Introduction
The incidence and mortality rates of lung cancer
have increased globally during the last few decades (1,2). The
majority of cases of lung cancer diagnosed were non-small cell lung
cancer (NSCLC), and ~40% of patients with NSCLC are affected by
advanced diseases (3). For patients
with advanced NSCLC with a good performance status, systemic
chemotherapy is the standard therapy at present. The first-line
treatment for such patients is platinum-based chemotherapy, which
improves symptom control, quality of life and survival as compared
with best supportive care (4). A good
response [complete response or partial response (CR/PR)] to
chemotherapy has typically been equated with the clinical benefit
of increased survival (5), but only
20–30% of patients achieve a good response in previous clinical
trials, while 40–50% maintain a stable disease (SD) status
(6–8).
Certain previous studies reported that an initial good response and
stable disease indicate similar survival benefits for
chemotherapeutical patients with advanced NSCLC (9,10). The
present retrospective study was undertaken to evaluate the similar
survival benefits of a good response and stable disease to
platinum-based chemotherapy in previously untreated NSCLC
patients.
Patients and methods
Patients
The patients enrolled in the present study were
those consecutively diagnosed with NSCLC and treated with
platinum-based chemotherapy as first-line treatment at the
University of Tsukuba Hospital and Tsukuba Medical Center Hospital
(both Tsukuba, Ibaraki, Japan) between January 1999 and December
2012. All the patients were histologically/cytologically confirmed
as presenting with NSCLC and unresectable advanced disease. The
histopathological diagnosis was defined by the World Health
Organization classification (11),
and patients were staged according to the Union for International
Cancer Control tumor-node-metastasis system (12).
Treatment and response
Enrolled patients received at least one cycle of
cisplatin- or carboplatin-based chemotherapy. The clinical,
pathological and radiological data, and the follow-up information
obtained until May 2013 were retrospectively reviewed. The patient
characteristics and efficacy were evaluated using patient data
extracted from the database. Tumor responses were classified as a
CR, PR, SD, progressive disease (PD) or not evaluable (NE),
according to the response evaluation criteria for solid tumors
(RECIST) version 1.1 (13). This
observational study conformed to the Ethical Guidelines for
Clinical Studies issued by the Ministry of Health, Labor and
Welfare of Japan.
The progression-free survival (PFS) time of each
patient was calculated from the day that chemotherapy was commenced
until disease progression. Overall survival (OS) time was
calculated from the day that chemotherapy was commenced until
mortality or the latest follow-up of the patient.
Statistical analysis
The survival rate was analyzed by the Kaplan-Meier
method, and comparisons were performed using the log-rank test in
univariate analysis. Significant variables identified in the
univariate analysis were included in the multivariate survival
analysis using Cox proportional hazards model to study the effects
of clinicopathological factors on survival. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using StatView software for
Windows, version 5.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Between January 1999 and December 2012, 322 patients
were diagnosed with advanced NSCLC and received platinum-based
chemotherapy at two hospitals. The median follow-up period was 10.2
months (range, 0.7–134.0 months). Table
I shows the patient characteristics. Of the 322 patients, 234
were male, and the median age was 64 years (range, 21–88 years). In
total, 85 patients were never-smokers. With regard to performance
status (PS), 258 patients exhibited a PS of 0–1 and 64 exhibited a
PS of 2–4. Overall, 33 patients presented with stage IIIA–B disease
and 289 patients with stage IV. Of the 322 NSCLC cases, 246 (76.4%)
were adenocarcinoma, 62 (19.3%) were squamous cell carcinoma, 11
(3.4%) were large cell carcinoma, and 3 (0.9%) were other types.
Epidermal growth factor receptor (EGFR) mutation positivity was
found in 19 patients, while 69 patients were negative for the
mutation and 234 patients were not evaluated for EGFR. The
chemotherapy regimens used are presented in Table II; 105 (32.6%) patients were
administered cisplatin-based chemotherapy, and 217 (67.4%) patients
were administered carboplatin-based chemotherapy as first-line
treatment. In the platinum-based chemotherapy regimens [carboplatin
(area under the serum concentration-time curve, 4–5 mg/ml/min; day
1; 3–4-week cycle) and cisplatin (60–80 mg/m2; day 1,
3–4-week cycle)], the cytotoxic drugs such as paclitaxel (180–200
mg/m2; day 1; 3–4-week cycle), doctaxel (60
mg/m2; day 1; 3–4-week cycle), gemcitabine (1000
mg/m2; days 1 and 8; 3–4-week cycle) and pemetrexed (500
mg/m2; day 1; 3–4-week cycle) were administered in 95,
62, 47 and 43 patients, respectively. The median number of cycles
of platinum-based chemotherapy was 2 (range, 1–7 cycles), and the
median number of cycles of maintenance therapy was 3 (range, 1–12
cycles).
