Introduction
Tumour tissue angiogenesis is often evaluated by
determining microvascular density (MVD) and endothelial area (EA)
(1–3).
MVD and EA have been experimentally proposed to be biomarkers
associated with biological aggressiveness and clinical outcome in
animal and human malignancies. In regards to pancreatic cancer, it
has been established that angiogenesis has a role in its
development and progression (4–17). In
addition, Ki-67 expression is an important parameter for biological
aggressiveness and prognosis in tumour tissue; Ki-67 antigens are
initially expressed in S-phase and increase throughout S- and
G2-phase until they reach their peak expression during
mitosis. Following division, cells progress into
G1-phase with high levels of Ki-67 antigen, which
gradually decrease during this phase and are low or no longer
expressed in cells in prolonged G1. Therefore, Ki-67
protein is expressed in proliferating cells throughout the cell
cycle, but not in quiescent (G0) cells (18–20). Ki-67
may be used as a proliferation index to characterise proliferating
and non-proliferating tumour cells (21,22);
however, no data have been reported regarding the correlation
between MVD or EA and Ki-67 proliferation index in pancreatic
ductal adenocarcinoma (PDAC).
The aim of the present study was to analyse MVD, EA
and Ki-67 expression in proliferating cells in a series of 31 human
PDAC tissues and in their corresponding adjacent normal tissue
(ANT) in order to evaluate differences in these tissue parameters
between normal and malignant tissue. In addition, the present study
aimed to evaluate the potential correlation of these factors with
each other. Furthermore, the correlation between each analysed
tissue index and the primary clinico-pathological features of PDAC
were investigated.
Patients and methods
Patients
The clinico-pathological features of selected
patients are summarised in Table I. A
total of 31 PDAC patients (PDACPs) underwent potentially curative
surgical resection at the Clinical Surgery Unit, University of
Catanzaro ‘Magna Graecia’ Medical School (Catanzaro, Italy).
Surgical approaches used included pancreaticoduodenectomy, distal
pancreatectomy and total pancreatectomy with lymph node dissection.
Tumour tissues and ANT were resected from patients and tumour
tissues were staged according to the American Joint Committee on
Cancer classification guidelines (7th edition) and the World Health
Organization classification guidelines (2000 version) for
pathologic grading (23,24). Computed tomography (CT) was conducted
on a Somatom Sensation CT scanner (Siemens AG, Munich, Germany) to
confirm that all patients did not have distant metastases and ten
patients underwent neo-adjuvant therapy with Gemcitabine or
Folfirinox prior to surgery. The present study was approved by the
Ethics Committee of ‘Mater Domini’ Hospital, ‘Magna Graecia’
University (Catanzaro, Italy). Full signed informed consent was
obtained from each patient enrolled in the present study, including
the authorisation to utilise each tissue sample for experimental
studies.
 | Table I.Clinico-pathological features of
patients. |
Table I.
Clinico-pathological features of
patients.
| Patient
characteristics | No. of patients |
|---|
| Overall series | 31 |
| Age |
|
|
<65 | 23 |
|
>65 | 8 |
| Gender |
|
| Male | 25 |
|
Female | 6 |
| Tumour site |
|
| Head | 13 |
|
Body-tail | 18 |
| TNM |
|
|
T2N0–1M0 | 14 |
|
T3N0–1M0 | 17 |
| Histologic type |
|
| Ductal
adenocarcinomas | 31 |
| Histologic grade |
|
|
G1-G2 | 19 |
| G3 | 12 |
Immunohistochemistry
For the evaluation of MVD, EA and Ki-67
proliferation index in PDAC tissue and ANT, a three-layer
biotin-avidin-peroxidase system was utilised, as previously
described (25). In brief, 4-µm thick
serial sections of formalin-fixed and paraffin-embedded tumour
samples and the corresponding ANT (2 of each sample) were
deparaffinised. Formalin and paraffin were purchased from Bio
Optica Milano SpA, (Milan, Italy). For antigen retrieval, sections
were then microwaved at 500 W for 10 min; following which,
endogenous peroxidase activity was blocked with 3% hydrogen
peroxide solution (Dako, Glostrup, Denmark). Subsequently, adjacent
sections were incubated with the mouse monoclonal anti-CD31
antibodies (dilution, 1:40; JC70a; Dako) for 30 min at room
temperature and mouse monoclonal anti-Ki-67 (dilution, 1:100;
MIB-1; Immunotech, Inc., Marseilles, France) for 1 h at room
temperature. The bound antibodies were visualised using a
biotinylated horse anti-mouse IgG (H+L) secondary antibody
(dilution, 1:100; BA-2000; Vector Laboratories, Inc., Burlingame,
CA, USA) incubated for 1 h at room temperature, followed by
avidin-biotin peroxidase complex and NovaRED (Vector Laboratories,
Inc.). Nuclear counterstaining was performed using Gill's
haematoxylin no. 2 (Polysciences, Inc., Warrington, PA, USA). For
the negative controls, slides were incubated with secondary
antibodies and primary antibody was omitted.
