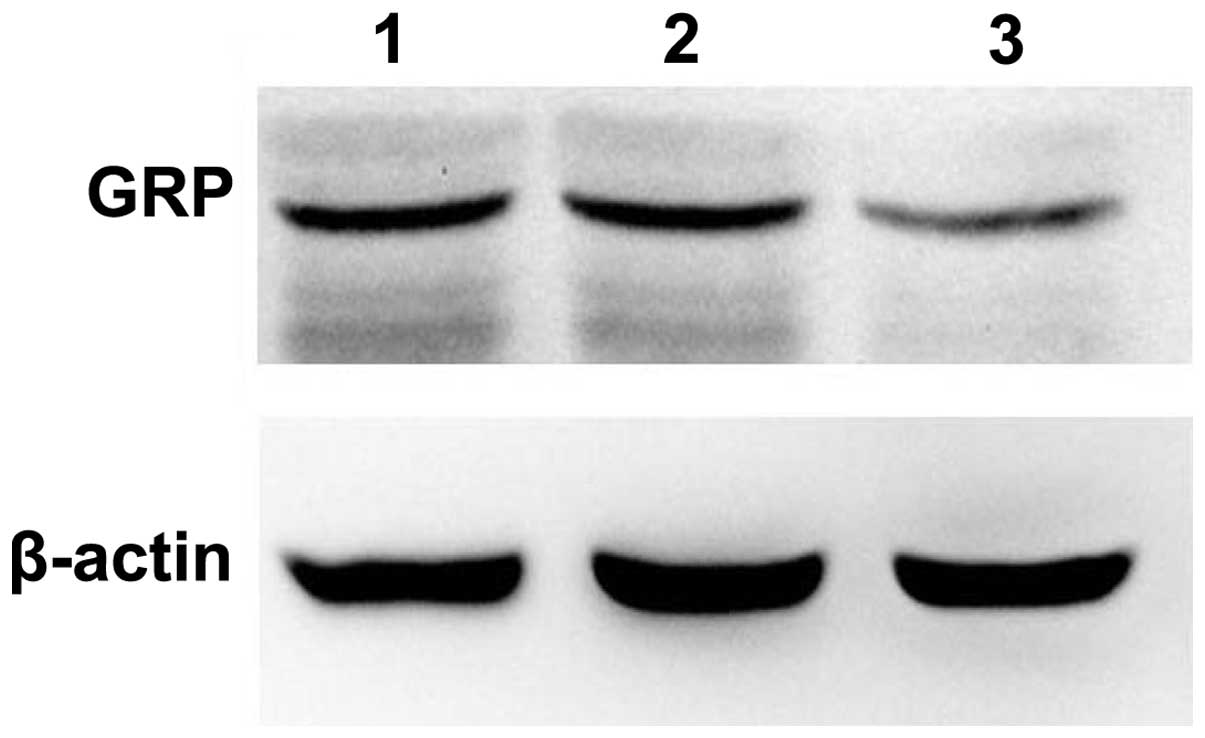
As shown in Fig. 3,
the expression level of GRP was calculated by the integral optical
density of the band. The mRNA level of GRP in the transfected cells
was significantly lower than that of the control transfected and
untransfected groups (0.36, 0.87 and 0.91, respectively;
P<0.05). The protein level of GRP was also markedly decreased in
the transfected group compared with the control transfected and
untransfected groups (0.13, 0.47 and 0.49, respectively; P<0.05;
Fig. 4). The results revealed that
the ES2 cells were successfully transfected by pGenesil-GRP-shRNA
and that the expression of GRP was significantly silenced.
