Introduction
A sinonasal inverted papilloma (IP) is a locally
aggressive, benign neoplasm with a high rate of recurrence.
Sinonasal IPs account for 0.5–4% of all sinonasal tumours (1), and are associated with malignant
transformation or concurrent invasive squamous cell carcinoma (SCC)
in ~9% of cases (2–4). The one, three and five-year survival
rates of sinonasal IP are are 80, 71 and 63%, respectively
(1). The five-year survival rate of
patients with sinonasal SCC is ~53% (5). It is difficult to distinguish a benign
sinonasal IP from an IP with SCC or other malignant transformations
during a routine workup with magnetic resonance imaging (MRI) or
computed tomography (CT), as the malignant transformation is always
local. Even histological examination of biopsied tissue may not
identify SCC residing within an IP (2,3). A
previous study indicated that positron-emission tomography (PET) or
PET/CT with 18F-fluorodeoxyglucose (FDG) may aid in the
detection of sinonasal IP malignancies (6). However, other studies have reported that
FDG-PET/CT is unable to differentiate between a benign sinonasal IP
and a malignancy (6,7).
To the best of our knowledge, there are no reported
cases of sinonasal IPs with SCC presenting as a cancer of unknown
primary (CUP). In the majority of cases of CUP, PET/CT is able to
