Introduction
Cholangiocarcinoma is a highly malignant epithelial
neoplasm arising from the bile duct epithelial cells or
cholangiocytes of the intra- or extrahepatic biliary system. The
incidence and mortality rates for cholangiocarcinoma are increasing
worldwide. Due to the difficulty of forming an early diagnosis,
more than two-thirds of cholangiocarcinomas have metastasis and
have no chance to benefit from surgical resection (1,2). Lymphatic
metastasis plays a significant role in the process of
cholangiocarcinoma metastasis, and the hepatic portal lymph node is
the most commonly affected node. Tumor metastasis is a complex and
multi-stage process in which a number of cytokines are now known to
be involved, particularly the vascular endothelial growth factor
(VEGF) family (3–5).
VEGF-C has been implicated as being a potent
stimulator of angiogenesis and lymphangiogenesis (6). By binding with VEGFR-2 and VEGFR-3,
VEGF-C exerts its biological functions in various cell types.
Recent studies revealed that VEGF-C can be expressed in several
cancer cell types, including ovarian carcinoma (7), lung adenocarcinoma (8), breast cancer (9), and head and neck squamous carcinoma
(10) cells, and that it can promote
lymphatic metastasis progression, thus promoting tumor malignancy.
Ishikawa et al (10) reported
that the expression of VEGF-C is negative in early-stage gastric
cancers due to the extremely low level of nodal metastasis, and
Yonemura et al (11) showed
that VEGF-C expression significantly correlates with lymph node
metastasis in advanced-stage gastric cancers. However, few studies
have been reported on the correlation between VEGF-C expression and
an invasive phenotype in cholangiocarcinoma tissues and cells.
The present study evaluated whether VEGF-C can be a
useful factor in predicting lymph node metastasis in human
cholangiocarcinoma tissues and the role played by VEGF in mediating
proliferation in the cholangiocarcinoma FRH-0201 cell line.
Materials and methods
Tissue collection and
immunohistochemistry (IHC)
A total of 65 patients with cholangiocarcinoma who
were diagnosed and treated with curative surgery using a standard
lymph node dissection, in Qilu Hospital of Shandong University
(Jinan, Shandong, China) between 2012 and 2015, were included in
the study. Written informed consent was obtained from all patients,
and the study was approved by the ethics committee of Qilu Hospital
of Shandong University. As normal controls, 5 cholangiolar biopsies
from patients (3 female and 2 male; age range, 64–74 years) with a
normal histology who underwent laparotomies were assessed.
Fragments of cholangiocarcinoma and normal cholangiolar biopsies
were processed for common histomorphology (hematoxylin-eosin and
Masson's stains) and for IHC. For IHC, frozen cholangiocarcinoma
tissue samples were stored at −80°C until use. Cryostat sections
were fixed in 4°C acetone (Beijing Zhongshan Biological Technology
Ltd., Beijing, China) for 20 min and air-dried for 30 min.
Endogenous peroxidase activity was removed using 3%
H2O2 in methanol (Beijing Zhongshan
Biological Technology Ltd.) for 10 min. Subsequent to being washed
three times with phosphate-buffered saline (PBS), the sections were
incubated with an affinity-purified goat polyclonal IgG antibody
against VEGF-C (catalog no. sc-7133; Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA; dilution, 1:100) overnight at room
temperature. Tissue sections were rinsed in PBS and then incubated
with a linked biotinylated polyclonal rabbit anti-goat
immunoglobulin secondary antibody (catalog no. E046601; Dako,
Glostrup, Denmark) for 15 min at room temperature. Subsequent to
rinsing with PBS, the sections were incubated with DAB and
counterstained with hematoxylin (Beijing Zhongshan Biological
Technology Ltd.). Negative controls were processed by omitting the
primary antibody. The sections were observed using an Olympus BX53
fluorescent microscope (Olympus Corporation, Tokyo, Japan). Cases
in which >5% of the cells showed positive immunostaining for
VEGF-C were judged to be positive.
