Introduction
Diabetes mellitus (DM) is a group of metabolic
diseases characterized by hyperglycemia resulting from the failure
of the production or action of insulin, which is secreted by the β
cells of the pancreas. Chronic hyperglycemia is associated with
damage, disorder and various organ failures, particularly of the
eyes, kidneys, nerves, heart and blood vessels. Mortality of
diabetic patients is primarily associated with late outcomes: Organ
failure (kidneys, heart and vessels), infections and immune system
disorders, or the result of untreated hyper- or hypoglycemia
(1,2).
The data presented by the International Federation of Diabetes
(3) indicates that, at present, DM is
detected in >250 million people worldwide, with >7 million
new cases diagnosed each year; it is also estimated that, by 2030,
the disease will affect >400 million people, of which 90–95% of
all cases will be DM type 2 (DM2), frequently resulting from
obesity (3). According to data and
predictions at the beginning of the 21st century, the World Health
Organization (WHO) estimated that, in 2015, excess weight would
affect 2.3 billion people and clinical obesity would manifest in
>700 million. The incidence of impaired glucose tolerance (IGT)
is dynamically increasing in developed countries, with an estimated
incidence in the year 2000 of >470 million individuals, which is
~9% of the general population. It is estimated that 50% of patients
exhibiting IGT will develop DM2 within the next 10 years (4).
The initial approach to treating type 2 diabetes
mellitus (DM2) and preDM relies on lifestyle changes, including
increased exercise and diet modification. If lifestyle
interventions are insufficient, the introduction of oral
hypogylcemic agents or insulin therapy may be necessary (1).
According to current DM2 management guidelines
(5), metformin treatment should be
initiated as early as possible in conjunction with lifestyle
modifications. Untreated DM2 typically requires the initiation of
exogenous insulin treatment after ~10 years. It has previously been
demonstrated that long-term treatment with oral hypoglycemic drugs
or exogenous insulin results in specific adverse effects, including
an increased risk of malignancy in the context of a chronic
inflammatory state (2). In the
present review, the oncogenic potential of metformin, insulin
analogues and incretinomimetics in the treatment of DM2 are
discussed (1).
Metformin, oral hypoglycemic agents and
neoplasia
Metformin is currently the first-line treatment for
DM2 in overweight and obese patients. Sulfonylurea derivatives are
an alternative treatment for patients of normal weight and for
cases where there is metformin intolerance. Acarbose, repaglinide,
glitazar, dipeptidyl peptidase-4 inhibitors and glucagon-like
peptide-1 analogues are used less frequently (1,5,6).
Several epidemiological studies have demonstrated
that metformin, as a monotherapy or in combination with other
hypoglycemic agents, reduces the risk of malignancy among patients
with impaired glucose metabolism (7).
A retrospective analysis of almost 63,000 patients with DM2,
diagnosed after the age of 40 in an English primary healthcare
setting, demonstrated an increased incidence of certain types of
cancer (pancreas, colon, breast and prostate) among patients
receiving sulfonylurea derivatives or insulin, when compared with
patients taking metformin. The associated increased cancer risk was
36% for sulfonylurea and 42% for insulin (8). Concurrent metformin treatment reduced
the aforementioned increased risks of sulfonylurea derivative or
insulin regimens. This phenomenon was most significant in the
insulin and metformin co-therapy cohort, with a risk-reduction of
46%. A case-control study evaluating patients with DM and
pancreatic adenocarcinoma, performed at the MD Anderson Cancer
Center (Houston, TX, USA), demonstrated a significant 62%
risk-reduction of adenocarcinoma development among patients treated
with metformin (9). By contrast,
patients treated with sulfonylurea derivatives or an insulin
variant exhibited an risk of developing pancreatic adenocarcinoma
that was increased by 2.5- and 5-fold, respectively. A reduced risk
of neoplasia development was also associated with metformin usage
in another observational cohort study of 4,000 Scottish patients
with a diagnosis of DM2 and 9 years of follow-up data (10). The study group consisted of a group of
diabetic patients who received metformin treatment and a control
group of diabetic patients who did not receive metformin. The
metformin-treated group demonstrated a 37% reduction in the
relative cancer risk and an even greater 54% reduction in the
absolute cancer risk when adjusted for co-variables, including age,
body mass index and history of tobacco use.