 | Table I.Characteristics of 322 patients with
non-small cell lung cancer who received platinum-based
chemotherapy. |
Table I.
Characteristics of 322 patients with
non-small cell lung cancer who received platinum-based
chemotherapy.
| Characteristic | Value |
|---|
| Median age (range),
years | 64 (21–88) |
| Gender, n (%) |
|
| Male | 234
(72.7) |
|
Female | 88
(27.3) |
| Smoking status, n
(%) |
|
|
Smoker | 237
(73.6) |
|
Never-smoker | 85
(26.4) |
| Performance status, n
(%) |
|
| 0–1 | 258 (80.1) |
| 2–4 | 64
(19.9) |
| Clinical stage, n
(%) |
|
|
IIIA–B | 33
(10.2) |
| IV | 289 (89.8) |
| Pathology, n (%) |
|
|
Adenocarcinoma | 246 (76.4) |
| Squamous
cell carcinoma | 62
(19.3) |
| Large
cell carcinoma | 11 (3.4) |
|
Other | 3
(0.9) |
| EGFR mutation, n
(%) |
|
|
Positive | 19 (5.9) |
|
Negative | 69
(21.4) |
| Not
evaluated | 234 (72.7) |
 | Table II.Regimens of chemotherapy. |
Table II.
Regimens of chemotherapy.
| Regimen | Value |
|---|
| Platinum, n (%) |
|
|
Cisplatin | 105 (32.6) |
|
Carboplatin | 217 (67.4) |
| Combined drugs, n
(%) |
|
|
Paclitaxel | 95
(29.5) |
|
Docetaxel | 62
(19.3) |
|
Gemcitabine | 47
(14.6) |
|
Pemetrexed | 43
(13.4) |
|
Vinorelbine | 38
(11.8) |
| S-1 | 13 (4.0) |
|
Etoposide | 12 (3.7) |
|
Vindesine | 11 (3.4) |
|
Bevacizumab | 11 (3.4) |
|
Irinotecan | 3
(0.9) |
| Median number of
platinum-based chemotherapy cycles (range) | 2
(1–7) |
| Median number of
maintenance therapy cycles (range) |
3 (1–12) |
Response/disease control and
survival
The median OS time for the 322 patients was 11.7
months. A total of 67 (20.8%) patients were responders (no CR and
67 PR) and 165 (51.2%) patients achieved SD, which amounted to a
disease control rate (DCR) of 72.0%. In the 260 patients with
non-squamous cell carcinoma, 50 (19.2%) were responders (no CR and
50 PR) and 135 (51.9%) achieved SD, which amounted to a DCR of
71.1%. In the 62 patients with squamous cell carcinoma, 17 (27.4%)
were responders (no CR and 17 PR) and 30 (48.4%) patients achieved
SD, which amounted to a DCR of 75.8%.