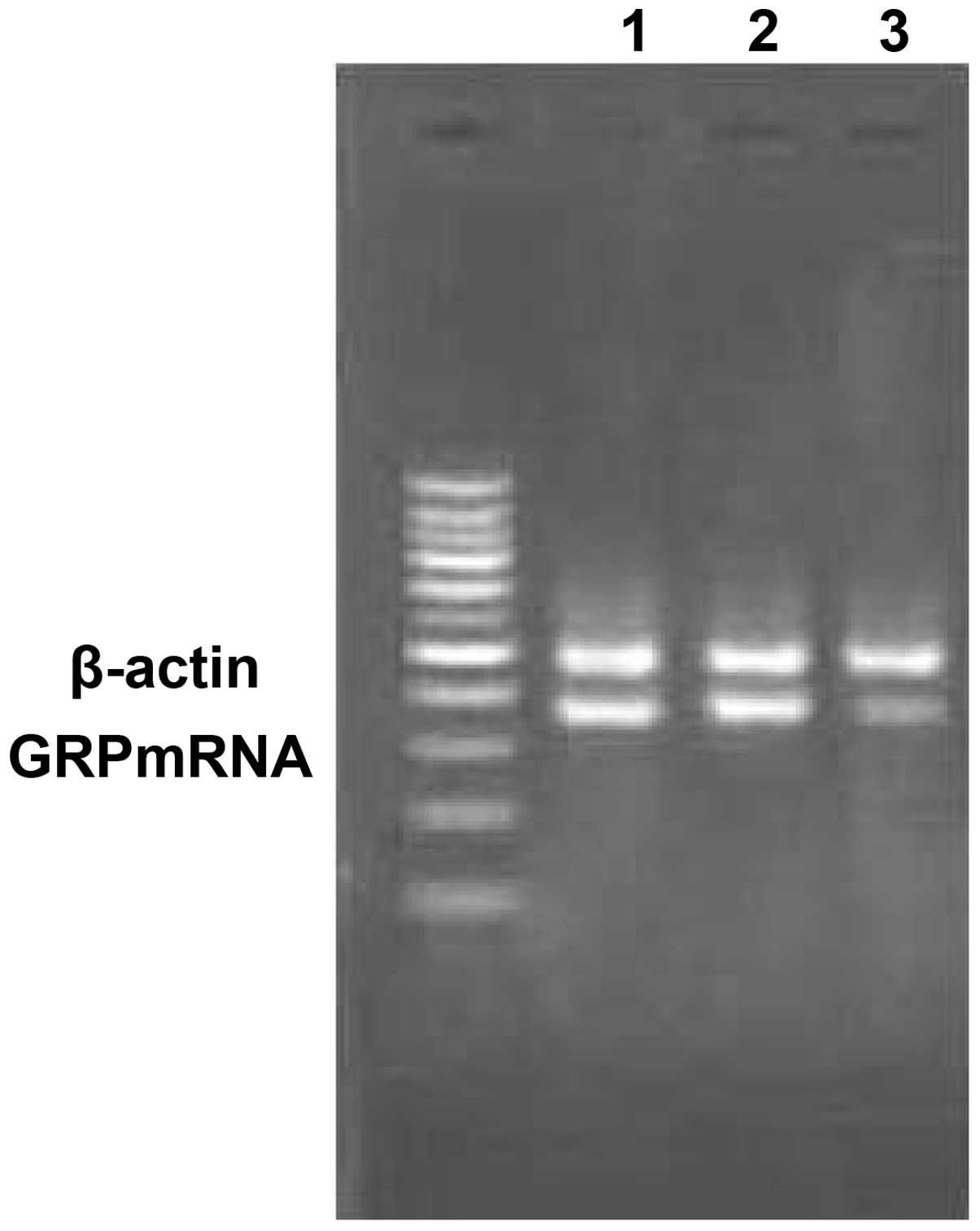 | Figure 3.Expression of GRP mRNA following
silencing. Lane Ma,100bp Plus II DNA marker with 1,500, 1,000, 900,
800, 700, 500, 400, 300, 200 and 100-bp bands (top to bottom) Lane
1, untransfected group; lane 2, empty vector-transfected group;
lane 3, GRP-shRNA-transfected group. GRP, gastrin-releasing
peptide; shRNA, short hairpin RNA. |
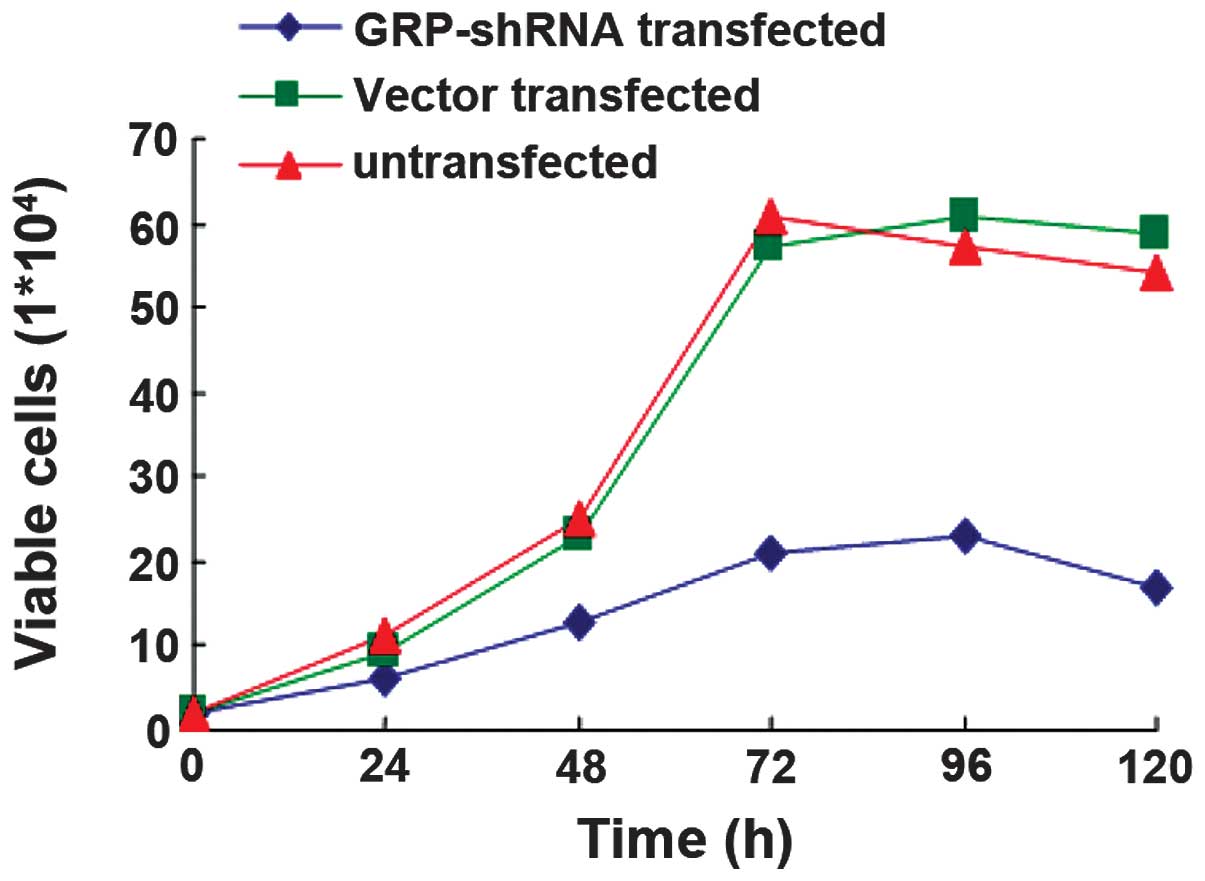
GRP silencing inhibits
proliferation
The ES2 cells were transfected with GRP-shRNA or
vector alone, untransfected cells were used as the control. The
cells were then cultured for 5 days and the cell viability was
detected each day. The results demonstrated that the ES2 cells
transfected with GRP-shRNA had a significantly lower proliferation
ability compared with the control (P<0.05, Fig. 5), while there was no observed
difference in the proliferation ability of the ES2 cells
transfected with the vector alone compared with the control
(P>0.05) (Fig. 5).