A previous study that analyzed the risk of
cancer-associated mortality and DM treatment observed a similar
pattern to the aforementioned result (7). Outcomes of the prospective ZODIAC study,
which included 1,300 patients with DM2 treated in DM clinics in
Holland, demonstrated a 57% reduction in cancer-associated
mortality among patients treated with metformin when compared with
those treated without metformin (11). Additionally, the study demonstrated a
dose-dependent correlation between metformin intake and the risk of
developing malignancy. A retrospective analysis from Canada
compared cancer-associated mortality between patients treated with
metformin and sulfonylurea derivatives, and between those treated
with or without insulin (12,13). The study demonstrated that the
relative risk of cancer-associated mortality was significantly
increased among patients treated with sulfonylurea derivatives when
compared with the metformin group. The study demonstrated that
insulin monotherapy significantly increased the risk of
cancer-associated mortality and that this increase was proportional
to the total daily dose of insulin.
Numerous studies have indicated that there may be a
correlation between metformin use and a reduced incidence of
neoplasia development (14–19). In vitro studies and animal
models support this finding (14).
The clinically observed protective action of metformin likely
results from the suppression of intracellular signaling pathways
that normally transduce activating signals from insulin receptors
(IRs) and insulin-like growth factor receptors (IGF-IRs) (15–18).
Conversely, hypoglycemic agents that act by increasing plasma
concentrations of insulin, endogenously, as in the case of
sulfonylurea derivatives, or exogenously, as in the case of insulin
therapy, may result in increased cancer incidence by stimulating
the aforementioned signal transduction pathways.
Human insulin, insulin analogues and
neoplasia
Insulin deficiency, either with or without insulin
resistance, requires treatment with exogenous insulin (7). Achievements in genetic engineering have
enabled the industrial-scale production of insulin that has a
similar structure to endogenous human insulin and exhibits
analogous properties. Furthermore, genetic engineers have altered
the molecular structure of insulin and its biochemical properties,
subsequently creating insulin analogues. Alterations in the
composition and amino acid sequences of insulin polypeptide chains
result in different pharmacokinetic and pharmacodynamic properties,
and change the binding affinity of the molecule for IRs and IGF-IRs
(7). Increased affinity towards these
receptors may amplify intracellular signaling associated with
insulin and insulin-like growth factors, and concomitantly induce
mechanisms that promote carcinogenesis. In light of these proposed
tumorigenesis pathways, insulin analogues have been increasingly
studied for their oncogenic properties. Kurtzhals et al
(20) compared the in vitro
oncogenic properties of human insulin, commercial insulin analogues
(aspart, lispro, glargine and detemir) and B10Asp; B10Asp is an
insulin analog with known carcinogenic potential, as previously
demonstrated by in vitro studies and animal models (19). A previous study analyzed the
affinities of different molecules to the IRs and IGF-IRs, along
with analyzing the metabolic and mitogenic effects of the molecules
on cell cultures (20). The
properties exhibited by rapid-acting insulin analogues, including
aspart and lispro, were comparable to insulin; however, long-acting
insulin analogues, including detemir and glargine, differed
significantly from insulin in their properties. Detemir
demonstrated a reduced affinity towards the two receptor types and
demonstrated lesser metabolic and mitogenic effects on cell
cultures. Glargine, by contrast, demonstrated a 6-fold stronger
affinity towards IGF-IR and an 8-fold greater mitogenic effect on
cell cultures, characteristics more similar to B10Asp than to human
insulin (20).
Sciacca et al (21) compared the signal transduction
activity of rapid-acting insulin analogues (aspart, lispro, and
glulisine) and long-acting insulin analogues (detemir and glargine)
with human insulin, insulin-like growth factor 1 and the insulin
analog B10Asp on cells in culture. The cells used in the study were
mice fibroblasts that expressed IR types A and B and IGF-IR. As in
the prior study by Kurtzhal et al (20), the characteristics of the rapid-acting
insulin analogues were similar to those of human insulin. The
long-acting insulin analogues, however, significantly increased the
intracellular signaling cascade dependent on type A IR and IGF-IR,
and therefore increased cellular proliferation. However, other than
B10Asp, which acts primarily via the type A IR, none of the
evaluated insulin analogues results in the oncogenic transformation
of the cell lines.
