Introduction
Spontaneous regression has been reported in a
variety of malignant diseases, including lung cancer, whereas the
types of cancer most commony exhibiting this rare phenomenon
include malignant melanoma, neuroblastoma and renal cell carcinoma
(1–3).
The standard definition of spontaneous regression is ‘the partial
or complete disappearance of a malignant tumor in the absence of
treatment or in the presence of therapy considered inadequate to
exert a significant influence on the disease’ (1,2). The
distinct pathogenetic mechanism underlying spontaneous regression
in malignant tumors remains unclear, as there is no consensus due
to its rare occurrence. This is the case report of a patient with
lung cancer who exhibited spontaneous regression of the primary and
metastatic lesions without having received treatment. The patient
provided written informed consent.
Case report
A 65-year-old man was referred to Mito Medical
Center, University of Tsukuba (Mito, Japan) with a mass in the left
side of the neck. The tumor had enlarged rapidly over the previous
2 weeks and was accompanied by severe pain in the left arm. Within
a week from the first presentation, paralysis developed gradually
below the level of T9 and the patient was admitted to our hospital.
The patient was a heavy smoker, with no family or past history of
disease. At the first presentation, the physical examination
revealed a sizeable, hard, fixed tumor on the left side of the
neck. The laboratory examination revealed a white blood cell count
of 9,800/µl, a C-reactive protein level of 8.59 mg/dl and a
cytokeratin-19 fragments level of 5.3 ng/ml. A computed tomography
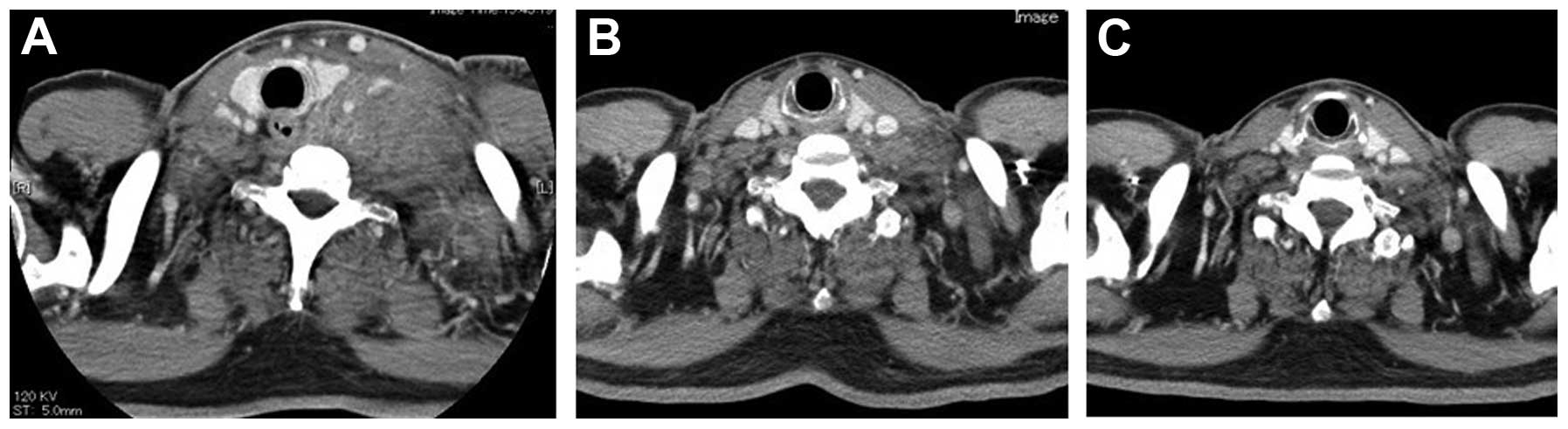
(CT) scan of the neck revealed enlarged, bulging left cervical
lymph nodes (Fig. 1A). A chest
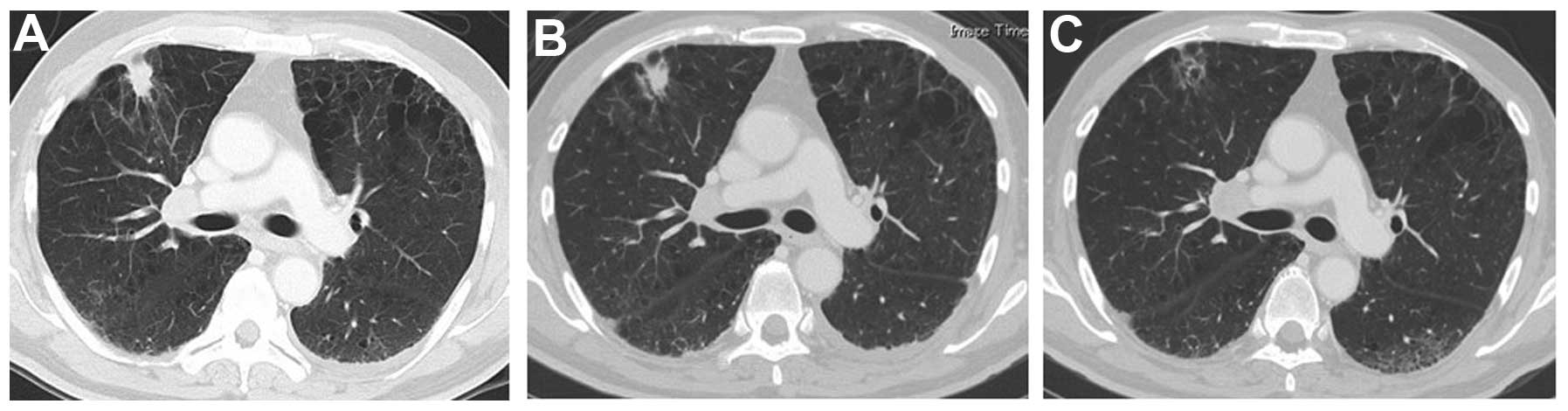
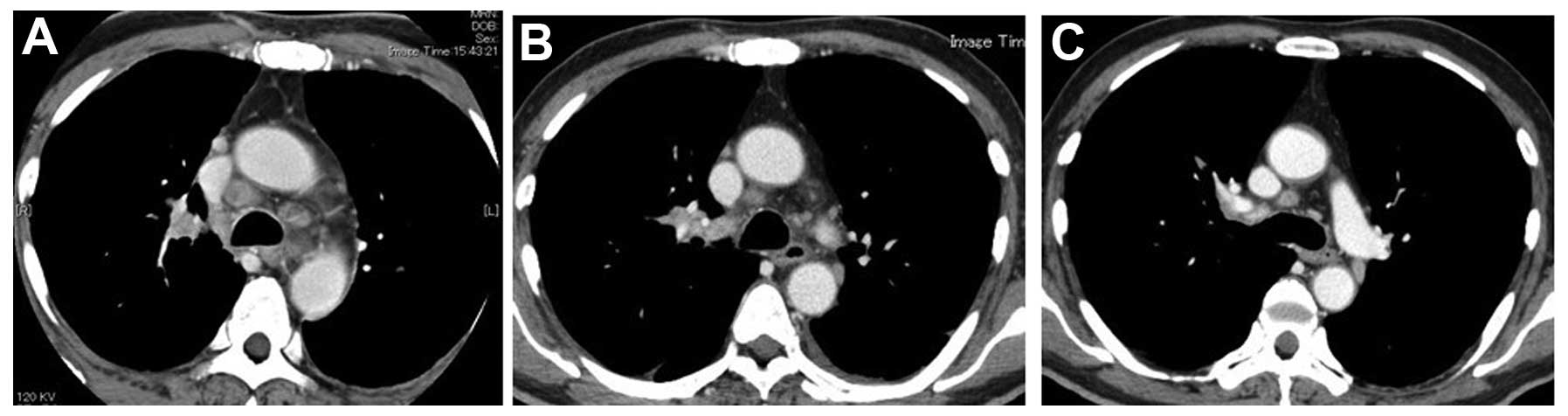
radiograph and CT scan revealed a solid peripheral tumor in the
right lung with enlarged lymph nodes on both sides of the
mediastinum (Figs. 2A and 3A). Magnetic resonance imaging (MRI) of the
thoracic vertebrae revealed metastasis to the vertebral body and
dura mater in T9. The findings of the abdominal CT and brain MRI
scans were normal.