Cell culture
The human hilar cholangiocarcinoma FRH-0201 cells
were supplied by Dr Wu (Department of General Surgery, Qilu
Hospital of Shandong University) and were maintained in Dulbecco's
modified Eagle's medium (Hyclone, GE Healthcare, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS), 100 µg/ml
streptomycina and 100 µg/ml penicillin (Zhejiang Tianhang
Biological Technology Ltd., Hangzhou, China) and grown at 37°C in
5% carbon dioxide. The cells were starved for 4 h in medium without
fetal bovine serum and then stimulated with VEGF-C at
concentrations of 1, 5, 10, 50 and 100 ng/m for 24, 48 and 72 h,
respectively, at 37°C.
Proliferation assay
The effects of VEGF-C on the FRH-0201 cells were
investigated by MTT assays (Promega Corporation, Madison, WI, USA).
FRH-0201 cells at 85–100% confluence were harvested, and then
5×103 cells per well were seeded onto a 96-well plate.
The cells were incubated in 200 µl medium in a humidified
incubator. After 24 h, the cells were stimulated with VEGF-C (Sino
Biological Inc., Beijing, China; catalog no. 10542-H08H) at 1, 5,
10, 50 and 100 ng/m for 24, 48 and 72 h, respectively, and the
medium was changed daily. Next, 20 µl MTT solution was added and
the cells were incubated for 4 h, solubilized with 200 µl DMSO
(Amresco LLC, Solon, OH, USA) and agitated for 10 min. The optical
density (OD) values were examined at 570 nm using a Safire 2
microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The
cell survival percentages were calculated by dividing the mean OD
of the VEGF-C-containing wells by that of the control wells.
Migration assay
Transwell chambers (8-µm, 24-well insert; Corning
Inc. Life Sciences, Tewksbury, MA, USA) were used for the migration
assay. Briefly, 600 µl 10% FBS-containing medium was added to the
lower chamber and 1×105 cells in 200 µl serum-free
medium were added to the upper chamber. The cells were incubated
for 48 h at 37°C, and the non-invading cells were then scraped off.
Finally, the insert membranes were stained with 0.1% crystal violet
(Amresco LLC) and the permeating cells were counted, with images
captured under an Olympus CKX41 inverted microscope (Olympus
Corporation). The migrated cells were counted in 10 random fields
at x200 magnification. At least three randomly selected fields were