Mayer and Chantelau (22) investigated the effects of human
insulin and insulin analogues on breast cancer cells. Breast cancer
cells were isolated from patients with type 1 DM, and were treated
with human insulin and insulin analogues. The cancer cells were
subsequently incubated with blood serum that lacked peptide C. The
mitogenic potential of the serum was increased by 11% when it
contained glargine compared with when it contained human insulin
(P=0.005). The mitogenic potential of the serum containing detemir
was slightly reduced compared with that containing human insulin
(~1%).
Previous epidemiological analyses studying the
oncogenic potential of insulin analogues have been inconclusive.
However, concerning findings were reported from a German
retrospective analysis of >127,000 patients (23). The study compared aspart, lispro or
glargine as monotherapy versus standard human insulin and analyzed
the subsequent development of malignancy. Multiple insulin
analogues demonstrated a positive correlation between daily doses
of insulin analogues and the risk of developing neoplasia. However,
following correction for dose differences and multiple population
variables, glargine was the only insulin analogue that demonstrated
a statistically significant correlation with an increased risk of
neoplasia development. By contrast, a Swedish study of 114,000
patient medical histories demonstrated no statistically significant
differences in the cancer risk between glargine and other insulin
analogues (24). A retrospective
review of 36,000 Scottish patients also reported no increase in the
cancer risk among glargine users (25). A meta-analysis of 31 randomized
studies, undertaken by Sanofi (Surrey, UK), a company that
manufactures insulin analogues, compared glargine usage with
different DM treatment regimens and demonstrated no increase in the
cancer risk (26,27). Furthermore, treating patients with
glargine resulted in a 10% reduction in the relative-risk of cancer
incidence compared with patients that received different
interventions. Nonetheless, funding sources and institutional
biases must be considered when considering findings from this
meta-analysis.
The risk of carcinogenesis following detemir
treatment has also been evaluated in multiple studies. In
vitro studies demonstrated that there was no additional risk of
an oncogenic transformation associated with detemir treatment
(28). Human studies involving
diabetics treated with detemir demonstrated similar results. A
meta-analysis of 16 studies comparing detemir with neutral
protamine Hagedorn and human insulin, in addition to 5 studies that
compared detemir with glargine, demonstrated a reduced risk of
developing neoplasia in detemir users (28). Compared with detemir, studies continue
to demonstrate the increased oncogenic potential of glargine. A
long-term, randomized, control trial of 1,340 patients with DM2
dependent on insulin therapy monitored patients for a mean time
period of 76 months (29). The study
matched every patient diagnosed with cancer in this study
population (n=112) with a control patient who did not develop
cancer, but had a similar follow-up time period, risk factors and
general characteristics. Patients diagnosed with cancer received an
increased mean daily dose of glargine compared with the control
group: 0.24 IU/kg of body weight vs. 0.16 IU/kg of body weight,
respectively (P=0.036). Cases that were diagnosed with cancer
correlated strongly with glargine daily doses ≥0.3 IU/kg of body
weight. This correlation remained following correction for
concomitant diseases, other insulin doses or concomitant metformin
usage. The absolute risk of increased cancer incidence was
increased 5.5-fold when compared with the control group (P=0.001).
Statistical analysis demonstrated no correlation between the
increased risk of developing cancer and the daily doses of human
insulin and insulin analogues other than glargine. Questions
regarding the safety of glargine have been the driving force behind
subsequent studies comparing different insulin analogues and
studies on novel insulin analogues, such as degludec (30).
It is notable that the United States Food and Drug
Administration (FDA) has warnings regarding the use of inhalational
insulin due to its possible increased cancer risk, specifically for
lung cancer. In a study comparing inhalational insulin with the
typical subcutaneous route of administration, 6 cases of non-small
cell lung cancer were reported in the inhalational insulin group
compared with 1 reported case in the control group (31). Given these findings, different routes
of insulin administration may pose an increased risk of cancer and
warrant further investigation.