detect the site of the primary tumour (6,7). However,
the efficacy of PET/CT for the detection of primary tumours in
patients with cervical carcinoma metastases remains to be
determined (6). In a previous study,
PET/CT was not able to identify a primary tumour or the use of
PET/CT did not confer an additional advantage in primary tumour
detection in patients with CUP (7).
In the present study, a case of right sinonasal IP
with poorly-differentiated SCC, which presented as a submandibular
lymph node metastasis, is reported. Notably, a high FDG uptake
level was not present in the right nasal cavity on PET/CT, and avid
local FDG uptake in the right maxillary sinus did not indicate the
presence of a coexistent malignancy. Written informed consent was
obtained from the patient's family.
Case report
A 66-year-old male who presented with a 6-month
history of a painless, progressive mass in the right submandibular
region was admitted to the First Affiliated Hospital, College of
Medicine (Hangzhou, China) in July 2010. The patient exhibited no
dysphagia, dyspnoea, nasal obstruction, nasal bleeding or fever. A
physical examination revealed a 3×4-cm smooth, non-tender mass in
the submandibular gland region. The oral cavity and opening of the
right submandibular duct were normal. The patient's past medical
history included two enucleations of right nasal polyps, 14 and 8
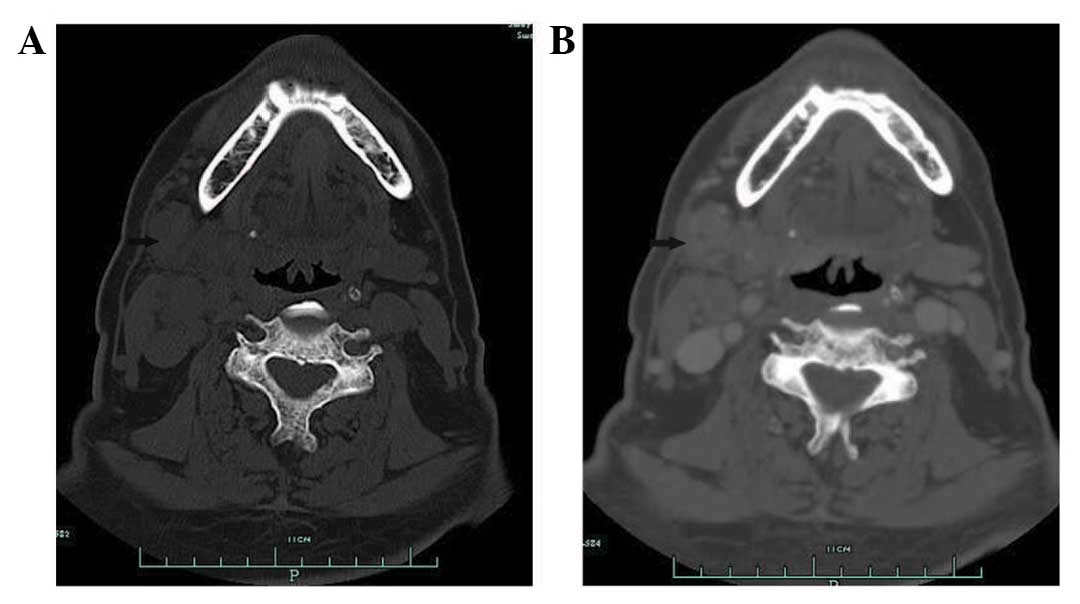
years ago, respectively. A CT scan of the submandibular regions
revealed a heterogeneous mass located outside of the right
submandibular gland. Contrast-enhanced imaging demonstrated
peripheral, but not central enhancement (Fig. 1). The initial diagnosis was of a
pleomorphic adenoma or adenoid cystic carcinoma of the right
submandibular gland. An excision of the right submandibular gland
was subsequently performed. During surgery, the lesion was
identified below the right submandibular gland, tightly adhered to
the gland and the right marginal mandibular branch of the facial
nerve. The lesion was completely excised and identified as a
metastatic carcinoma by frozen section, which revealed large tumour
cells with rich cytoplasm, a nested distribution and invasion of
the lymph node tissue. The post-operative pathological analysis
revealed atypical tumour cells, with a nested arrangement, and
infiltration of the submandibular and cervical lymph nodes,
confirming a diagnosis of poorly-differentiated SCC of the
submandibular lymph node. A mass in the right nasal cavity was
identified during nasal endoscopy, and the nasopharynx was normal.