counted, and the average number of cells was calculated per
field.
Statistical analysis
Statistical analysis was performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). Data were expressed as the
mean ± standard deviation, and differences between groups were
analyzed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of VEGF-C in
cholangiocarcinoma tissues
The clinical and pathological characteristics of the
65 patients are summarized in Table
I. The expression of VEGF-C was measured by IHC in the
cholangiocarcinoma tissues and normal human bile duct specimens.
Strong immunoreactivity was present for VEGF-C in the
cholangiocarcinoma specimens, but minimal immunoreactivity was
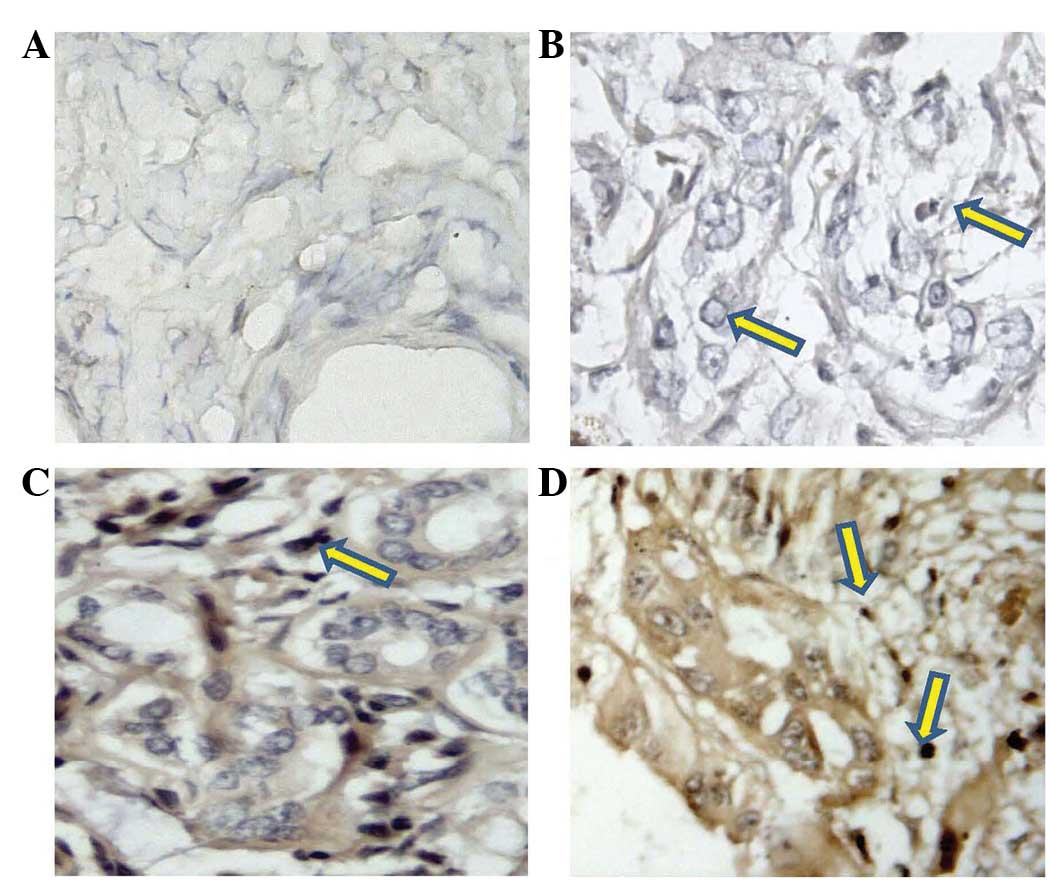
observed in the normal specimens (Fig. 1A
and B). In the normal bile duct specimens, moderate- to
low-level expression was only observed in 5% of the normal bile
duct specimens. By contrast, high-level VEGF-C expression was
observed in the majority of cholangiocarcinoma specimens, and the
positive rate was 75.4% (49/65) (Fig. 1C
and D).
 | Table I.Summary of the clinicopathological
features of 65 patients with cholangiocarcinoma. |
Table I.
Summary of the clinicopathological
features of 65 patients with cholangiocarcinoma.
| Feature | Value |
|---|
| Age, years (mean ±
standard deviation) | 55±8.5 |
| Gender
(male/female) | 38/27 |
| Tumor location,
n |
|
| Hilar /
Intrahepatic bile duct | 34 |
|
Extrahepatic bile duct | 31 |
| Histological
differentiation, n |
|
| Well | 15 |
|
Moderate | 19 |
| Poor | 31 |
| Lymphatic invasion,
n |
|
|
Present | 42 |
|
Absent | 23 |
Association between the expression of
VEGF-C and clinicopathological data
The association between clinicopathological data and
the expression of VEGF-C is shown in Table II. No significant difference in tumor
size was observed among the positive and negative expression types.
There was also no significant difference between the expression of
VEGF-C and age, gender and tumor location. However, VEGF-C was
expressed at a significantly higher level in the patients with
lymph node metastasis than in those without lymph node metastasis
(P<0.01).
 | Table II.Correlation between VEGF-C expression
and pathological features. |
Table II.
Correlation between VEGF-C expression
and pathological features.