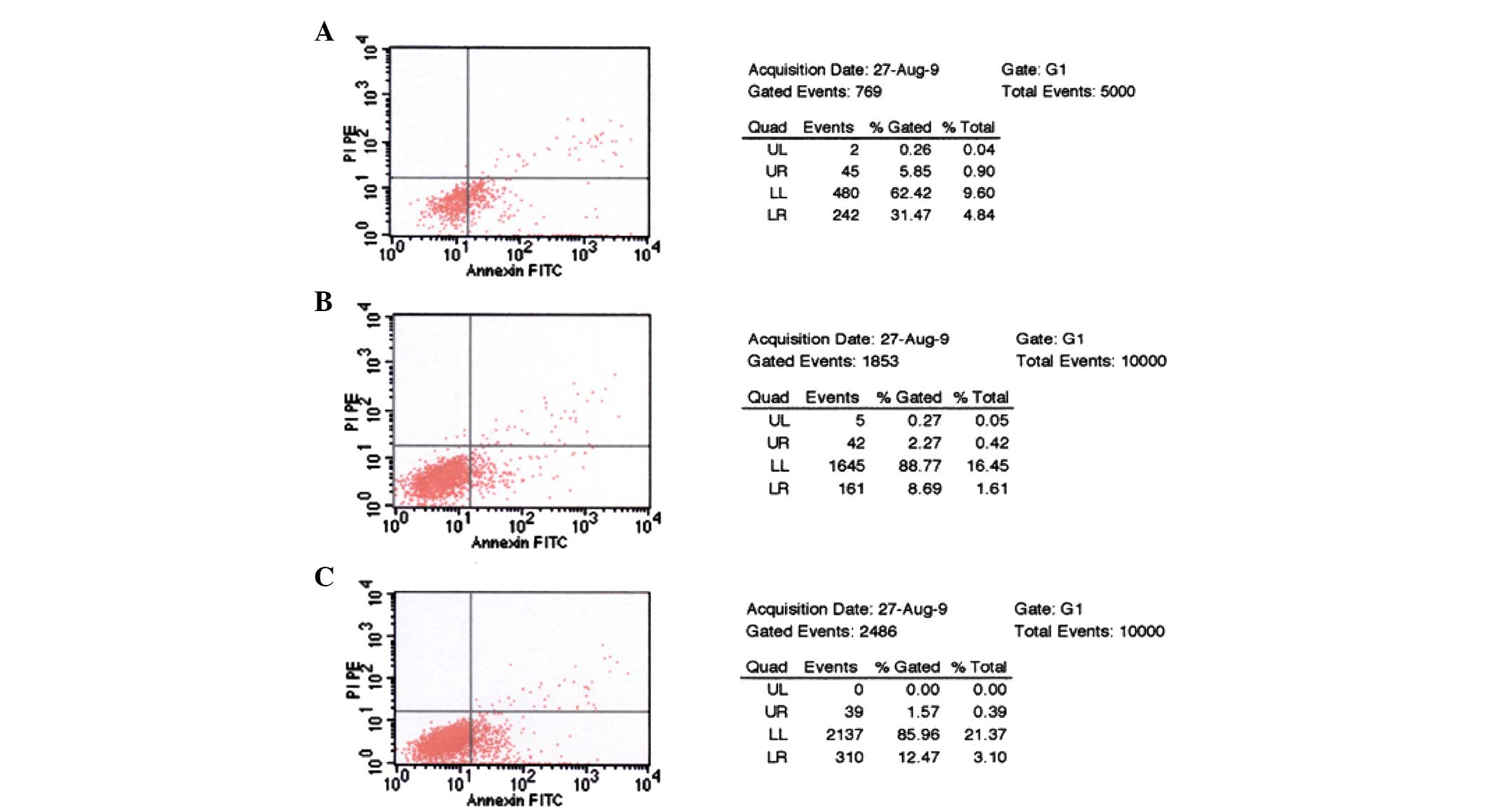
GRP silencing induces apoptosis
As shown in Fig. 6,
following transfection with pGenesil-GRP-shRNA, the apoptosis rate
of the ES2 cells significantly increased (from 8.69 to 31.47%),
while that of the vector group increased only slightly (from 8.69
to 11.47%); a significant difference was observed between the two
groups (P<0.05).