A biopsy of the right nasal cavity lesion revealed chronic
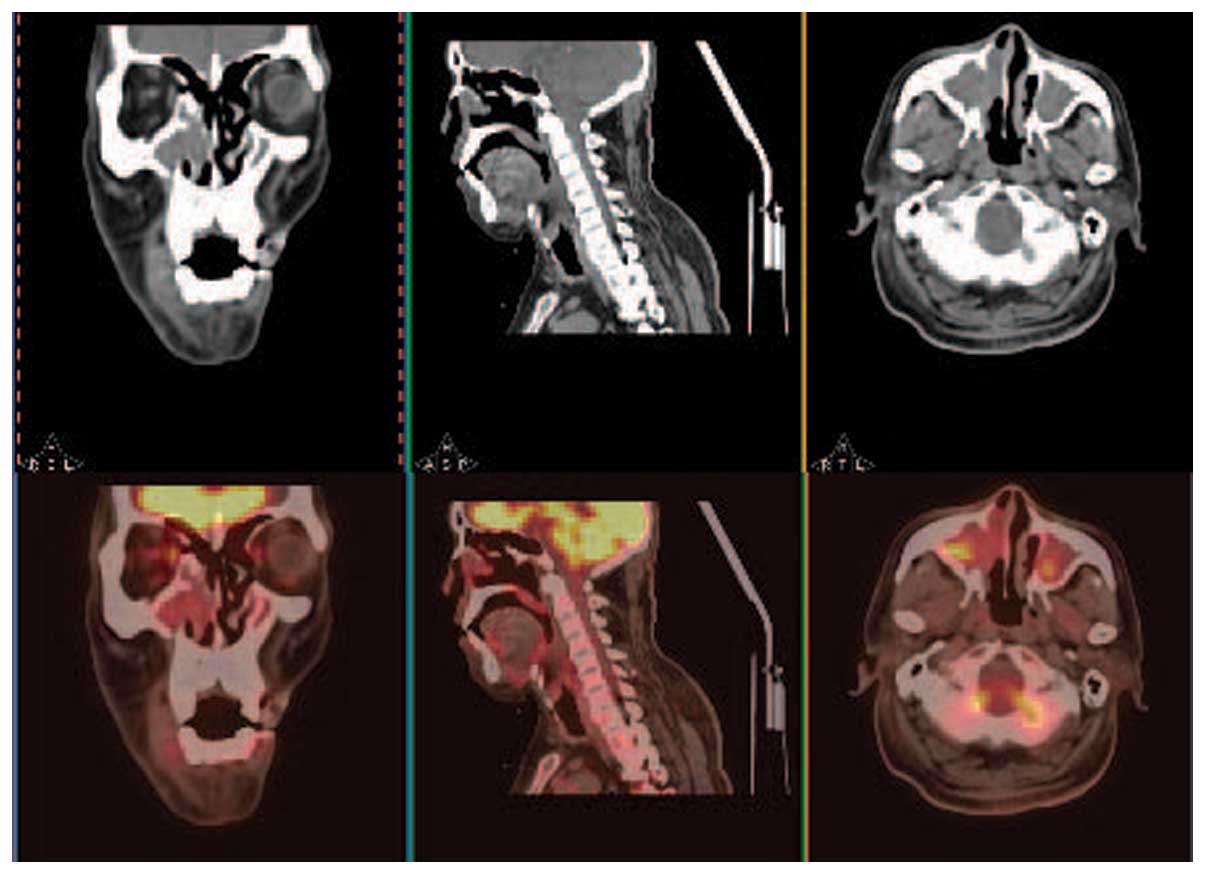
inflammation. During PET/CT, local FDG uptake [maximum standardised
uptake value (SUVmax), 4.02] was observed in the
bilateral maxillary sinuses, but not in other sites in the body,
including the right nasal cavity (Fig.
2). Therefore, the primary site was not identified. The patient
received radiotherapy (60 Gy).
In September 2011, 14 months after the initial
presentation, the patient presented with a 2-month history of a
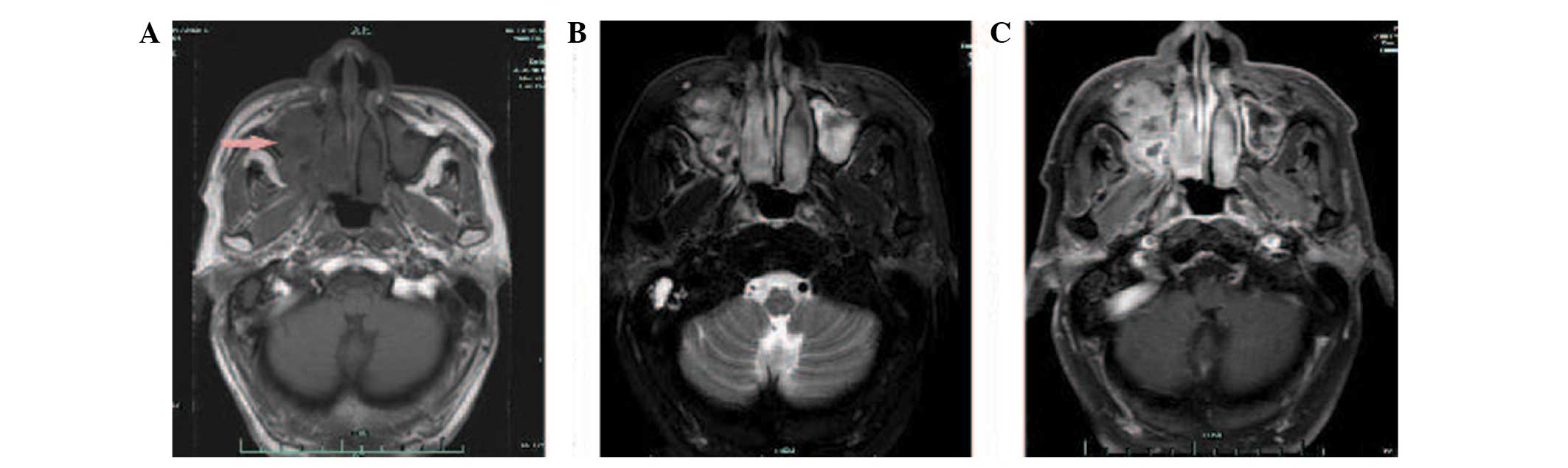
progressive right nasal obstruction. An MRI of the nasal sinuses
revealed a soft-tissue mass in the right nasal cavity and maxillary
sinus. The medial, lateral and anterior walls of the right
maxillary sinus had been destroyed by the tumour. Hyperintensity
was observed on T1- and T2-weighted imaging, and there was marked
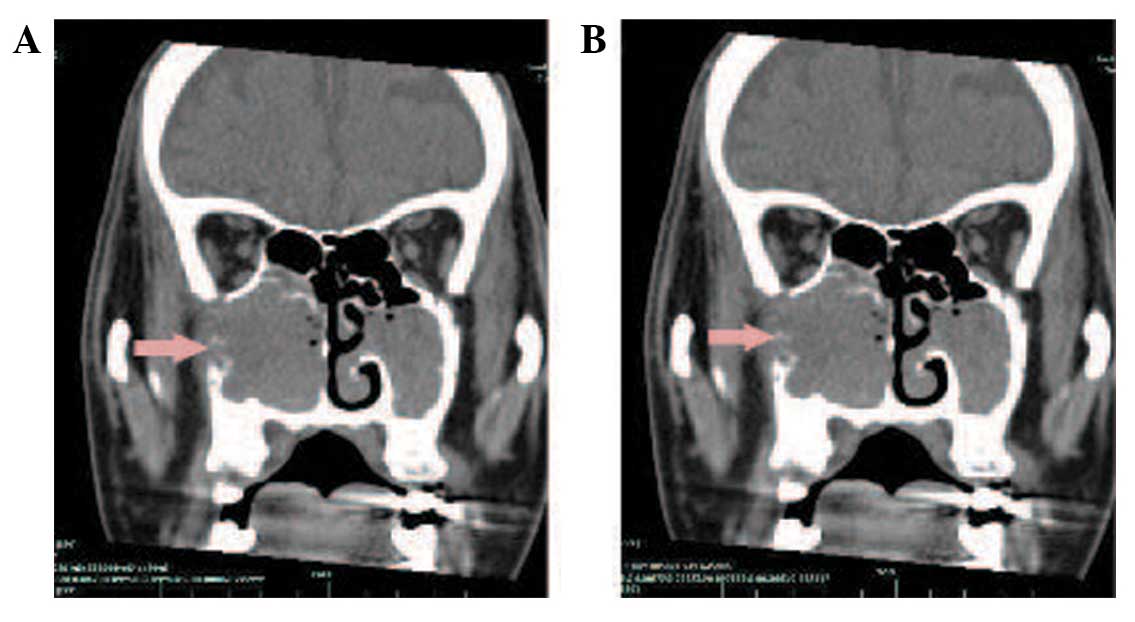
enhancement following gadolinium-DTPA administration (Fig. 3). A CT scan also showed soft-tissue
masses within the right maxillary sinus and nasal cavity. The CT
value was 28 HU. Contrast-enhanced imaging demonstrated markedly
enhanced lesions at 812 HU (Fig. 4).
A nasal endoscopy revealed that the right nasal cavity and right
maxillary sinus were filled with oedematous, polypoid lesions.