Morphometrical assay
A Quantimet 500 image analysis system (Leica
Microsystems GmbH, Wetzlar, Germany) with a connected Nikon Eclipse
E400 Biological Microscope (Nikon Corporation, Tokyo, Japan) was
employed. The five most vascularised areas (‘hot spots’) were
selected at low magnification and the individual vessels (Fig. 1A) and Ki-67 proliferation index
(Fig. 1B and C) were counted at x400
magnification (0.19 mm2 area) in tumour tissue and ANT.
A method of morphometric evaluation was used as described in a
previous study (26). Single
red-stained endothelial cells, endothelial cell clusters and
microvessels, clearly separated from adjacent microvessels, tumour
cells, normal epithelial cells and other connective tissue elements
were also counted. The immunostained area was evaluated identifying
and manually delimiting the perimeter of each red immunostained
area in a field of 0.19 mm2 on the computer, then the
software calculated the delimited area. In tumour tissue, areas of
necrosis were not included in the counting area. Single red-stained
endothelial cells were also evaluated in terms of immunostained
area at x400 magnification (0.19 mm2 area) (25). Ki-67 expression was determined in
corresponding areas to MVD ‘hot spots’ and only specific red
nuclear staining was considered. The number of MIB-1
positively-stained nuclei was counted at x400 magnification (0.19
mm2 area). The fraction of Ki-67-positive cells was
calculated as the ratio of positively-stained tumour cells to all
tumour cells. Furthermore, morphological detail, including the
microvessel, its wall and the red blood cells in the lumen.