Percutaneous aspiration cytology of the tumor on the
left side of the neck and transbronchial biopsy of the upper lobe
of the right lung were performed and the pathological examination
established the diagnosis of poorly differentiated non-small-cell
lung cancer, with metastases to the cervical lymph nodes. A week
after these biopsies, spontaneous regression of the neck tumor was
identified on a CT scan of the neck (Fig.
1B) and regression of the primary tumor of the lung and
mediastinal lymph nodes was observed on chest radiography. One
month after the first presentation, spontaneous regression of the
primary tumor and mediastinal lymph nodes was confirmed by chest
radiograph and CT scan (Figs. 2B and
3B). Thereafter, the patient
underwent irradiation of the vertebra and dura mater at the T9
metastatic site. Despite receiving no additional therapy for the
primary tumor or the tumor of the neck, the size of these lesions
continued to decrease, almost disappearing completely 2 months
after the first presentation (Figs.
1C, 2C and 3C).
Discussion
Lung cancer metastasis to vascular- and
lymphatic-associated organs is frequent and the most common
metastatic sites are the lung, bone and brain (4–6). Lymph
node involvement is primarily regional in cancers of the internal
organs, including lung cancer (4,7).
Therefore, common sites of lymph node metastasis in lung cancer are
the hilar, mediastinal and supraclavicular nodes. Metastasis to the
cervical lymph nodes and thoracic vertebra are occasionally
observed at advanced stages, suggesting that cancer cells may reach
several sites throughout the body via the bloodstream and lymphatic
system.
Spontaneous regression of malignant disease,
according to a generally accepted definition, is a complete or
partial, temporary or permanent disappearance of all or at least
certain relevant parameters of a soundly diagnosed malignant
disease, without any medical treatment or with treatment that is
considered inadequate to produce the resulting regression (1,2). The
present case may be classified as a partial spontaneous regression
according to this definition. Everson and Cole (2) reported only 176 cases of spontaneous
regression between 1900 and 1964, with an estimated incidence of
1/60,000–100,000 cancer patients. Challis and Stam (3) updated that review. According to their
review, 741 cases of spontaneous regression of malignant diseases
were reported in the literature between 1900 and 1987 (3). Although a variety of malignant diseases
were reported, renal cell carcinoma, neuroblastoma, malignant
melanoma, choriocarcinoma, bladder cancer, retinoblastoma,
lymphoma, leukemia and breast cancer accounted for two-thirds of
all the cases (3). Despite its
incidence in a variety of malignant diseases, spontaneous
regression in lung cancer is considered to be a particularly rare
event (1,2,8,9). Everson and Cole (2) reported only two cases, whereas Lowy and
Erickson had only found four previous cases in the medical
literature when they reported an additional case of spontaneous
regression in a patient with metastatic small-cell lung cancer in
1986 (10).
In the present case, the histological diagnosis of
lung cancer was established by specimens obtained during the
transbronchial procedure; the neck lesion was also histologically
confirmed to be metastatic from lung cancer by specimens obtained
during the skin biopsy. Although histological confirmation of
metastatic lung adenocarcinoma was obtained, the metastatic neck
lesion disappeared shortly following the skin biopsy. Certain
previous studies have reported spontaneous regression of metastatic
lesions following removal of the primary lesion (11–15),
whereas other studies have reported spontaneous regression
associated with infection (1,16). In the present case, however,
spontaneous regression of the metastatic lesion was observed
without any treatment of the primary or metastatic lesions; in
addition, there was no infection of the primary or metastatic
sites. It was recently reported that immunological mechanisms,
including certain cytokines, such as interferon and tumor necrosis
factor, may stimulate cytotoxic function against cancer cells,
leading to spontaneous regression (17–19).
Unfortunately, blood samples were not obtained for immunological
evaluation, as there was no expectation of tumor regression at the
time. Therefore, data elucidating the precise immunological
association are lacking and the exact mechanism of spontaneous
regression of the metastatic lesion remains unknown.