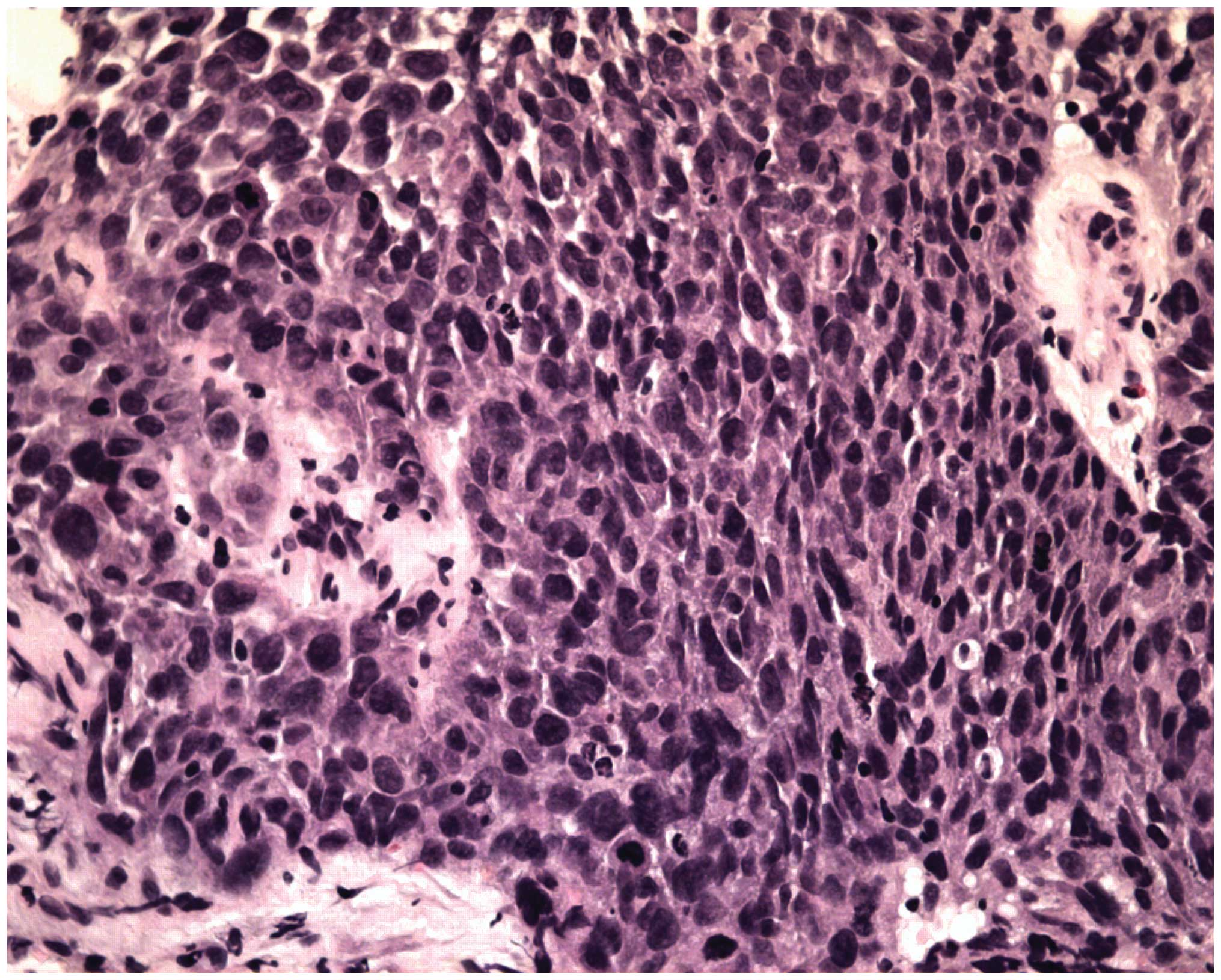
Biopsies of these lesions contained IP with SCC in the right
maxillary sinus and nasal cavity (Fig.
5). The patient received pre-operative half-dose radiotherapy
(30 Gy). The patient underwent a total right maxillectomy 1 month
after the pre-operative radiotherapy, followed by another half-dose
of radiotherapy (30 Gy) post-operatively. However, the patient
succumbed to a brain metastasis in August 2013, 37 months after the
initial presentation.