The overall scientific literature regarding the
effects of the myriad of diabetic treatment regimens and modalities
remain limited. At present, there is no consensus on the safety and
oncogenic potential of insulin analogues; however, the positive
role of metformin in DM management and its protective effects on
cancer development, as monotherapy and in combination with other
hypoglycemic drugs, can confidently be affirmed (6–11,14–18). By
contrast, drugs that act via increasing endogenous insulin
concentrations, in addition to exogenous insulin itself, may
increase the cancer risk (7–9,12,13,32–44).
Despite in vitro studies indicating that glargine may
stimulate carcinogenesis, the available evidence from
epidemiological analyses indicates it is premature to advocate a
causative association between glargine use and the increased risk
of developing cancer. The possibility that individual differences
in glargine metabolism or the use of atypically high doses of
insulin analogues may increase the cancer risk cannot be excluded
(20–27,29–30).
Expert recommendations from multiple professional committees (FDA,
EASD, EMEA, IDF and ADA) state that there is no reason for limiting
glargine use in the general DM population. However, in cases of
existing proliferative diseases, such as cancer, or in patient
populations with a known higher risk of cancer development, such as
women with BRCA1 or BRCA2 mutations, different insulin analogues
other than glargine should be considered as the first choice
treatment (45–47).
Incretinomimetics and neoplasia
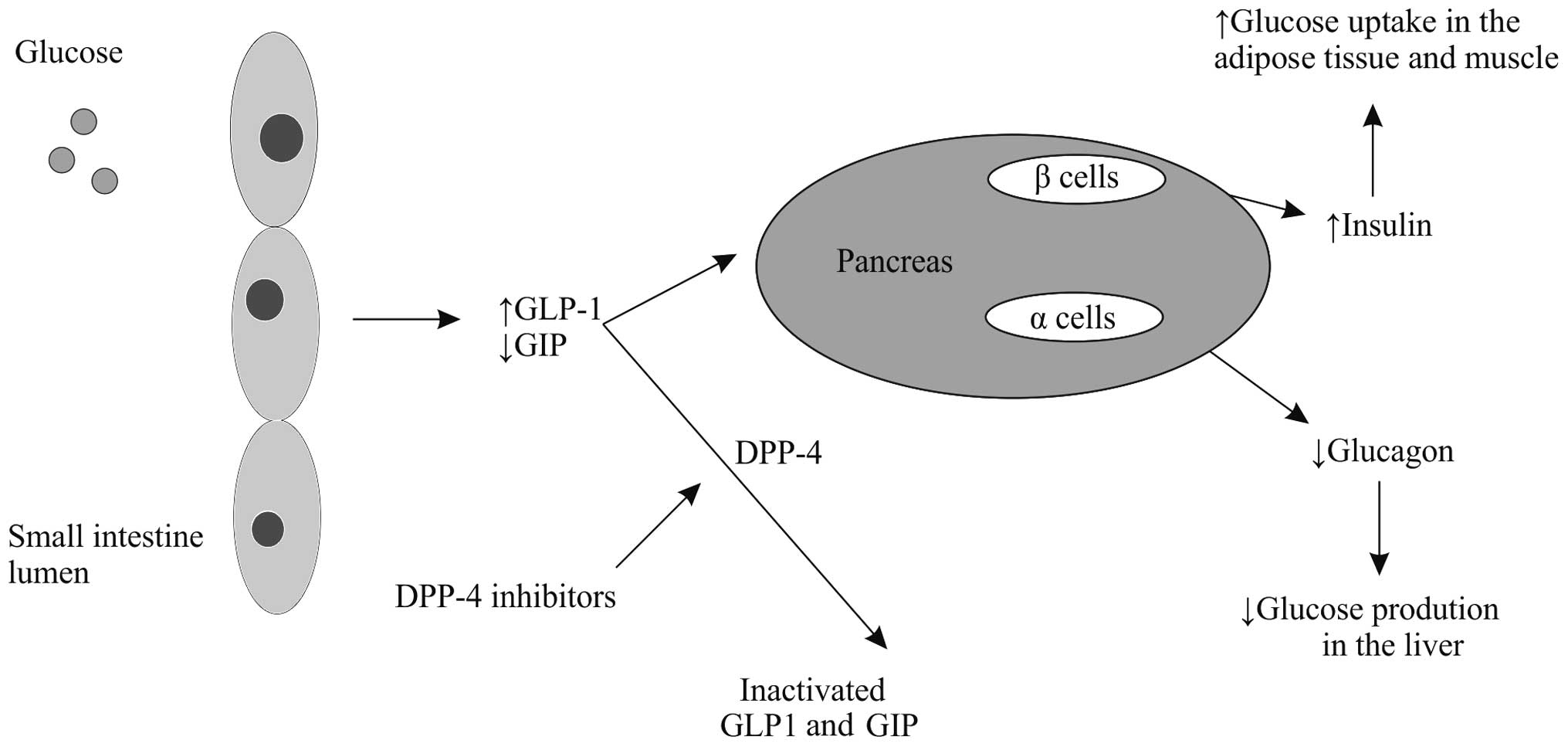
One of the most recent medication classes developed
for DM2 treatment are the incretinomimetics (Fig. 1) (48).
This drug class has been used in the USA since 2006 and in Europe
since 2008. In the pathogenesis of DM2, rising insulin resistance
is accompanied by the concomitant dysfunction of pancreatic β
cells. It is hypothesized that β cell dysfunction may be a result
of the abnormal regulation of a class of gastrointestinal hormones,
termed incretins. Reduction of glucagon-like peptide-1 (GLP-1)
secretion and simultaneous impairment of glucose-dependent
insulinotropic peptide (GIP) function, despite appropriate hormonal
concentrations, results in the loss of normal insulin secretion