Notably, the diameter of the lumen was very small compared with the
red proliferating nuclei that were clearly visible near the
microvessel. This was observed at x1,000 magnification in oil
(Fig. 1D).
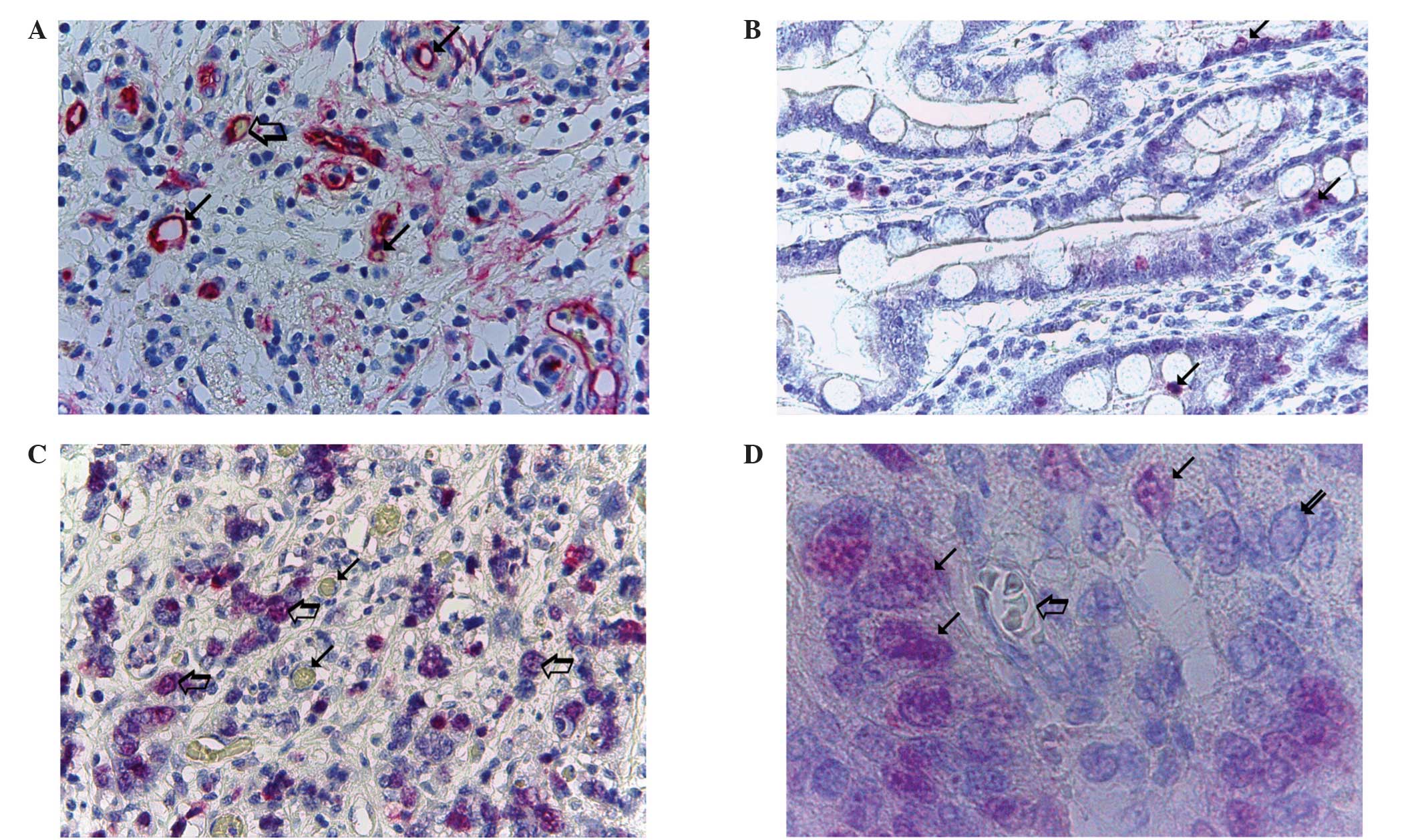 | Figure 1.Morphometrical analysis of MVD and
Ki-67 proliferation index in PDAC tissue. (A) Highly vascularised
pancreatic ductal adenocarcinoma sample stained with anti-CD-31
antibodies. Numerous red immunostained microvessels are visible:
Small arrows, microvessels with a visible lumen; large arrow,
microvessel with a red blood cell in its lumen, this acted as an
internal positive control (magnification, x400). (B)
Well-differentiated and (C) poorly-differentiated PDAC samples
stained with anti-Ki-67 antibodies; low and high rates of
proliferation are observed, respectively. Arrows, single
red-stained proliferating nuclei (magnification, x400). (D)
Poorly-differentiated PDAC sample stained with anti-Ki-67
antibodies. Small arrows, single red-stained proliferating nuclei;
large arrow, microvessel with several red blood cells in its lumen,
this acted as an internal positive control. Red proliferating
nuclei are visible near the microvessels (magnification, x1,000, in
oil). MVD, microvascular density; PDAC, pancreatic ductal
adenocarcinoma. |
Statistical analysis
Significant differences in the angiogenic indexes
and Ki-67-positive fraction between PDAC and ANT were assessed
using the Student's t-test. Linear correlations among MVD, EA and
Ki-67 proliferation index were compared with each other and were
quantified using the Pearson's correlation coefficient (r).