We previously reported the case of a patient with
lung adenocarcinoma exhibiting spontaneous regression of a scalp
metastasis (15). Notably, in that
previous case, spontaneous regression of the scalp metastatic
lesion developed after 1 month following biopsy of the scalp mass
and the regression of the primary lung lesion also developed 1
month following a transbronchial biopsy (15). In the present case, spontaneous
regression occurred not only in the cervical lymph node metastatic
lesion, but also in the primary lung lesion 1 month after obtaining
the pathological specimens. It is noteworthy that, in these two
patients, spontaneous regression developed shortly after a direct
invasive approach to the tumor lesions, which is highly suggestive
of an association between the onset of the regression and a change
in the intratumoral immunological mechanism between the host and
the tumor. A likely explanation is that there may be a stimulus
associated with the direct invasive approach to the lesions, which
initiates the spontaneous regression. However, the precise
mechanism remains unclear and future studies are required to
elucidate this mechanism. The patient should be closely followed up
to monitor the clinical course of this unusual case. A later report
may reveal the causes and broaden the knowledge regarding this
uncommon phenomenon.
References
|
1
|
Cole WH: Effects to explain spontaneous
regression of cancer. J Surg Oncol. 17:201–209. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Everson TC and Cole WH: Spontaneous
regression of cancer. Philadelphia WB Sounders; 1966
|
|
3
|
Challis GB and Stam HJ: The spontaneous
regression of cancer. A review of cases from 1900–1987. Acta Oncol.
29:545–550. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gueron G, De Siervi A and Vazquez E: Key
questions in metastasis: new insights in molecular pathways and
therapeutic implications. Curr Pharm Biotechnol. 12:1867–1880.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oikawa A, Takahashi H, Ishikawa H,
Kurishima K, Kagohashi K and Satoh H: Application of conditional
probability analysis to distant metastases from lung cancer. Oncol
Lett. 3:629–634. 2012.PubMed/NCBI
|
|
6
|
Nakazawa K, Kurishima K, Tamura T, et al:
Specific organ metastases and survival in small cell lung cancer.
Oncol Lett. 4:617–620. 2012.PubMed/NCBI
|
|
7
|
Sampath L, Kwon S, Hall MA, Price RE and
Sevick-Muraca EM: Detection of cancer metastases with a
dual-labeled near-infrared/positron emission tomography imaging
agent. Transl Oncol. 3:307–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kappauf H, Gallmeier WM and Wunch PH:
Complete spontaneous remission in a patient with metastatic
non-small-cell lung cancer. Case report, review of literature and
discussion of possible biological pathways involved. Ann Oncol.
8:1031–1039. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cafferata MA, Chiaramondia M, Monetti F
and Ardizzoni A: Complete spontaneous remission of non-small-cell
lung cancer: a case report. Lung Cancer. 45:263–266. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lowy AD Jr and Erickson ER: Spontaneous
19-year regression of oat cell carcinoma with scalene metastasis.
Cancer. 58:978–980. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deweerd JH, Hawthorne NJ and Adson MA:
Regression of renal cell hepatic metastasis following removal of
primary lesions. J Urol. 117:790–792. 1977.PubMed/NCBI
|
|
12
|
Marces SG, Choyke PL, Reiter R, et al:
Regression of metastatic renal cell carcinoma after cytoreductive
nephrectomy. J Urol. 150:463–466. 1993.PubMed/NCBI
|
|
13
|
Gohji K, Kizaki T and Fujii A: Spontaneous
regression of pulmonary metastasis from nonfunctioning
adrenocortical carcinoma after removal of the primary lesion: a
case report. J Urol. 154:1854–1855. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hammad AM, Paris GR, van Heuven WA,
Thompson IM and Fitzsimmons TD: Spontaneous regression of choroidal
metastasis from renal cell carcinoma. Am J Ophthalmol. 135:911–913.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyazaki K, Masuko H, Satoh H and Ohtsuka
M: Lung cancer with spontaneous regression of scalp metastasis.
Respir Med Extra. 3:83–85. 2007. View Article : Google Scholar
|
|
16
|
Wadsworth SJ, Davies CW, Gray W and
Gleeson FV: Spontaneous regression of pulmonary metastases
demonstrated by CT. Br J Radiol. 72:304–307. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stoll BA: Spontaneous regression of
cancer: new insights. Biotherapy. 4:23–30. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moroda T, Iiai T and Suzuki S: Autologous
killing by a population of intermediate T-cell receptor cells and
its NK1.1+ and NK1.1 subsets, using Fas ligand/Fas
molecules. Immunology. 91:219–226. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawamura T, Seki S and Takeda K:
Protective effect of NK1.1+ cells as well as NK cells
against intraperitoneal tumors in mice. Cell Immunol. 193:219–225.
1999. View Article : Google Scholar : PubMed/NCBI
|