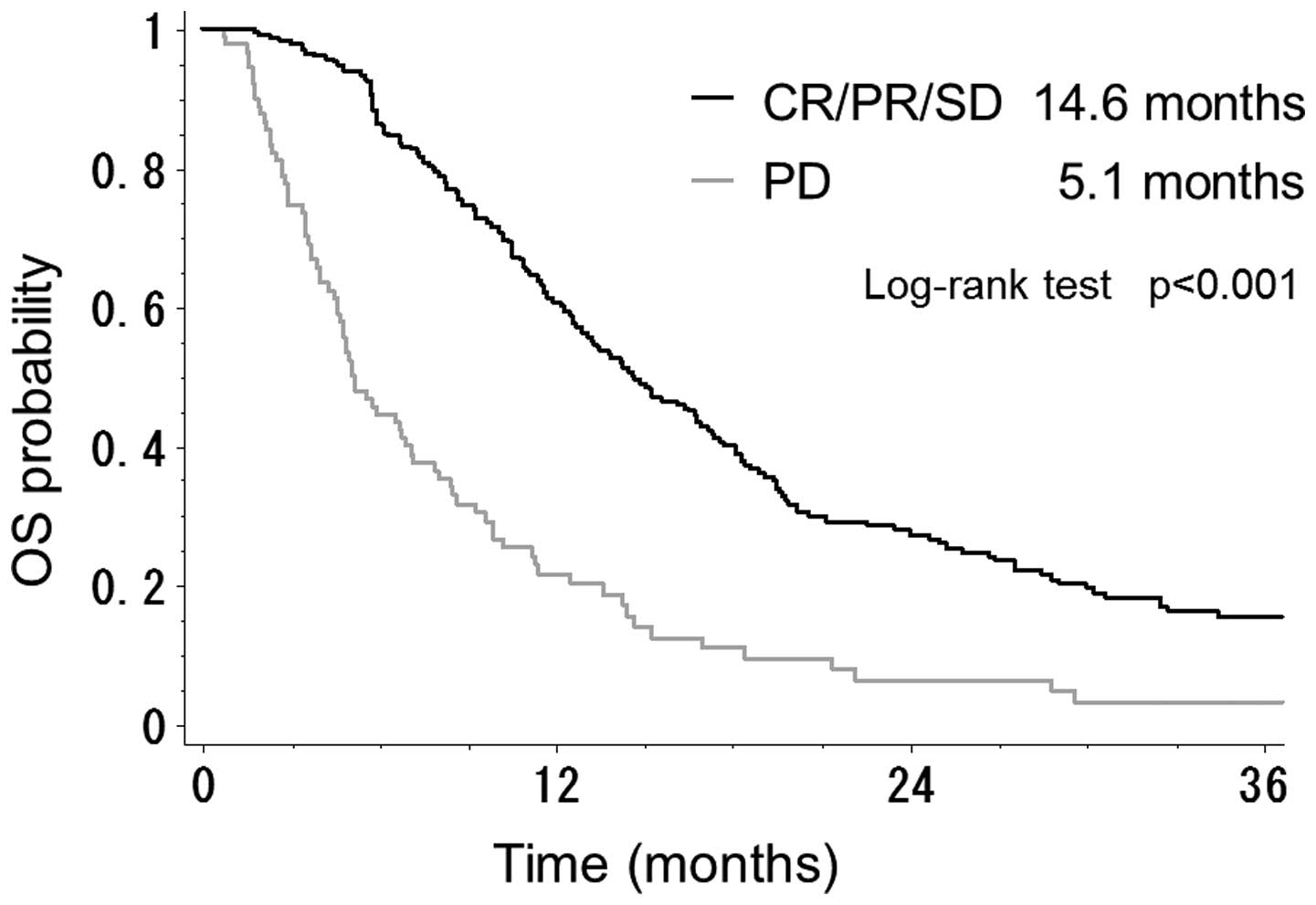
As shown in Fig. 1,
the median OS time in the 232 patients with CR/PR or SD was better
than that of the 90 patients with PD (14.6 vs. 5.1 months;
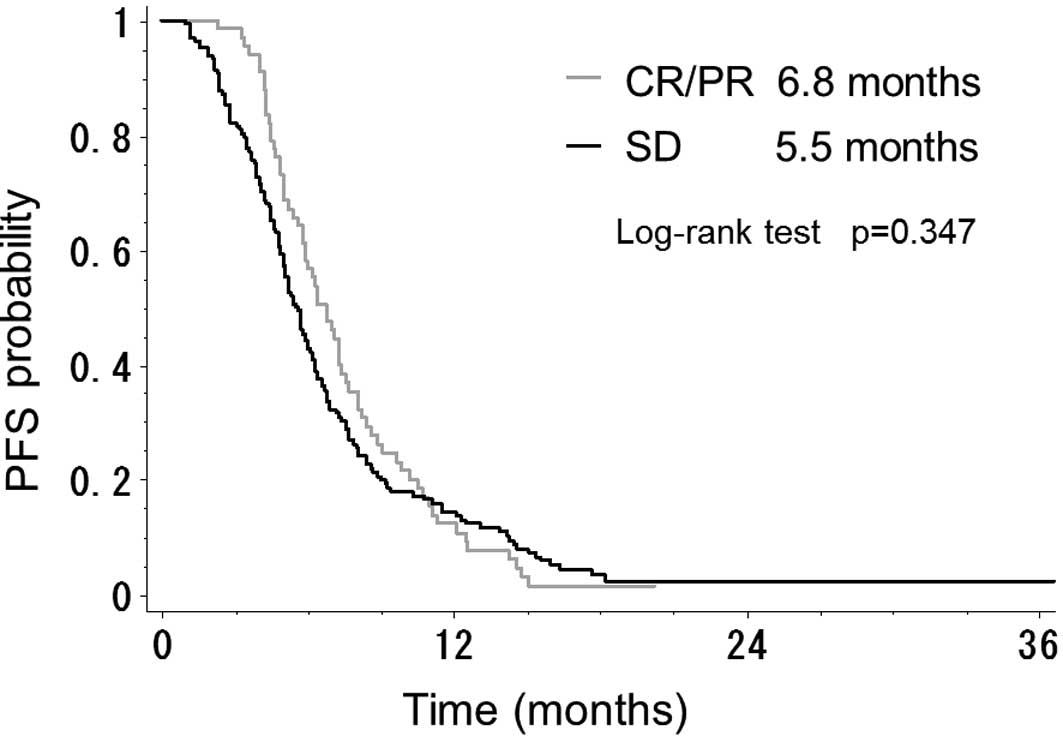
P<0.001). By contrast, there was no difference in PFS time
between the 67 patients with CR/PR and the 165 patients with SD
(6.8 vs. 5.5 months; P=0.347) (Fig.
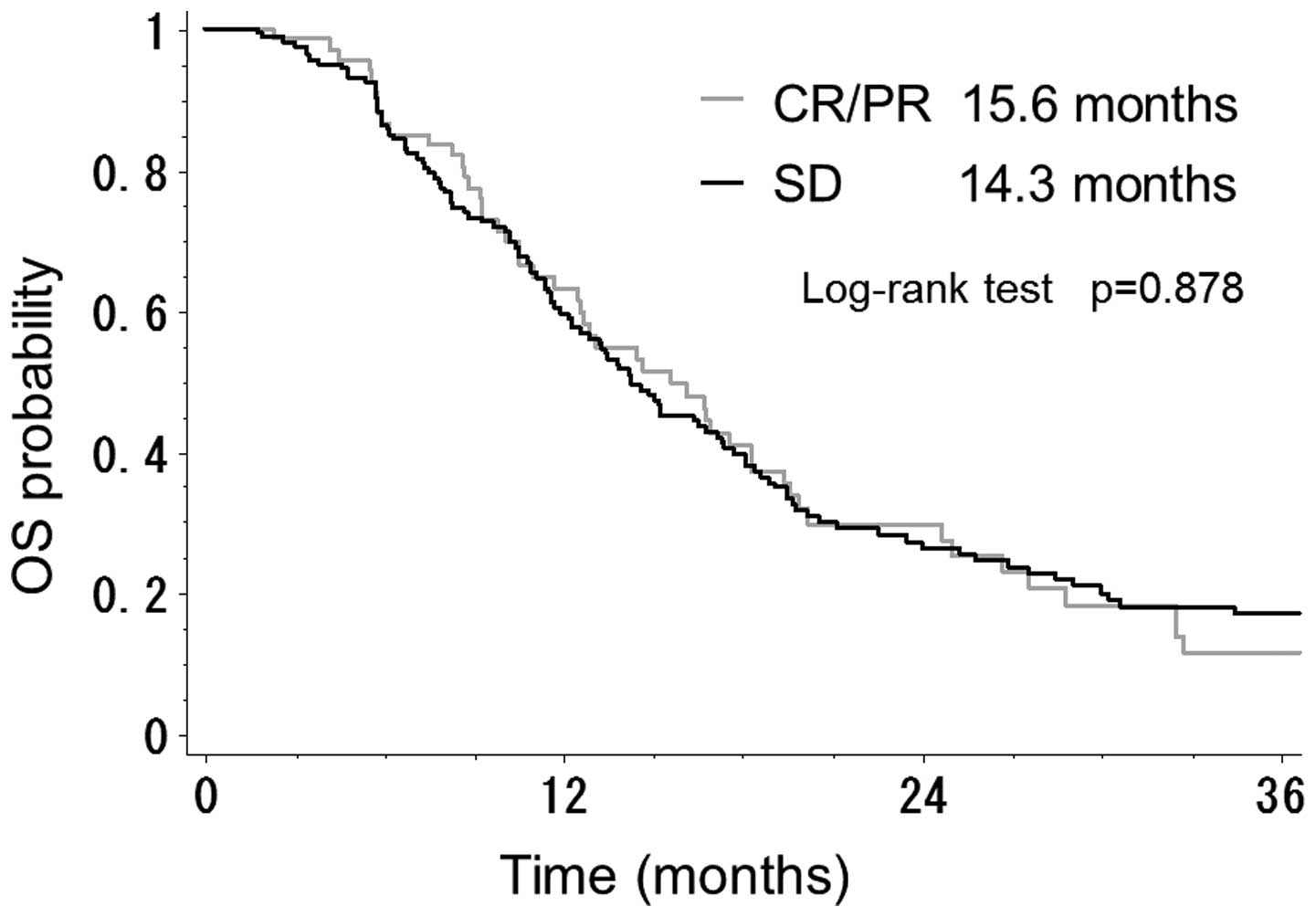
2). There was also no significant difference between patients
with CR/PR and those with SD with regard to OS time (15.6 vs. 14.3
months; P=0.878) (Fig. 3).
In the patients with non-squamous cell carcinoma,
the median OS time in the patients with CR/PR and SD was better
than that in the patients with PD (16.1 vs. 5.2 months;