|
| VEGF-C expression,
n |
|---|
|
|
|
|---|
| Feature | n | Positive | Negative | P-value |
|---|
| Age, years |
|
|
| P>0.05 |
| ≥60 | 30 | 21 | 9 |
|
<60 | 35 | 28 | 7 |
|
| Gender |
|
|
| P>0.05 |
| Male | 38 | 28 | 10 |
|
|
Female | 27 | 21 | 6 |
|
| Tumor location |
|
|
| P>0.05 |
|
Hilar/Intrahepatic | 34 | 26 | 8 |
|
|
Extrahepatic | 31 | 23 | 8 |
|
| Tumor size, cm |
|
|
| P>0.05 |
| ≥2 | 21 | 18 | 3 |
|
<2 | 44 | 31 | 13 |
|
| Differentiation |
|
|
| P>0.05 |
|
Well/moderate | 34 | 25 | 9 |
| Poor | 31 | 24 | 7 |
|
| Lymphatic
invasion |
|
|
| P<0.01 |
|
Present | 42 | 36 | 6 |
|
Absent | 23 | 13 | 10 |
|
Effect of VEGF-C on the proliferation
of FRH-0201 cells
The cell proliferation activities of the FRH-0201
cells were monitored by MTT assay. The results showed that VEGF-C
exhibited marked growth stimulatory effects, compared with the
control group, at concentrations of 1 ng/ml (P<0.05) and ≥5
ng/ml (P<0.01). However, when the concentrations of VEGF-C
exceeded 10 ng/ml, the rate of increase in cell proliferation
slowed, and this increase was abolished by 100 ng/ml (Fig. 2).
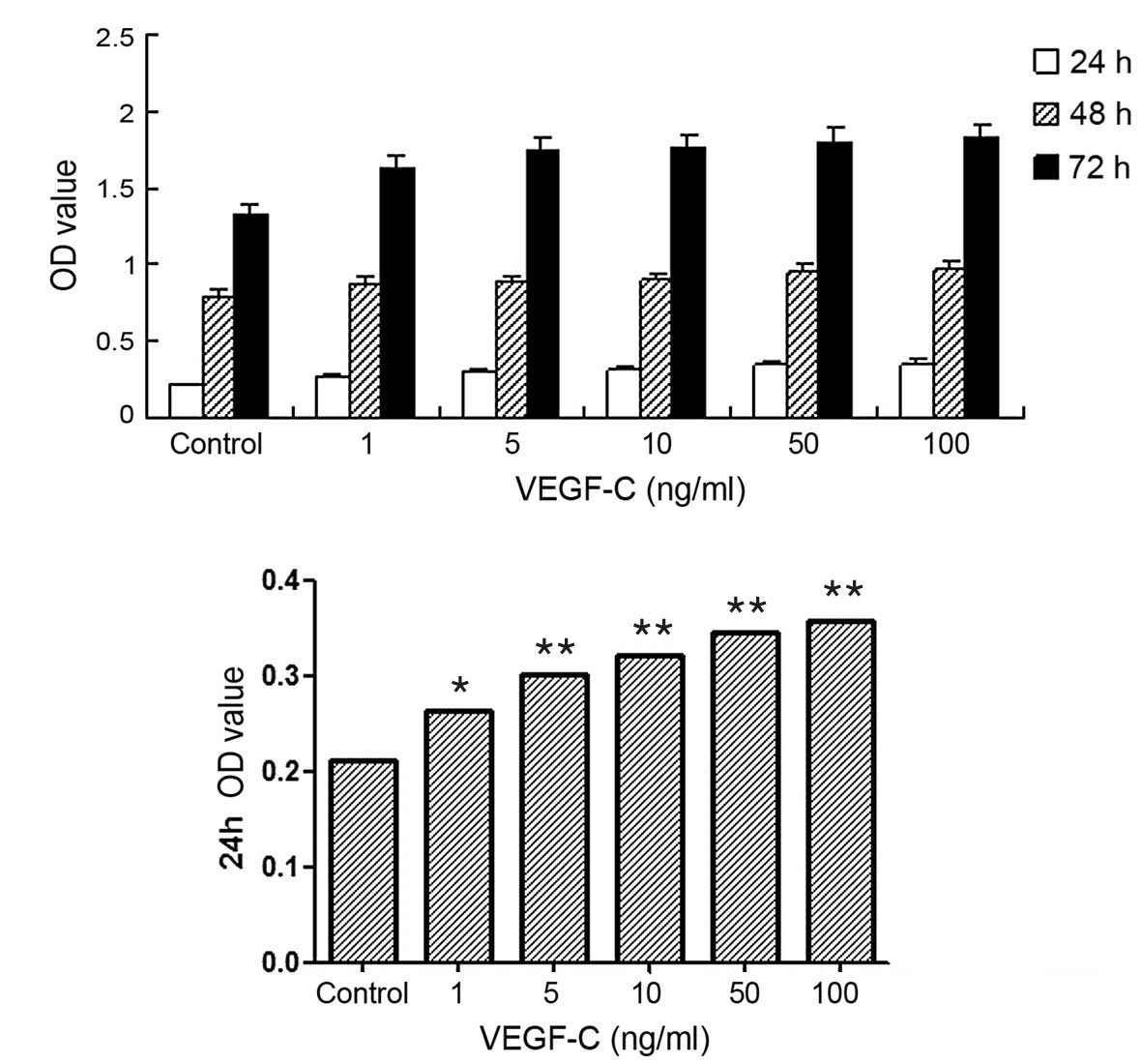 | Figure 2.Effect of vascular endothelial growth
factor C (VEGF-C) on the proliferation of FRH-0201
cholangiocarcinoma cells treated for different times, assessed by
MTT assay. (A) The proliferation of cholangiocarcinoma cells
treated with VEGF-C at concentrations of 1, 5, 10, 50 and 100 ng/ml
for 24, 48 and 72 h, respectively; (B) The proliferation of
cholangiocarcinoma cells treated with VEGF-C at concentrations of