patterns observed during the second phase of insulin release. GLP-1
analogues and GLP-1 receptor agonists, in addition to inhibitors of
dipeptidyl peptidase-4 (DPP-4), an enzyme that degrades incretins,
have been intensively studied as potential novel drug therapies for
DM2 (48). When administered to DM2
patients, GLP-1 demonstrates superiority to GIP, as it improves the
early and late phases of insulin release. There are two biochemical
approaches in treating DM2 that utilize the incretin hormonal
pathway. One approach involves the stimulation of GLP-1 receptors
with a receptor agonist, such as exenatide, or a GLP-1 analog, such
as liraglutide. The second approach involves blocking DDP-4 by
specific inhibitors, including sitagliptin, vildagliptin and
saxagliptin. The two approaches reduce the blood glucose
concentration, improve metabolic homeostasis, and improve quality
of life through improved diabetic control. GLP-1 targeted therapy
has also been demonstrated to reduce body weight (49).
Long-term preclinical trials on animal models
demonstrated a lack of carcinogenic potential of DPP-4 inhibitors,
despite such indications from in vitro studies. In theory,
DPP-4 may promote tumor progression due to the fact that the
inhibited enzyme is a suppressor protein (33–35). A
number of previous studies have indicated that there is a
correlation between GLP-1 analogues and DPP-4 inhibitors and an
increased risk of medullary thyroid cancer and a more aggressive
course of multiple endocrine neoplasia type 2. This effect may be
due to increased GLP-1 receptor-dependent intracellular signaling
transduction pathways (35–40,50).
Another possibility is due to a mutation in the proto-oncogene RET,
although this has not been confirmed (41). Changes in DPP-4 expression levels have
been identified in hematological malignancies and various types of
cancer, including cancers of the ovary, uterus and prostate,
non-small cell lung cancer, and neuroendocrine tumors of the gut
and pancreas (42). Loss of DPP-4
activity has been shown to correlate with a more aggressive
malignancy and an earlier metastatic presentation (50). By contrast, expression of GLP-1
receptors in colon cancer presented as a positive prognostic factor
for treatment efficacy (43,44).