P<0.001). There was no difference in PFS time between the
patients with CR/PR and the patients with SD (7.3 vs. 5.7 months;
P=0.253). Additionally, there was no significant difference between
the patients with CR/PR and those with SD with regard to OS (16.9
vs. 15.1 months; P=0.938).
In the patients with squamous cell carcinoma, the OS
time in the patients with CR/PR and SD was better than that in the
patients with PD (13.1 vs. 4.8 months; P<0.001). There was no
difference in PFS time between the patients with CR/PR and the
patients with SD (5.0 vs. 5.1 months; P=0.978). Also, there was no
significant difference between the patients with CR/PR and those
with SD with regard to OS time (13.1 vs. 13.3 months; P=0.732).
Prognostic factors
Next, the prognostic factors of the 322 NSCLC
patients were evaluated. Table III
presents the results of the univariate and multivariate analyses.
In the univariate analysis, the female gender, a good PS (PS of
0–1), never-smoker status, non-squamous cell carcinoma,
cisplatin-based chemotherapy, pemetrexed-containing chemotherapy,
bevacizumab-containing chemotherapy and disease-controlled patients
(CR/PR and SD) were associated with a longer OS time. According to
the multivariate Cox proportional hazards model, a good PS (PS of
0–1), never-smoker status, pemetrexed-containing chemotherapy and
disease-controlled patients (CR/PR and SD) were favorable
prognostic factors.
 | Table III.Prognostic factors of the 322
non-small cell lung cancer patients. |
Table III.
Prognostic factors of the 322
non-small cell lung cancer patients.