Correlations among the MVD, EA and Ki-67 proliferation index groups
and the main clinico-pathological features were analysed using the
Chi-square test. Values are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference between values. All statistical analyses
were performed using the SPSS statistical software package, version
20.0 (IBM SPSS, Armonk, NY, USA).
Results
All the tissue parameters evaluated, including MVD,
EA and Ki-67 proliferation index, were compared between PDAC tissue
and ANT; the results are reported in Table II. The number of microvessels and EA
were significantly higher in PDAC tissue (27±8 and 186.06±65.89
µ2, respectively) compared with the corresponding values
in ANT (8±4 and 73.12±27.88 µ2; P=0.001 and P=0.003,
respectively). In addition, a significantly higher number of
Ki-67-positive proliferative cells were observed in PDAC tissue
compared with ANT (66±25 vs. 15±6; P=0.001) (Table II). Immunohistochemical staining was
performed using anti-Ki-67 antibodies and the evaluation at x1,000
magnification in oil demonstrated that in highly vascularised
cancer tissue, red-immunostained proliferating nuclei were highly
expressed and were primarily located in perivascular position
(Fig. 1D).
 | Table II.Comparison of MVD, EA and
Ki-67-positive cells in PDAC tissue and ANT. |
Table II.
Comparison of MVD, EA and
Ki-67-positive cells in PDAC tissue and ANT.
| Tissue type | MVD (no. of
microvessels) | EA
(µ2) | Ki-67 positive
fraction (no. of MIB-1-positive nuclei) |
|---|
| ANT | 8±4 | 73.12±27.88 | 15±6 |
| PDAC | 27±8 | 186.06±65.89 | 66±25 |
| P-value | 0.001 | 0.003 | 0.001 |
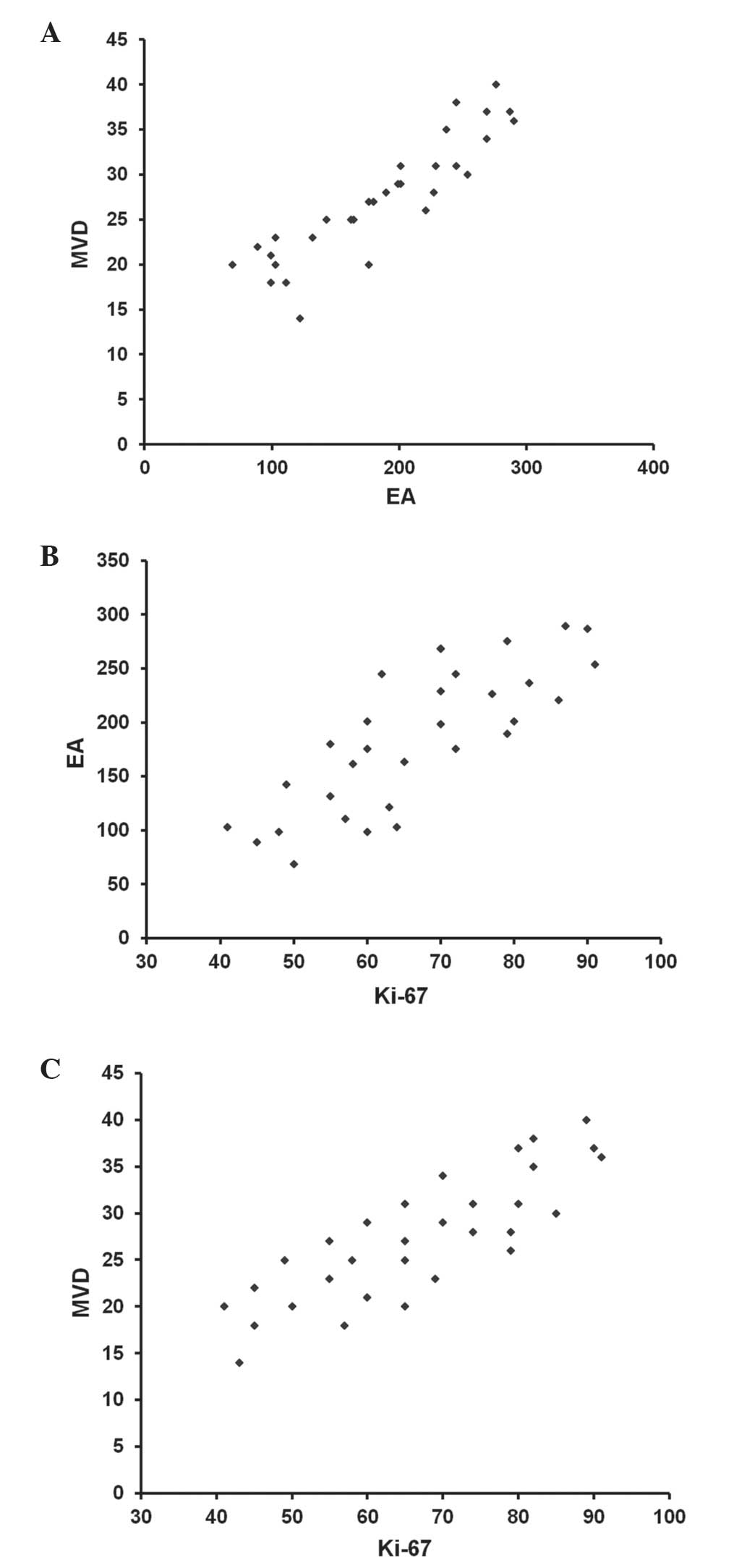
As shown in Fig. 2, in
tumour tissue there were significant correlations between MVD and
EA (r=0.80; P=0.001), between MVD and Ki-67 (r=0.72; P=0.003) and
between EA and Ki-67 (r=0.65; P=0.004). No correlation was detected
concerning the parameters MVD, EA or Ki-67 and the main
clinico-pathological features of PDAC.
Discussion
Previous studies have demonstrated that angiogenesis
and Ki-67-positive fraction are essential for the development and
progression of tumours (27–31). In addition, numerous studies have
suggested that increased tissue MVD and Ki-67 proliferation index,
which were individually assessed, were correlated with a poor