Further studies on incretinomimetics and
carcinogenesis are required, since the available data is limited
compared with older hypoglycemic agents. The majority of cited
authors advise moderate optimism and caution when using
incretinomimetics in patients with an increased baseline risk of
cancer and in patients with known malignancy. Future investigations
may aid in the evaluation of the oncogenic safety of GLP-1 agonists
and DPP-4 inhibitors. Until then, clinicians should attentively
consider every case individually, weighing the risks and benefits,
prior to initiating treatment with incretinomimetics.
Summary
Metformin use in patients with preDM and DM2
significantly reduces the risk of developing malignancy and
prolongs survival rates. Sulfonylurea derivatives and certain
insulin analogues, primarily high daily doses of glargine, may
increase the risk of developing malignancy; however, further
studies are warranted. At present, expert recommendations state
that there is no reason to delay initiation of insulin analogues
for DM2 treatment in the typical diabetic population; however,
attention should be given when using glargine or incretinomimetics
in patients with an increased cancer risk and in patients with a
known malignancy. Nonetheless, the appropriate treatment of preDM
and DM2, beginning with lifestyle modifications and possibly
starting metformin treatment, is the best initial strategy to
reduce the cancer risk.
Acknowledgements
This study has been supported by the National
Science Centre (grant no. UMO-2012/05/D/NZ5/01844).
Glossary
Abbreviations
Abbreviations:
|
DM
|
diabetes mellitus
|
|
DM2
|
type 2 DM
|
|
IR
|
insulin receptors
|
|
IGF-IR
|
insulin-like growth factor
receptor
|
|
GLP-1
|
glucagon-like peptide-1
|
|
GIP
|
glucose-dependent insulinotropic
peptide
|
|
DPP-4
|
dipeptidyl peptidase-4
|
References
|
1
|
Sieradzki JC: Diabetes mellitusSzczeklik's
Internal Medicine. Szczeklik A and Gajewski P: Medycyna Praktyczna,
Kraków; pp. 1353–1402. 2014, (In Polish).
|
|
2
|
Matyszewski A, Czarnecka A, Solarek W, et
al: Molecular basis of carcinogenesis in diabetic patients
(Review). Int J Oncol. 46:1435–1443. 2015.PubMed/NCBI
|
|
3
|
World Health Organization, . Obesity and
overweight. http://www.who.int/mediacentre/factsheets/fs311/en/Accessed.
November 30–2010
|
|
4
|
International Diabetes Federation, .
Diabetes Atlas. 6th. http://www.idf.org/diabetesatlas/content/europe%3b%20Accessed.
November 30–2010
|
|
5
|
Polish Diabetes Association, . Clinical
recommendation concerning the management in patients with diabetes
in 2014. Clinical Diabetology. 3 (Suppl A):2014.http://www.cukrzyca.info.pl/zalecenia_kliniczne/zalecenie_kliniczne_dotyczace_postepowania_u_chorych_na_cukrzyce_2014In
Polish; Accessed. September 30–2014
|
|
6
|
Inzucchi SE, Bergenstal RM, Buse JB, et al
American Diabetes Association (ADA); European Association for the
Study of Diabetes (EASD): Management of hyperglycaemia in type 2
diabetes: A patient-centered approach: Position statement of the
American Diabetes Association (ADA) and the European Association
for the Study of Diabetes (EASD). Diabetes Care. 35:1364–1379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piątkiewicz P and Czech A: Glucose
metabolism disorders and the risk of cancer. Arch Immunol Ther Exp
(Warsz). 59:215–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Currie CJ, Poole CD and Gale EA: The
influence of glucose-lowering therapies on cancer risk in type 2
diabetes. Diabetologia. 52:1766–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li D, Yeung SC, Hassan MM, Konopleva M and
Abbruzzese JL: Antidiabetic therapies affect risk of pancreatic
cancer. Gastroenterology. 137:482–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: A cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Landman GW, Kleefstra N, van Hateren KJ,
Groenier KH, Gans RO and Bilo HJ: Metformin associated with lower
cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care.
33:322–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin. Diabetes Care.
29:254–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bowker SL, Yasui Y, Veugelers P and
Johnson JA: Glucose-lowering agents and cancer mortality rates in
type 2 diabetes: Assessing effects of time-varying exposure.