1, 5, 10, 50 and 100 ng/ml for 24 h. Cell proliferation in the
VEGF-C group was significantly different compared with that in the
control group (*P<0.05, **P<0.01 vs. control), but the rate
of increase in cell proliferation slowed when VEGF-C concentrations
exceeded 10 ng/ml. |
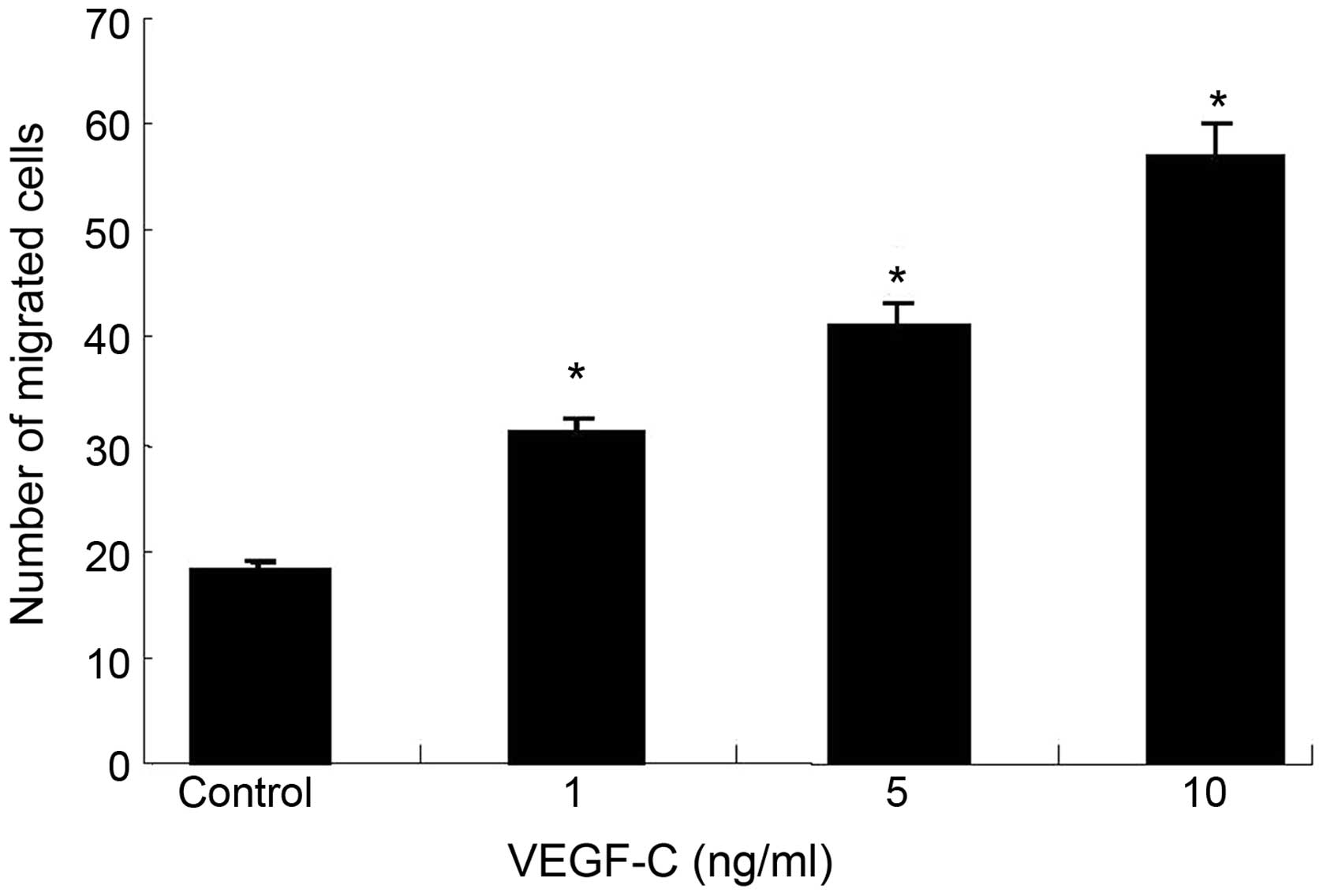
VEGF-C promotes tumor cell
migration
The effect of VEGF-C on FRH-0201 cell migration, a
key determinant of malignant metastasis, was further examined. As
shown in Fig. 3, ectopic high
expression levels of VEGF-C led to significantly increased
abilities of cell migration compared with the control cells
(P<0.01), while more migrated cells were observed in the VEGF-C
group compared with the control group (P<0.05).
Discussion
Tumor metastasis, an important factor in determining
patient survival in cancer, occurs via the blood vascular and
lymphatic systems to transport carcinogenic fluid and cells. A
number of cytokines are now known to be involved in the process of
tumor metastasis. VEGF-C is one of these factors, and is an
essential predictor of tumor growth, lymphangiogenesis and
metastatic behavior, as well as a variety of other significant
pathologies (12,13). However, there have been few studies on
the expression of VEGF-C in cholangiocarcinoma, and the association
between VEGF-C and lymph node metastasis has not been made entirely
clear.
The present study clearly demonstrated the close
association between VEGF-C expression and lymph node metastasis.
Tumors with high expression levels of VEGF-C exhibited more remote
lymph node involvement than those with low VEGF-C expression