prognosis in PDACP (21,22). However, studies into PDAC are limited
and no data have been previously published regarding the
correlation between MVD, EA angiogenic indexes and Ki-67 fraction
in PDAC. The results of the present study demonstrated that MVD, EA
and Ki-67 proliferation index were all significantly increased in
PDAC tissue compared with ANT; in addition, it was revealed that in
tumour tissue, these parameters were increased in parallel to each
other. Of note, a close spatial association was observed between
proliferating tumour cells and microvessels, as determined using
image analysis data in the present study; this therefore indicated
an anatomical association between neovessel formation and tumour
cell proliferation. In addition, this anatomical link may be
supported by the overexpression of several angiogenic cytokines,
including vascular endothelial growth factor, fibroblast growth
factor, thymidine phosphorylase and tryptase, which have been
previously demonstrated to be present in PDAC tissue (32–35). These
cytokines, secreted from tumoral and stromal cells, have a role in
the autocrine and paracrine growth stimulation of tumoral and
endothelial cells (36–38).
In conclusion, the data presented in the current
study suggested a pre-clinical background for the future evaluation
of novel therapeutic treatments, which combine angiogenesis
inhibitors and anti-proliferative chemotherapeutic drugs for PDAC.
The study demonstrated that in association with angiogenesis, MVD,
EA and Ki-67 proliferation index, are significantly correlated to
each other in PDAC tumour tissues, and were significantly increased
in these tissues compared with levels in normal tissues. Following
future confirmation of the data, the analyzed biomarkers may
identify an integrated panel of clinical aggressiveness which may
be useful in the future to select patients who are suitable to
receive potential adjuvant treatment.
References
|
1
|
Folkman J: Tumor angiogenesis and tissue
factor. Nat Med. 2:167–168. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ranieri G, Coviello M, Chriatti A, et al:
Vascular endothelial growth factor assessment in different blood
fractions of gastroenterology cancer patients and healthy controls.