Discussion
Currently no routine diagnostic methods are capable
of differentiating between benign sinonasal IP and IP coexistent
with carcinoma, since MRI and CT scans cannot differentiate between
these diagnoses (3). Recently, a few
studies found that PET or PET/CT was useful in detecting malignant
transformation in sinonasal IPs. Shojaku et al (2007)
reported that the SUVmax of two patients with sinonasal
IP and coexistent SCC were 8.9 and 20.9, which was higher than
those of patients with IPs without SCC, which ranged from 4.9 to
7.3 (4). The study suggested that IPs
with a high SUVmax may be associated with malignancy
even if the pre-operative biopsy is benign, and that a gross total
excision of the IP and cancer should be performed in these cases,
rather than removal of the IP alone (4). Allegra et al (8) found that FDG-PET/CT may aid in the
detection of IP recurrence. The study showed that 21 patients
exhibited an FDG uptake value that ranged from 4.5 to 8.1, and that
an SUVmax value of 8.1 was detected in a patient with a
histological diagnosis of recurrent IP with SCC (8). However, Lee et al (2007) reported
a case of benign IP in the maxillary sinus that had high
SUVmax values (9.0 at 1 h and 18.1 at 2 h post-FDG
injection), which were indistinguishable from those of malignant
tumours (9). Cohen et al
(2009) suggested that the degree of FDG uptake on PET/CT may be not
a reliable predictor of malignancy in sinonasal IPs, as moderate to
extremely high FDG uptake was also observed in benign sinonasal
papillomas (2). Jeon et al
(2009) reported that the SUVmax of 6 sinonasal IPs with
coexistent SCC ranged from 13.3 to 31.9 (mean ± standard deviation,
20.2±6.6), and that this range was higher than that of benign IPs
(8.2 to 7.8; mean, 8.0) (3). The
study also suggested that PET/CT cannot be used to reliably
differentiation between benign IP and malignancy (3). The inability of PET/CT to differentiate
between benign and malignant processes may be as FDG uptake is not
specific to malignant processes, and other conditions, such as
benign tumours or infectious processes, can also increase the rate
of glycolysis (2,3). Therefore, the role of FDG-PET/CT in the
diagnosis of sinonasal IP and the differentiation of benign IPs
from those with coexistent malignancies requires further
investigation.
Cases of IP malignant transformation with cervical
metastasis are rare (10). Only two
cases of sinonasal IPs with SCC and cervical metastasis have
previously been reported in the English-language literature
(10,11). Mathew et al reported a case of
sinonasal IP with coexistent malignancy that progressed to the
cervical lymph nodes in regions IIB and III (10). Mazlina et al reported a case of
multicentric IP in the sinonasal region and middle ear in which the
patient developed a cervical metastasis secondary to malignant
transformation of the IP in the middle ear (11). Recently, Karam et al reported a case
of sinonasal IP coexistent with high-grade esthesioneuroblastoma in
which the patient developed metastases bilaterally in the cervical
lymph nodes of regions I and II (12). To the best of our knowledge, sinonasal
IP with coexistent SCC presenting as a CUP has not previously been
reported. In the present case, the patient initially presented with
enlarged cervical lymph nodes in region I. FDG-PET/CT revealed an
SUVmax of 4.02 in the bilateral maxillary sinuses, but
increased FDG uptake was not observed in the right nasal cavity.
Based on previous studies and our experience, the FDG-PET/CT
findings in the present case did not suggest a coexistent
malignancy in the nasal cavities or sinuses. Additionally, a biopsy
of the right nasal cavity found inflammation. These findings
indicate that biopsied tissue may not always identify SCC within a
sinonasal IP, particularly if the SCC only resides in a small
portion of the sinonasal IP (3).
Thus, multiple biopsies should be conducted to improve diagnostic
accuracy.