levels. The IHC study showed that VEGF-C was overexpressed in the
cholangiocarcinoma tissues (positive ratio, 75.4%), but the normal
bile duct tissues were negative for this cytokine. Moreover, the
concrete expression of VEGF-C was associated with lymphatic
metastasis. In vitro, VEGF-C at 1 to 5 ng/ml exhibited
marked growth stimulation compared with the control group. In
Transwell chamber assays, VEGF-C led to significantly increased
cell migratory ability. This is compatible with previous studies
and suggests that VEGF-C induces tumor cell metastasis in
cholangiocarcinoma (14,15).
In the present study, certain clinically lymph
node-negative cholangiocarcinoma specimens also showed VEGF-C
expression. It is possible that the VEGF-C-positive
cholangiocarcinoma patients without clinical lymph node metastasis
in the present study may have exhibited lymph node metastasis at a
microscopic level. Nevertheless, more importantly, the expression
of VEGF-C was significantly higher in the lymph node-positive
patients than in lymph node-negative patients, suggesting that the
examination of VEGF-C expression in cholangiocarcinoma tissues may
be an indicator of the presence of lymph node metastasis.
Taken together, these findings suggested that VEGF-C
could promote proliferation and migration significantly in
cholangiocarcinoma cells, and that there is a significant
correlation between the expression of VEGF-C and lymphatic
invasion. Hence, the targeting of VEGF-C may be a useful
therapeutic strategy for certain patients with
cholangiocarcinoma.