| A, Univariate
survival analysis (log-rank test) |
|
|
|
|
|
|---|
|
|
|---|
| Prognostic
factors |
| Median OS time,
months |
|
| P-value |
|---|
| Gender
(female/male) |
|
16.7/10.9 |
|
| <0.001 |
| Age, years
(<70/≥70) |
|
11.2/13.3 |
|
|
0.528 |
| PS (0–1/2–4) |
| 13.6/5.8 |
|
| <0.001 |
| Smoking status
(never-smoker/smoker) |
|
18.1/11.0 |
|
| <0.001 |
|
Non-squamous/squamous cell carcinoma |
|
12.3/10.5 |
|
|
0.046 |
| CDDP/CBDCA |
|
13.5/11.4 |
|
|
0.041 |
| PEM (+/-) |
|
21.4/10.2 |
|
| <0.001 |
| Bevacizumab
(+/-) |
|
21.4/11.3 |
|
|
0.004 |
| Maintenance
(+/-) |
|
18.1/11.6 |
|
|
0.191 |
| CR+PR/SD+PD |
|
15.6/11.2 |
|
|
0.064 |
| CR+PR+SD/PD |
|
14.6/5.1 |
|
| <0.001 |
|
| B, Multivariate
analysis (Cox's proportional hazards model) |
|
|
|
|
|
|
| Prognostic
factors | Hazard ratio |
| 95% CI |
| P-value |
|
| Female | 0.75 |
| 0.52–1.07 |
| 0.106 |
| PS 0–1 | 0.48 |
| 0.35–0.66 |
| <0.001 |
| Never-smoker | 0.64 |
| 0.44–0.93 |
| 0.020 |
| Non-squamous cell
carcinoma | 0.98 |
| 0.71–1.36 |
| 0.896 |
| CDDP | 0.95 |
| 0.71–1.27 |
| 0.722 |
| PEM | 0.47 |
| 0.32–0.68 |
| <0.001 |
| Bevacizumab | 0.60 |
| 0.32–1.14 |
| 0.117 |
| CR+PR+SD | 0.37 |
| 0.28–0.48 |
| <0.001 |
Discussion
In the present study evaluating daily practice in
NSCLC patients, four main results were found. First, in the overall
group of NSCLC patients, the DCR (CR/PR and SD rates) was 72.0%,
although the response rate that was composed of CR/PR alone was
only 20.8%. Second, the OS time was 11.7 months in the overall
group of NSCLC patients, and 14.6 months in the disease-controlled
patients (patients with CR, PR and SD). Third, the OS time in the
disease-controlled patients was longer than that in the patients
with PD, but there was no statistical significant difference in the
OS time between the patients with CR/PR and those with SD. The same
results were observed in the patients with squamous cell lung
carcinoma and those with non-squamous cell carcinoma. Fourth, the
OS time in the disease-controlled patients treated with platinum
and pemetrexed was 21.4 months.
Thus, these results illustrated that prolongation of
survival time was associated not with the response rate, but with
the disease control rate, and a high response rate may have scarce
clinical meaning in daily practice. If a cure would not be
achieved, these results implied that it would be important not to
merely achieve shrinkage of the tumor, but to maintain the patent's
condition for a long time without any tumor progression.
In previous studies, there have been various
opinions with regard to the survival of patients with SD; certain
studies have insisted that patients with SD were associated with a
favorable, long OS time compared with those with CR/PR (9,10), and
another study described a longer OS time obtained in patients with
CR/PR compared with those with SD (14). Lara et al (9) suggested that patients who achieved SD at
8 weeks experience a survival time equal to that of PR/CR patients.
The study claimed that DCR (CR, PR and SD) is stronger than
response (CR/PR alone) in the prediction of the OS time of patients
with advanced NSCLC. He et al (10) reported that initial CR/PR and SD
result in similar PFS and OS times for patients with advanced NSCLC
receiving platinum-based chemotherapy. One previous study suggested
that SD may be representative of a potential survival benefit of
chemotherapy. Therefore, the differentiation between SD and CR/PR
may not be of any practical importance (15). By contrast, Coudert et al
(14) reported that SD after
first-line chemotherapy was a significant negative prognostic
factor compared with CR/PR. Recently, Mandrekar et al
(16) indicated that patients with PD
experienced worse survival compared with those with non-PD, with a
certain degree of separation between the NSCLC categories of SD and
CR/PR. Controversy remains with regard to whether initial CR/PR and
SD indicate similar survival benefits or not in advanced NSCLC
patients receiving chemotherapy. This may be due to the complexity
of SD that exhibits minor increases and decreases: When SD is
achieved, some patients experience tumor shrinkage of <30% in
the diameter of the target lesions, whilst others experience tumor
increases of <20% in the diameter of the target lesions. These
‘decreased’ SD and ‘increased’ SD may have different behavior. In
the present patients, there was no statistical significant
difference in survival time between the patients with SD and those
with CR/PR.