It has been reported that PET/CT aids in the
detection of primary tumours in CUP cases (6,7). In our
previous study, the sensitivity of FDG PET/CT in detecting primary
tumours was 73.3% and the positive predictive value was 52.4%
(6). These findings indicated that
PET/CT has certain limitations in detecting the primary site of the
CUP. Although several studies have demonstrated that PET/CT imaging
may be useful in the evaluation of IPs with SCC, particularly group
2 and 3 lesions (13), the findings
in the present case, as well as in other studies (2,3,13), demonstrated that PET/CT does not
reliably identify sinonasal IP with SCC. Therefore, we suggest that
low FDG uptake by a sinonasal IP does not exclude the possibility
of malignant transformation or coexistent malignancy, even in IPs
with benign pre-operative biopsies.
In conclusion, sinonasal IPs with coexistent
malignancy and cervical metastasis are rare. The present study
describes the first reported case of a sinonasal IP with coexistent
SCC that presented as a CUP. FDG uptake on PET/CT may be not a
reliable predictor of malignancy in sinonasal IPs, and we suggest
the use of multiple biopsies of suspected IPs in order to improve
diagnostic accuracy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172562).
References
|
1
|
Tanvetyanon T, Qin D, Padhya T, Kapoor R,
McCaffrey J and Trotti A: Survival outcomes of squamous cell
carcinoma arising from sinonasal inverted papilloma: Report of 6
cases with systematic review and pooled analysis. Am J Otolaryngol.
30:38–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen EG, Baredes S, Zuckier LS, Mirani
NM, Liu Y and Ghesani NV: 18F-FDG PET evaluation of sinonasal
papilloma. AJR Am J Roentgenol. 193:214–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeon TY, Kim HJ, Choi JY, et al: 18F-FDG
PET/CT findings of sinonasal inverted papilloma with or without
coexistent malignancy: Comparison with MR imaging findings in eight
patients. Neuroradiology. 51:265–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shojaku H, Fujisaka M, Yasumura S, et al:
Positron emission tomography for predicting malignancy of sinonasal
inverted papilloma. Clin Nucl Med. 32:275–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turner JH and Reh DD: Incidence and
survival in patients with sinonasal cancer: A historical analysis
of population-based data. Head Neck. 34:877–885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao K, Luo XM, Zhou SH, et al:
18F-fluorodeoxyglucose positron emission
tomography/computed tomography as an effective diagnostic workup in
cervical metastasis of carcinoma from an unknown primary tumor.
Cancer Biother Radiopharm. 27:685–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JS, Yim JJ, Kang WJ, et al: Detection
of primary sites in unknown primary tumors using FDG-PET or
FDG-PET/CT. BMC Res Notes. 4:562011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allegra E, Lombardo N, Cascini G, La Boria
A, Garozzo A and Tamburrini O: Possible role of 18FDG-PET/CT for
the surveillance of sinonasal inverted papilloma. Clin Otolaryngol.
35:249–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee KW, Kuo WR, Tsai CC, Chen YW, Chai CY,
Su YC and Lin CS: Positive positron emission tomography/computed
tomography in early inverted papilloma of the maxillary sinus. J
Clin Oncol. 25:4848–4850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathew P and Idiculla JJ: Malignant
sinonasal papilloma with neck metastasis: a rare report and
literature review. Int J Oral Maxillofac Surg. 41:368–370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazlina S, Shiraz MA, Hazim MY, Amran AR,
Zulkarnaen AN and Wan Muhaizan WM: Sinonasal inverted papilloma
with malignant transformation in the middle ear: a multicentric
origin? J Laryngol Otol. 120:597–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karam SD, Jay AK, Anyanwu C, Steehler MK,
Davidson B, Debrito P and Harter KW: Pathologic collision of
inverted papilloma with esthesioneuroblastoma. Front Oncol.
4:442014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barnes L, Vervin RS and Gnepp DR: Diseases
of the nose, paranasal sinuses, and nasopharynxSurgical Pathology
of the Head and Neck. Barnes L: 1. Marcel Dekker; New York, NY: pp.
403–451. 1985
|