Acknowledgements
This study was supported by the National Science
Foundation of China (grant no. 81302123) and the Promotive Research
Fund for Excellent Young and Middle-aged Scientists of Shandong
Province (grant no. BS2013YY029).
References
|
1
|
Patel T: Increasing incidence and
mortality of primary intrahepatic cholangiocarcinoma in the United
States. Hepatology. 33:1353–1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gores GJ: Cholangiocarcinoma: Current
concepts and insights. Hepatology. 37:961–969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu L, Li X, Ye L, et al: Vascular
endothelial growth inhibitor is a negative regulator of
aggressiveness and microvascular density in human clear cell renal
cell carcinoma. Anticancer Res. 34:715–722. 2014.PubMed/NCBI
|
|
4
|
Lozano-Santos C, Martinez-Velasquez J,
Fernandez-Cuevas B, et al: Vascular endothelial growth factor A
(VEGFA) gene polymorphisms have an impact on survival in a subgroup
of indolent patients with chronic lymphocytic leukemia. PLoS One.
27:e1010632014. View Article : Google Scholar
|
|
5
|
Zhao R, Liu XQ, Wu XP, et al: Vascular
endothelial growth factor (VEGF) enhances gastric carcinoma
invasiveness via integrin alpha(v)beta6. Cancer Lett. 287:150–156.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skobe M, Hamberg LM, Hawighorst T, et al:
Concurrent induction of lymphangiogenesis, angiogenesis and
macrophage recruitment by vascular endothelial growth factor-C in
melanoma. Am J Pathol. 159:893–903. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Decio A, Taraboletti G, Patton V, et al:
Vascular endothelial growth factor c promotes ovarian carcinoma
progression through paracrine and autocrine mechanisms. Am J
Pathol. 184:1050–1061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Liu C, Qiu L, Li J, Zhang P and Sun
Y: Overexpression of both platelet-derived growth factor-BB and
vascular endothelial growth factor-C and its association with
lymphangiogenesis in primary human non-small cell lung cancer.
Diagn Pathol. 9:1282014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciobanu M, Eremia IA, Crăiţoiu S, et al:
Lymphatic microvessels density, VEGF-C and VEGFR-3 expression in 25
cases of breast invasive lobular carcinoma. Rom J Morphol Embryol.
54:925–934. 2013.PubMed/NCBI
|
|
10
|
Ishikawa M, Kitayama J, Kazama S and
Nagawa H: Expression of vascular endothelial growth factor C and D
(VEGF-C and -D) is an important risk factor for lymphatic
metastasis in undifferentiated early gastric carcinoma. Jpn J Clin
Oncol. 33:21–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yonemura Y, Endo Y, Fujita H, et al: Role
of vascular endothelial growth factor C expression in the
development of lymph node metastasis in gastric cancer. Clin Cancer
Res. 5:1823–1829. 1999.PubMed/NCBI
|
|
12
|
Kono M, Watanabe M, Abukawa H, Hasegawa O,
Satomi T and Chikazu D: Cyclo-oxygenase-2 expression is associated
with vascular endothelial growth factor C expression and lymph node
metastasis in oral squamous cell carcinoma. J Oral Maxillofac Surg.
71:1694–1702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rubbia-Brandt L, Terris B, Giostra E,
Dousset B, Morel P and Pepper MS: Lymphatic vessel density and
vascular endothelial growth factor-C expression correlate with
malignant behavior in human pancreatic endocrine tumors. Clin
Cancer Res. 10:6919–6928. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aishima S, Nishihara Y, Iguchi T, Taguchi
K, Taketomi A, Maehara Y and Tsuneyoshi M: Lymphatic spread is
related to VEGF-C expression and D2-40-positive myofibroblasts in
intrahepatic cholangiocarcinoma. Mod Pathol. 21:256–264. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang WB, Li YH, Liu B, Wang HS, Cui AR and
Zhnag XH: Correlation between PPARgamma and VEGF-C expression in
extrahepatic cholangioadenocarcinoma (EHCAC) and their prognostic
significance. Zhonghua Zhong Liu Za Zhi. 31:773–777. 2009.(In
Chinese). PubMed/NCBI
|