In clinical trials and in practice, prolongation of
survival time appears to have been recorded in NSCLC patients in
recent years, which may have been due to the appearance of more
effective and less toxic drugs (17),
molecular targeting agents (18–22) and
the improvement of supportive therapy, such as G-CSF (23) and antiemetic drugs (24). In the present study, the survival of
all consecutive NSCLC patients in daily practice was evaluated,
therefore, the study included ʻunfitʼ patients, who are usually
excluded from clinical trials. However, it was notable that the OS
time in these patients was not shorter than that observed in recent
clinical trials (25–30). In addition, in the present patients
treated with platinum and pemetrexed, the OS time was as long as
that observed in the PARAMOUNT trial (16.9 months) (31). In patients treated with bevacizumab
and those with maintenance therapy with the drug, the OS time in
the present study was evaluated to be as good as that of previous
clinical trials (32–33). Our ʻdaily clinical practiceʼ results
provide information for the near future treatment of NSCLC
patients. We believe that these favorable results are largely
dependent on the power of novel antitumor drugs, such as
pemetrexed, tyrosine kinase inhibitors and bevacizumab.
Despite these significant findings, the present
study has certain limitations. The first limitation was inherent to
the retrospective design of the study: lead time and length time
biases could not be avoided. Second, OS time may have been affected
by other factors, such as the effects of second-line and subsequent
therapies. Third, the study period was so long that various
regimens were enrolled. Not only novel antitumor drugs, but also
improvements in supportive care and advances in imaging techniques
may have conferred favorable effects for the survival of patients
in recent years. Fourth, the RECIST criteria were not always
applied to clinical chemotherapy decisions in daily practice. If SD
is achieved in daily practice, careful consideration is required to
decide whether or not to continue the chemotherapy using the same
regimen.
Regardless of these limitations, the findings of the
present study have certain clinical significance for the management
of future NSCLC patients of unselected groups. The results
confirmed that careful consideration is required in treating NSCLC
patients who experience SD with chemotherapy.
In conclusion, if the primary outcome of
chemotherapy for NSCLC at present is not shrinkage of the tumor,
but is the prolongation of survival, chemotherapy would provide a
clinical benefit not only for the 20% of patients with a good
response who have CR/PR, but also for the 70% of disease-controlled
patients who have SD, PR and CR.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Little AG, Gay EG, Gaspar LE and Stewart
AK: National survey of non-small cell lung cancer in the United
States: Epidemiology, pathology and patterns of care. Lung Cancer.
57:253–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: Recent
advances and future directions. Oncologist. 13:(Suppl 1). 5–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Therasse P: Measuring the clinical
response. What does it mean? Eur J Cancer. 38:1817–1823. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelly K, Crowley J, Bunn PA Jr, et al:
Randomized phase III trial of paclitaxel plus carboplatin versus
vinorelbine plus cisplatin in the treatment of patients with
advanced non-small-cell lung cancer: A Southwest Oncology Group
trial. J Clin Oncol. 19:3210–3218. 2001.PubMed/NCBI
|
|
7
|
Yamamoto N, Nambu Y, Fujimoto T and
Koshiji M: A landmark point analysis with cytotoxic agents for
advanced NSCLC. J Thorac Oncol. 4:697–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paz-Ares L, de Marinis F, Dediu M, Thomas
M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et
al: Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): A double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lara PN Jr, Redman MW, Kelly K, Edelman
MJ, Williamson SK, Crowley JJ and Gandara DR: Southwest Oncology
Group: Disease control rate at 8 weeks predicts clinical benefit in
advanced non-small-cell lung cancer: Results from Southwest
Oncology Group randomized trials. J Clin Oncol. 26:463–467. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He L, Teng Y, Jin B, Zhao M, Yu P, Hu X,
Zhang J, Li S, Gao Y and Liu Y: Initial partial response and stable
disease according to RECIST indicate similar survival for
chemotherapeutical patients with advanced non-small cell lung
cancer. BMC Cancer. 10:6812010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis WD, Colby TV and Sobin LH:
Histological classification of lung and pleural tumours. World
Health Organization - International Histological Classification of
Tumours: Histological Typing of Lung and Pleural Tumours (3rd).
Springer. (Geneva). 21–24. 1999.
|
|
12
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coudert B, Ciuleanu T, Park K, Wu YL,
Giaccone G, Brugger W, Gopalakrishna P and Cappuzzo F: SATURN
Investigators: Survival benefit with erlotinib maintenance therapy
in patients with advanced non-small-cell lung cancer (NSCLC)
according to response to first-line chemotherapy. Ann Oncol.