Diabetologia. 53:1631–1637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chong CR and Chabner BA: Mysterious
metformin. Oncologist. 14:1178–1181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buzzai M, Jones RG, Amaravadi RK, et al:
Systemic treatment with the antidiabetic drug metformin selectively
impairs p53-deficient tumor cell growth. Cancer Res. 67:6745–6752.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kisfalvi K, Eibl G, Sinnett-Smith J and
Rozengurt E: Metformin disrupts crosstalk between G protein-coupled
receptor and insulin receptor signaling systems and inhibits
pancreatic cancer growth. Cancer Res. 69:6539–6545. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonzalez-Angulo AM and Meric-Bernstam F:
Metformin: A therapeutic opportunity in breast cancer. Clin Cancer
Res. 16:1695–1700. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rozengurt E, Sinnett-Smith J and Kisfalvi
K: Crosstalk between insulin/insulin-like growth factor-1 receptors
and G protein-coupled receptor signaling systems: a novel target
for the antidiabetic drug metformin in pancreatic cancer. Clin
Cancer Res. 16:2505–2511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jorgensen LN, Dideriksen LH and Drejer K:
Carcinogenic Effect Of The Human Insulin Analog B10Asp In Female
Rats. Diabetologia. 35:A31992.
|
|
20
|
Kurtzhals P, Schäffer L, Sørensen A, et
al: Correlations of receptor binding and metabolic and mitogenic
potencies of insulin analogs designed for clinical use. Diabetes.
49:999–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sciacca L, Cassarino MF, Genua M, et al:
Insulin analogues differently activate insulin receptor isoforms
and post-receptor signalling. Diabetologia. 53:1743–1753. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayer D and Chantelau E: Treatment with
insulin glargine (Lantus) increases the proliferative potency of
the serum of patients with type-1 diabetes: a pilot study on MCF-7
breast cancer cells. Arch Physiol Biochem. 116:73–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hemkens LG, Grouven U, Bender R, et al:
Risk of malignancies in patients with diabetes treated with human
insulin or insulin analogues: A cohort study. Diabetologia.
52:1732–1744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jonasson JM, Ljung R, Talbäck M, Haglund
B, Gudbjörnsdòttir S and Steineck G: Insulin glargine use and
short-term incidence of malignancies - a population-based follow-up
study in Sweden. Diabetologia. 52:1745–1754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colhoun HMSDRN Epidemiology Group: Use of
insulin glargine and cancer incidence in Scotland: A study from the
Scottish Diabetes Research Network Epidemiology Group.
Diabetologia. 52:1755–1765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Home PD and Lagarenne P: Combined
randomised controlled trial experience of malignancies in studies
using insulin glargine. Diabetologia. 52:2499–2506. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosenstock J, Fonseca V, McGill JB, et al:
Similar risk of malignancy with insulin glargine and neutral
protamine Hagedorn (NPH) insulin in patients with type 2 diabetes:
Findings from a 5 year randomised, open-label study. Diabetologia.
52:1971–1973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dejgaard A, Lynggaard H, Råstam J and
Krogsgaard Thomsen M: No evidence of increased risk of malignancies
in patients with diabetes treated with insulin detemir: A
meta-analysis. Diabetologia. 52:2507–2512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mannucci E, Monami M, Balzi D, et al:
Doses of insulin and its analogues and cancer occurrence in
insulin-treated type 2 diabetic patients. Diabetes Care.
33:1997–2003. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heise T, Tack CJ, Cuddihy R, et al: A
new-generation ultra-long-acting basal insulin with a bolus boost
compared with insulin glargine in insulin-naive people with type 2
diabetes: A randomized, controlled trial. Diabetes Care.
34:669–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
U.S. Food and Drug Administration, .
Safety Alerts for Human Medical Products - Exubera (insulin human
rDNA origin) Inhalation Powder. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm085319.htmAccessed.
September 30–2014
|
|
32
|
Pro B and Dang NH: CD26/dipeptidyl
peptidase IV and its role in cancer. Histol Histopathol.