Oncol Rep. 11:435–439. 2004.PubMed/NCBI
|
|
3
|
Ranieri G, Labriola A, Achille G, et al:
Microvessels density, mast cell density and thymidine phosphorylase
expression in oral squamous carcinoma. Int J Oncol. 21:1317–1323.
2002.PubMed/NCBI
|
|
4
|
Ranieri G, Patruno R, Lionetti A, et al:
Endothelial area and micrivascular density in a canine
non-Hodgkin's lymphoma: An interspecies model of tumor
angiogenesis. Leuk Lymphoma. 46:1639–1643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamazaki K, Nagao T, Yamaguchi T, et al:
Expression of basic fibroblast growth factor (FGF-2)-associated
with tumour proliferation in human pancreatic carcinoma. Virchows
Arch. 431:95–101. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Díaz VM, Planaguma J, Thomson TM, et al:
Tissue plasminogen activator is required for the growth, invasion
and angiogenesis of pancreatic tumor cells. Gastroenterology.
122:806–819. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumour angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soucek L, Lawlor ER, Soto D, et al: Mast
cells are required for angiogenesis and macroscopic expansion of
Myc-induced pancreatic islet tumours. Nat Med. 13:1211–1218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pang B, Fan H, Zhang IY, et al: HMGA1
expression in human gliomas and its correlation with tumor
proliferation, invasion and angiogenesis. J Neurooncol.
106:543–549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma SG, Aggarwal N, Gupta SD, et al:
Angiogenesis in renal cell carcinoma: Correlation of microvessel
density and microvessel area with other prognostic factors. Int
Urol Nephrol. 43:125–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanci M, Dikis C, Inan S, et al:
Immunolocalization of VEGF, VEGF receptors, EGF-R and Ki-67 in
leiomyoma, cellular leiomyoma and leiomyosarcoma. Acta Histochem.
113:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gravdal K, Halvorsen OJ, Haukaas SA and
Akslen LA: Proliferation of immature tumor vessels is a novel
marker of clinical progression in prostate cancer. Cancer Res.
69:4708–4715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koide N, Saito H, Suzuki A, et al:
Clinicopathologic features and histochemical analyses of
proliferative activity and angiogenesis in small cell carcinoma of
the esophagus. J Gastroenterol. 42:932–938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tenderenda M: Potential prognostic value
of angiogenesis, cell proliferation and metastasing in patients
with surgically treated gastric cancer-current knowledge. Wiad Lek.
59:855–860. 2006.(In Polish). PubMed/NCBI
|
|
15
|
Imamura M, Yamamoto H, Nakamura N, et al:
Prognostic significance of angiogenesis in gastrointestinal stromal
tumor. Mod Pathol. 20:529–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujita S, Nagamachi S, Nishii R, et al:
Relationship between cancer cell proliferation, tumour angiogenesis
and 201Tl uptake in non-small cell lung cancer. Nucl Med Commun.
27:989–997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Zhang S, Chen YP and Lin JY:
Increased expression of angiogenin in gastric carcinoma in
correlation with tumor angiogenesis and proliferation. World J
Gastroenterol. 12:5135–5139. 2006.PubMed/NCBI
|
|
18
|
Patruno R, Zizzo N, Zito AF, et al:
Microvascular density and endothelial area correlate with Ki-67
proliferative rate in the canine non-Hodgkin's lymphoma spontaneous
model. Leuk Lymphoma. 47:1138–1143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlüter C, Duchrow M, Wohlenberg C, et
al: The cell proliferation-associated antigen of antibody Ki-67: A
very large, ubiquitous nuclear protein with numerous repeated
elements, representing a new kind of cell cycle-maintaining
protein. J Cell Biol. 123:513–522. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalogeraki A, Tzardi M, Panagiotides I, et
al: MIB1 (Ki-67) expression in non-Hodgkin' s lymphomas. Anticancer
Res. 17:487–491. 1997.PubMed/NCBI
|
|
21
|
Stipa F, Lucandri G, Limiti MR, et al:
Angiogenesis as a prognostic indicator in pancreatic ductal
adenocarcinoma. Anticancer Res. 22:445–449. 2002.PubMed/NCBI
|
|
22
|
Karademir S, Sökmen S, Terzi C, et al:
Tumor angiogenesis as a prognostic predictor in pancreatic cancer.