Effects of 3.6 GRP siRNA on the
invasion ability of ES2 cells
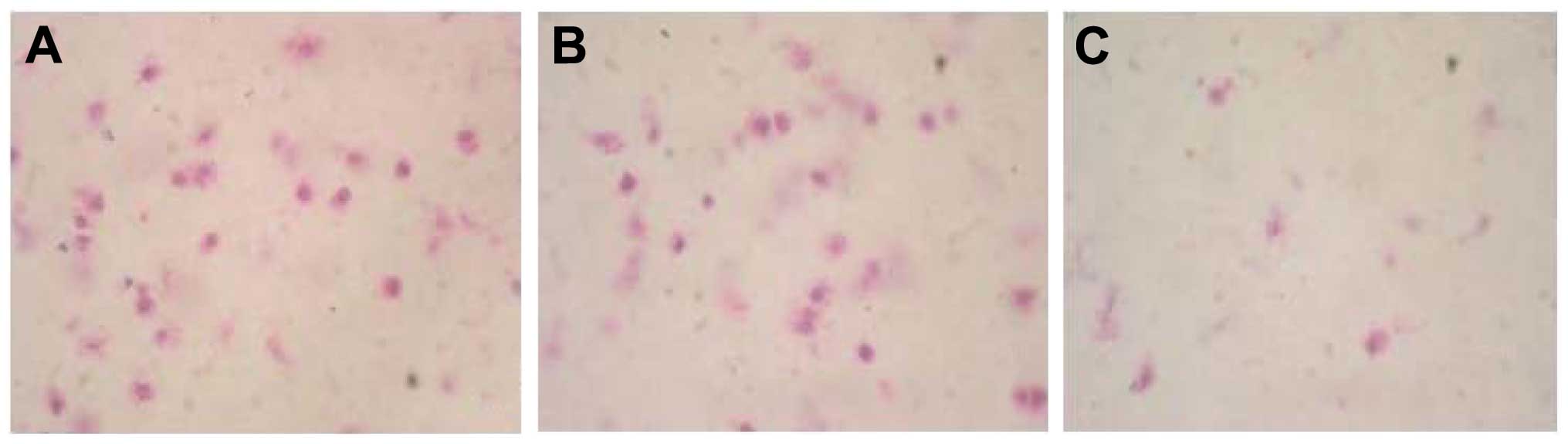
Cells that had migrated over the microporous
membrane were stained by methylene blue and were counted under an
inverted microscope (IX71; Olympus Corporation, Tokyo, Japan)
(Fig. 7). The invasive ability of the
cells (the number of cells that had migrated over the microporous
membrane) in the transfected group was 21±5.3, which was
significantly lower than that of the control groups (49±11.8 for
the vector transfected group; 43±10.1 for the untransfected group)
(P<0.05). The results suggested that silencing of GRP
significantly weakened the invasive ability of ES2 cells, and
revealed that the GRP may be important for the invasion of ovarian
cancer.
Discussion
GRP and its receptor, GRPR, are overexpressed in a
number of different tumors, and are believed to have an important
role in carcinogenesis and tumor progression (12). It has been hypothesized that the
carcinogenic ability of GRP relies on its affinity to GRPR. It was
previously reported that in neurofibromatosis, silencing of GRPR
through specific siRNA resulted in a significant reduction in
proliferation and cell migration, and was accompanied by the
inactivation of Akt and the activation of phosphatase and tensin
homolog, which is a known inhibitor of the phosphoinositide
3-kinase/Akt (PI3K/Akt) pathway. This indicated that the PI3K/Akt
signaling pathway may be regulated by GRP/GRPR (13). Another study concerning small cell
lung cancer indicated that the overexpression of GRP led to an
elevated level of Akt, which is known to be the initiating factor
of the epidermal growth factor receptor (EGFR) signaling pathway
(14). As a high level of EGFR is
considered to contribute to the resistance of small lung cancer to
gemcitabine, the results of the present study indicate that GRP may
also have a role in chemotherapy resistance through activating the
EGFR signaling pathway in tumors.
In the present study, the expression of GRP in the
ES2 cells was downregulated using siRNA technology. The results
revealed that the protein level of GRP significantly decreased
following transfection, which was accompanied by a decrease in the
proliferation and migration ability of ES2 cells, and a marked
increase in the rate of apoptosis. This suggested that GRP has a
role in the proliferation and cell migration of human ovarian
cancer cells. This finding is consistent with that of previous
studies concerning other types of tumor (15–18).
Ischia et al (15) reported
that GRP and GRPR are overexpressed in prostate cancer and can
stimulate the growth of prostate cancer cells. In addition, GRP was
found to be associated with the migration and metastasis of cancer
cells in neuroblastoma and breast cancer (16–18). It
can therefore be concluded that GRP may be a potential novel target
for the treatment of ovarian cancers.