19:1345–1351. 2004.PubMed/NCBI
|
|
33
|
Wesley UV, McGroarty M and Homoyouni A:
Dipeptidyl peptidase inhibits malignant phenotype of prostate
cancer cells by blocking basic fibroblast growth factor signaling
pathway. Cancer Res. 65:1325–1334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masur K, Schwartz F, Entschladen F,
Niggemann B and Zaenker KS: DPPIV inhibitors extend GLP-2 mediated
tumour promoting effects on intestinal cancer cells. Regul Pept.
137:147–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duntas LH: Clinical comments related to
medullary thyroid cancer diagnosis and management. Thyroid Res. 6
(Suppl 1):S62013.PubMed/NCBI
|
|
36
|
Chiu WY, Shih SR and Tseng CH: A review on
the association between glucagon-like peptide-1 receptor agonists
and thyroid cancer. Exp Diabetes Res. 2012:9241682012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vangoitsenhoven R, Mathieu C and Van der
Schueren B: GLP1 and cancer: Friend or foe? Endocr Relat Cancer.
19:F77–F88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arora S, Mehrotra A and Gulati SC:
Incretins and thiazolidinediones in glucose homeostasis and cancer:
Role of common polymorphisms. Cancer Lett. 323:128–134. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gier B, Butler PC, Lai CK, Kirakossian D,
DeNicola MM and Yeh MW: Glucagon like peptide-1 receptor expression
in the human thyroid gland. J Clin Endocrinol Metab. 97:121–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Waser B, Beetschen K, Pellegata NS and
Reubi JC: Incretin receptors in non-neoplastic and neoplastic
thyroid C cells in rodents and humans: relevance for incretin-based
diabetes therapy. Neuroendocrinology. 94:291–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Madsen LW, Knauf JA, Gotfredsen C, et al:
GLP-1 receptor agonists and the thyroid: C-cell effects in mice are
mediated via the GLP-1 receptor and not associated with RET
activation. Endocrinology. 153:1538–1547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Waser B, Rehmann R, Sanchez C, Fourmy D
and Reubi JC: Glucose-dependent insulinotropic polypeptide
receptors in most gastroenteropancreatic and bronchial
neuroendocrine tumors. J Clin Endocrinol Metab. 97:482–488. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kissow H, Hartmann B, Holst JJ, et al:
Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4
inhibition does not accelerate neoplasia in carcinogen treated
mice. Regul Pept. 179:91–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koehler JA, Kain T and Drucker DJ:
Glucagon-like peptide-1 receptor activation inhibits growth and
augments apoptosis in murine CT26 colon cancer cells.
Endocrinology. 152:3362–3372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
European Association for the Study of
Diabetes: Lantus insulin: a possible link with cancer which
requires further investigation. http://webcast.easd.org/press/glargine/glargine.htmAccessed.
September 30–2014
|
|
46
|
International Diabetes Federation, .
Statement from the International Diabetes Federation Related to
Studies Published in Diabetologia Suggesting Possible Link Between
Insulin Glargine and Cancer. http://www.idf.org/statement-international-diabetes-federation-related-studies-published-diabetologia-suggesting-possibAccessed.
September 30–2014
|
|
47
|
American Diabetes Association, . Studies
Find No Increase in Cancer Risk from Insulin Glargine. http://www.diabetes.org/newsroom/press-releases/2012/sci-sessions-insulin-cancer.htmlAccessed.
September 30–2014
|
|
48
|
Matuszek B, Lenart-Lipińska M and
Nowakowski A: Incretin hormones in the treatment of type 2
diabetes. Part II. Incretins - new possibilities for
pharmacotherapy of type 2 diabetes. Endokrynol Pol. 59:322–329.
2008.(In Polish). PubMed/NCBI
|
|
49
|
Znaniecka M, Rutkowska J and
Bandurska-Stankiewicz E: A new direction in the treatment of
diabetes - inncretinomimetics and DPP-4 inhibitors. Przegląd
Kardiodiabetologiczny. 5:171–181. 2010.(In Polish).
|
|
50
|
Labuzek K, Kozłowski M, Szkudłapski D,
Sikorska P, Kozłowska M and Okopień B: Incretin-based therapies in
the treatment of type 2 diabetes - more than meets the eye? Eur J
Intern Med. 24:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|