23:388–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cesano A, Lane SR, Ross GA and Fields SZ:
Stabilization of disease as an indicator of clinical benefit
associated with chemotherapy in non-small cell lung cancer
patients. Int J Oncol. 17:587–590. 2000.PubMed/NCBI
|
|
16
|
Mandrekar SJ, An MW, Meyers J, Grothey A,
Bogaerts J and Sargent DJ: Evaluation of alternate categorical
tumor metrics and cut points for response categorization using the
RECIST 1.1 data warehouse. J Clin Oncol. 32:841–850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ho C, Ramsden K, Zhai Y, Murray N, Sun S,
Melosky B and Laskin J: Less toxic chemotherapy improves uptake of
all lines of chemotherapy in advanced non-small-cell lung cancer: A
10-year retrospective population-based review. J Thorac Oncol.
9:1180–1186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosell R, Carcereny E, Gervais R, et al:
Spanish Lung Cancer Group in collaboration with Groupe Français de
Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica:
Erlotinib versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation-positive
non-small-cell lung cancer (EURTAC): A multicentre, open-label,
randomised phase 3 trial. Lancet Oncol. 13:239–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang JC, Wu YL, Schuler M, M, Popat S,
Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al: Afatinib
versus cisplatin-based chemotherapy for EGFR mutation-positive lung
adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall
survival data from two randomised, phase 3 trials. Lancet Oncol.
16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lyman GH, Reiner M, Morrow PK and Crawford
J: The effect of filgrastim or pegfilgrastim on survival outcomes
of patients with cancer receiving myelosuppressive chemotherapy.
Ann Oncol. Apr 7–2015.pii: mdv174 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uchino J, Hirano R, Tashiro N, Yoshida Y,
Ushijima S, Matsumoto T, Ohta K, Nakatomi K, Takayama K, Fujita M,
et al: Efficacy of aprepitant in patients with advanced or
recurrent lung cancer receiving moderately emetogenic chemotherapy.
Asian Pac J Cancer Prev. 13:4187–4190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fossella F, Pereira JR, von Pawel J,
Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna
A, Fidias P, et al: Randomized, multinational, phase III study of
docetaxel plus platinum combinations versus vinorelbine plus
cisplatin for advanced non-small-cell lung cancer: The TAX 326
study group. J Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fidias PM, Dakhil SR, Lyss AP, Loesch DM,
Waterhouse DM, Bromund JL, Chen R, Hristova-Kazmierski M, Treat J,
Obasaju CK, et al: Phase III study of immediate compared with
delayed docetaxel after front-line therapy with gemcitabine plus
carboplatin in advanced non-small-cell lung cancer. J Clin Oncol.
27:591–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciuleanu T, Brodowicz T, Zielinski C, Kim
JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: A
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cappuzzo F, Ciuleanu T, Stelmakh L,
Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W,
Melezínek I, et al: Erlotinib as maintenance treatment in advanced
non-small-cell lung cancer: A multicentre, randomised,
placebo-controlled phase 3 study. Lancet Oncol. 11:521–529. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pérol M, Chouaid C, Pérol D, Barlési F,
Gervais R, Westeel V, Crequit J, Léna H, Vergnenègre A, Zalcman G,
et al: Randomized, phase III study of gemcitabine or erlotinib
maintenance therapy versus observation, with predefined second-line
treatment, after cisplatin-gemcitabine induction chemotherapy in
advanced non-small-cell lung cancer. J Clin Oncol. 30:3516–3524.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paz-Ares LG, de Marinis F, Dediu M, Thomas
M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et
al: PARAMOUNT: Final overall survival results of the phase III
study of maintenance pemetrexed versus placebo immediately after
induction treatment with pemetrexed plus cisplatin for advanced
nonsquamous non-small-cell lung cancer. J Clin Oncol. 31:2895–2902.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barlesi F, Scherpereel A, Gorbunova V,
Gervais R, Vikström A, Chouaid C, Chella A, Kim JH, Ahn MJ, Reck M,
et al: Maintenance bevacizumab-pemetrexed after first-line
cisplatin-pemetrexed-bevacizumab for advanced nonsquamous
nonsmall-cell lung cancer: Updated survival analysis of the AVAPERL
(MO22089) randomized phase III trial. Ann Oncol. 25:1044–1052.
2014. View Article : Google Scholar : PubMed/NCBI
|