References
|
1
|
Roesler R, Kapczinski F, Quevedo J, et al:
The gastrin-releasing peptide receptor as a therapeutic target in
central nervous system disorders. Recent Pat CNS Drug Discov.
2:125–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang J, Lu Y, Ouyang K, et al: Specific
antibodies elicited by a novel DNA vaccine targeting
gastrin-releasing peptide inhibit murine melanoma growth in vivo.
Clin Vaccine Immunol. 16:1033–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiao J, Kang J, Ishola TA, et al:
Gastrin-releasing peptide receptor silencing suppresses the
tumorigenesis and metastatic potential of neuroblastoma. Proc Natl
Acad Sci USA. 105:12891–12896. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fleischmann A, Waser B and Reubi JC:
Overexpression of gastrin-releasing peptide receptors in
tumor-associated blood vessels of human ovarian neoplasms. Cell
Oncol. 29:421–433. 2007.PubMed/NCBI
|
|
5
|
Patel O, Shulkes A and Baldwin GS:
Gastrin-releasing peptide and cancer. Biochim Biophys Acta.
1766:23–41. 2006.PubMed/NCBI
|
|
6
|
Fan DM, Shi HR, Chen ZM, Wu QH, Liu HN and
Zhang RT: Early detection of ovarian carcinoma by proteome
profiling based on magnetic bead separation and matrix-assisted
laser desorption/ionization time of flight mass spectrometry.
African Journal of Microbiology Research. 4:940–951. 2010.
|
|
7
|
Patel O, Dumesny C, Giraud AS, et al:
Stimulation of proliferation and migration of a colorectal cancer
cell line by amidated and glycine-extended gastrin-releasing
peptide via the same receptor. Biochem Pharmacol. 68:2129–2142.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chinnappan D, Qu XP, Xiao DM, et al: Human
gastrin-releasing peptide receptor gene regulation requires
transcription factor binding at two distinct CRE sites. Am J
Physiol Gastrointest Liver Physiol. 295:G153–G162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schroeder RP, de Visser M, van Weerden WM,
et al: Androgen-regulated gastrin-releasing peptide receptor
expression in androgen-dependent human prostate tumor xenografts.
Int J Cancer. 126:2826–2834. 2010.PubMed/NCBI
|
|
10
|
Rassouli FB and Matin MM: Gene silencing
in human embryonic stem cells by RNA interference. Biochem Biophys
Res Commun. 390:1106–1110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu XL, Liang L and Ding YQ:
Overexpression of FMNL2 is closely related to metastasis of
colorectal cancer. Int J Colorectal Dis. 23:1041–1047. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ischia J, Patel O, Shulkes A and Baldwin
GS: Gastrin-releasing peptide: different forms, different
functions. Biofactors. 35:69–75. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiao J, Kang J, Ishola TA, et al:
Gastrin-releasing peptide receptor silencing suppresses the
tumorigenesis and metastatic potential of neuroblastoma. Proc Natl
Acad Sci USA. 105:12891–12896. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Carlisle DL, Swick MC, et al:
Gastrin-releasing peptide activates Akt through the epidermal
growth factor receptor pathway and abrogates the effect of
gefitinib. Exp Cell Res. 313:1361–1372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ischia J, Patel O, Bolton D, Shulkes A and
Baldwin GS: Expression and function of gastrin-releasing peptide
(GRP) in normal and cancerous urological tissues. BJU Int. 113
(Suppl 2):S40–S47. 2014. View Article : Google Scholar
|
|
16
|
Paul P, Gillory LA, Kang J, Qiao J and
Chung DH: Targeting gastrin-releasing peptide as a new approach to
treat aggressive refractory neuroblastomas. Surgery. 149:425–432.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee S, Qiao J, Paul P and Chung DH:
Integrin β1 is critical for gastrin-releasing peptide
receptor-mediated neuroblastoma cell migration and invasion.
Surgery. 154:369–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ni C, Zhao X, Sun T, Liu Y, Gu Q and Sun
B: Role of gastrin-releasing peptides in breast cancer metastasis.
Hum Pathol. 43:2342–2347. 2012. View Article : Google Scholar : PubMed/NCBI
|