J Hepatobiliary Pancreat Surg. 7:489–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th. Springer; New York, NY: pp. 241–249.
2010
|
|
24
|
Hamilton SR and Aaltonen LA: Pathology and
Genetics. Tumours of the Digestive SystemWorld Health Organization
Classification of Tumours. International Agency for Research on
Cancer (IARC). 3rd. 2. IARC Press; Lyon, France: pp. 1–307.
2000
|
|
25
|
Ranieri G, Grammatica L, Patruno R, et al:
A possible role of thymidine phosphorylase expression and
5-fluorouracil increased sensitivity in oropharyngeal cancer
patients. J Cell Mol Med. 11:362–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ranieri G, Labriola A, Achille G, et al:
Microvessel density, mast cell density and thymidine phosphorylase
expression in oral squamous carcinoma. Int J Oncol. 21:1317–1323.
2002.PubMed/NCBI
|
|
27
|
Ammendola M, Sacco R, Sammarco G, et al:
Mast cells positive to tryptase and C-Kit receptor expressing cells
correlates with angiogenesis in gastric cancer patients surgically
treated. Gastroenterol Res Pract. 2013:7031632013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ammendola M, Sacco R, Donato G, et al:
Mast Cell positive to tryptase correlates with metastatic lymph
nodes in gastrointestinal cancers patients treated surgically.
Oncology. 85:111–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Y, Hwang RF, Logsdon CD and Ullrich SE:
Dynamic mast cell-stromal cell interactions promote growth of
pancreatic cancer. Cancer Res. 73:3927–3937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Chen X, Fang J and Yang C:
Overexpression of both VEGF-A and VEGF-C in gastric cancer
correlates with prognosis and silencing of both is effective to
inhibit cancer growth. Int J Clin Exp Pathol. 6:586–597.
2013.PubMed/NCBI
|
|
31
|
Ammendola M, Zuccalà V, Patruno R, et al:
Tryptase-positive mast cells and angiogenesis in keloids: A new
post-surgical target for prevention. Updates Surg. 65:53–57. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Patsouras D, Papaxoinis K, Kostakis A, et
al: Fibroblast activation protein and its prognostic significance
in correlation with vascular endothelial growth factor in
pancreatic adenocarcinoma. Mol Med Rep. 11:4585–4590.
2015.PubMed/NCBI
|
|
33
|
Matsuda Y, Yoshimura H, Suzuki T, et al:
Inhibition of fibroblast growth factor receptor 2 attenuates
proliferation and invasion of pancreatic cancer. Cancer Sci.
105:1212–1219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong SP, Shin SK, Bang S, et al:
Prognostic value of thymidine phosphorylase expression for
pancreatic cancer. Hepatogastroenterology. 56:1178–1182.
2009.PubMed/NCBI
|
|
35
|
Ammendola M, Sacco R, Sammarco G, Donato
G, et al: Mast cells density positive to tryptase correlates with
angiogenesis in pancreatic ductal adenocarcinoma patients having
undergone surgery. Gastroenterol Res Pract. 2014:9519572014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ranieri G, Mammì M, Donato Di Paola E, et
al: Pazopanib a tyrosine kinase inhibitor with strong
anti-angiogenetic activity: A new treatment for metastatic soft
tissue sarcoma. Crit Rev Oncol Hematol. 89:322–329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Humbert M, Castéran N, Letard S, et al:
Masitinib combined with standard gemcitabine chemotherapy: In vitro
and in vivo studies in human pancreatic tumour cell lines and
ectopic mouse model. PLoS One. 5:e94302010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marech I, Patruno R, Zizzo N, et al:
Masitinib (AB1010), from canine tumor model to human clinical
development: Where we are? Crit Rev Oncol Hematol. 91:98–111. 2014.
View Article : Google Scholar : PubMed/NCBI
|