Introduction
Intraepithelial neoplasia describes the atypical
proliferations observed in the squamous epithelium, such as in the
uterine cervix, and has been reported to have ‘precancerous’
characteristics (1). Therefore,
intraepithelial neoplasia has been used to indicate cancer
precursors and lesions with high risk for cancer in certain types
of adenocarcinomas, including those of the breast, pancreas and
endometrium (2–4). The typical histological appearance of
intraepithelial neoplasia is characterized by the cytological
features of cancer, for which it is a precursor, with atypical cell
proliferation in anatomical structures, including normal ducti and
acini (1–5).
Prostatic intraepithelial neoplasia (PIN) is a
well-defined entity, which exhibits cytological changes and the
proliferation of secretory cells, features which are comparable to
those of cancer cells within the prostatic ducti and acini
(5–8).
Although PIN is defined as low- or high-grade, low-grade is
generally not considered relevant due to its low prognostic
significance and low degree of interobserver agreement among
pathologists (7,9). Therefore, only the expression of
high-grade PIN (HGPIN) was investigated in the present study. The
acinar type of HGPIN is known to be closely associated with
prostate adenocarcinoma, as HGPIN was reported to be observed in
85–100% of the materials examined following prostatectomies
performed due to adenocarcinoma (9).
In addition, a close association was reported between PIN and
atypical glandular foci suspicious for malignancy (10). Furthermore, it was determined that the
risk of malignancy increases in core prostate biopsies with the
increased number of cores with PIN (11).
PIN has four basic architectural patterns: Tufting,
micropapillary, cribriform and flat; in addition, other less common
patterns, such as signet-ring cell, small-cell neuroendocrine,
foamy, mucinous, squamous differentiation and inverted patterns
have been reported (5–12). The inverted (hobnail) pattern is
composed of secretory cell nuclei, which are histologically
localized at the luminal surface of the prostate gland, rather than
the periphery, and exhibit reverse polarity (12). The hobnail PIN pattern (variant) is a
rare histological type in the prostate; at present, only one
previous study has investigated this pattern, which evaluated a
total of 15 cases of hobnail HGPIN (12). This inverted pattern was found to be
primarily localized in the peripheral zone and was associated with
adenocarcinoma in 45% of the cases (12).
The aim of the present study was to discuss the
frequency of the hobnail PIN variant/pattern in core needle
biopsies of prostate glands as well as to investigate the
histological features and association with adenocarcinoma.
Materials and methods
Patients and histological samples
In the present study, a total of 2,034 prostate
transrectal ultrasonographic (TRUS) biopsy samples were evaluated
for the presence of the hobnail PIN pattern. The samples were
evaluated at the Pathology Clinic of the Istanbul Education and
Research Hospital (Istanbul, Turkey) between January 2010 and 2014.
Out of the 2,034 TRUS biopsy samples, 13 cases of inverted HGPIN
were identified. A total of 12 core biopsies were performed for
each of the 13 patients. Tissue samples (4–6 µm) were fixed in
formalin, paraffin-embedded onto glass slides and stained with
hematoxylin and eosin for histological evaluation. The slides were
then examined under a light microscope (BW51; Olympus Corporation
Tokyo, Japan). The following criteria were required for the
positive diagnosis of hobnail PIN: Localization of the nuclei on
the luminal surface of the prostate gland, rather than the
periphery, which indicates reverse polarity in the proliferated
cells of the acini and ducts; and observation of less prominent
nucleoli in the nuclei of cells compared with adjacent non-inverted
cell nuclei. Cases diagnosed with hobnail PIN were then categorized
according to whether the PIN was pure or associated with other
variants or prostate adenocarcinoma (13). In addition, the Gleason score
(14) was determined for cases with
prostatic adenocarcinoma accompanying hobnail PIN. The Gleason
grading system is used to score the histological growth pattern and
glandular differentiation of prostatic adenocarcinoma cells. It
includes five basic grade patterns ranging from grade 1–5 (grade 1,
well-differentiated tumor pattern; grade 5, no glandular
differentiation). The final Gleason score, which ranges from 2–10,
is calculated by the addition of the two most common grades: the
primary (most common) pattern and the secondary (second most
common) pattern. Thus, the most well-differentiated tumors exhibit
a Gleason score of 2, and the least-differentiated tumors exhibit a
score of 10 (14). Written informed
consent was obtained from all patients and the study was approved
by the ethics committee of Istanbul Education and Research
Hospital.
Immunohistochemical analysis
Immunohistochemical studies were performed for p63,
34βE12 and α-methylacyl-coenzyme A racemase (AMACR) for the
differential diagnosis with adenocarcinoma in cases diagnosed with
inverted PIN. Immunohistochemistry was conducted using the
Benchmark XT staining system (Ventana Medical Systems, Inc.,
Tucson, AZ, USA) and antibodies against 34βE12 (monoclonal mouse
anti-human; cat. no. CM 127 AC; 1:50; Biocare Medical Inc.,
Concord, CA, USA), AMACR (monoclonal rabbit anti-human; cat. no.
504S-14; 1:100; Cell Marque Corporation, Rocklin, CA, USA) and p63
(monoclonal mouse anti-human; cat. no. VP 163 GG25; 1:200; Biocare
Medical Inc.). Briefly, the tissue sections were deparaffinized
with EZ Prep solution (Ventana Medical Systems, Inc.) at 75°C,
pretreated with cell conditioning 1 (CC1) solution (Ventana Medical
Systems, Inc.) for antigen retrieval at 95°C, and incubated with
hydrogen peroxide (Ventana Medical Systems, Inc.) for 4 min to
block endogenous peroxidase activity. The sections were then
incubated with the AMACR, p63 and 34βE12 primary antibodies for 32
min at 37°C. Next, the sections were blocked using the Endogenous
Biotin Blocking Kit (Ventana Medical Systems, Inc.) followed by
incubation with a streptavidin-horseradish peroxiade-conjugated
secondary antibody (monoclonal goat anti-rat; cat. no. 760-500;
1:200; Ventana Medical Systems, Inc.) for 8 min at 37°C. The
immunolocalized AMACR, p63 and 34βE12 were visualized using a
copper-enhanced DAB reaction. The slides were counterstained with
hematoxylin II (Ventana Medical Systems, Inc.) for 4 min and Bluing
Reagent (Ventana Medical Systems, Inc.) for 4 min and coverslips
were applied using an automated coverslipper (Tissue-Tek Film
Automated Coverslipper; Sakura Finetek Japan Co., Ltd., Tokyo,
Japan). Cytoplasmic staining for AMACR and 34βE12, and nuclear
staining for p63 were considered positive. The clinical data of the
patients, including age and medical history, were obtained from the
patient charts. None of the cases involved in the present study
with adenocarcinoma underwent surgery at the hospitals affiliated
with the authors; therefore, radical prostatectomy materials were
not examined.
Results
Identification of hobnail PIN
Out of the 2,034 biopsy samples that were examined
in the present study, the hobnail PIN pattern was identified in a
total of 13 (0.63%) samples. The age range of the 13 patients was
53–83 years, with a mean age of 64 years. Hobnail PIN was observed
in 4 cores in 3 patients, 2 cores in 5 patients and 1 core in 5
patients. In addition, prostatic acinar type adenocarcinoma was
identified in 7 cases. The Gleason score was 7 in all of the
adenocarcinoma cases.
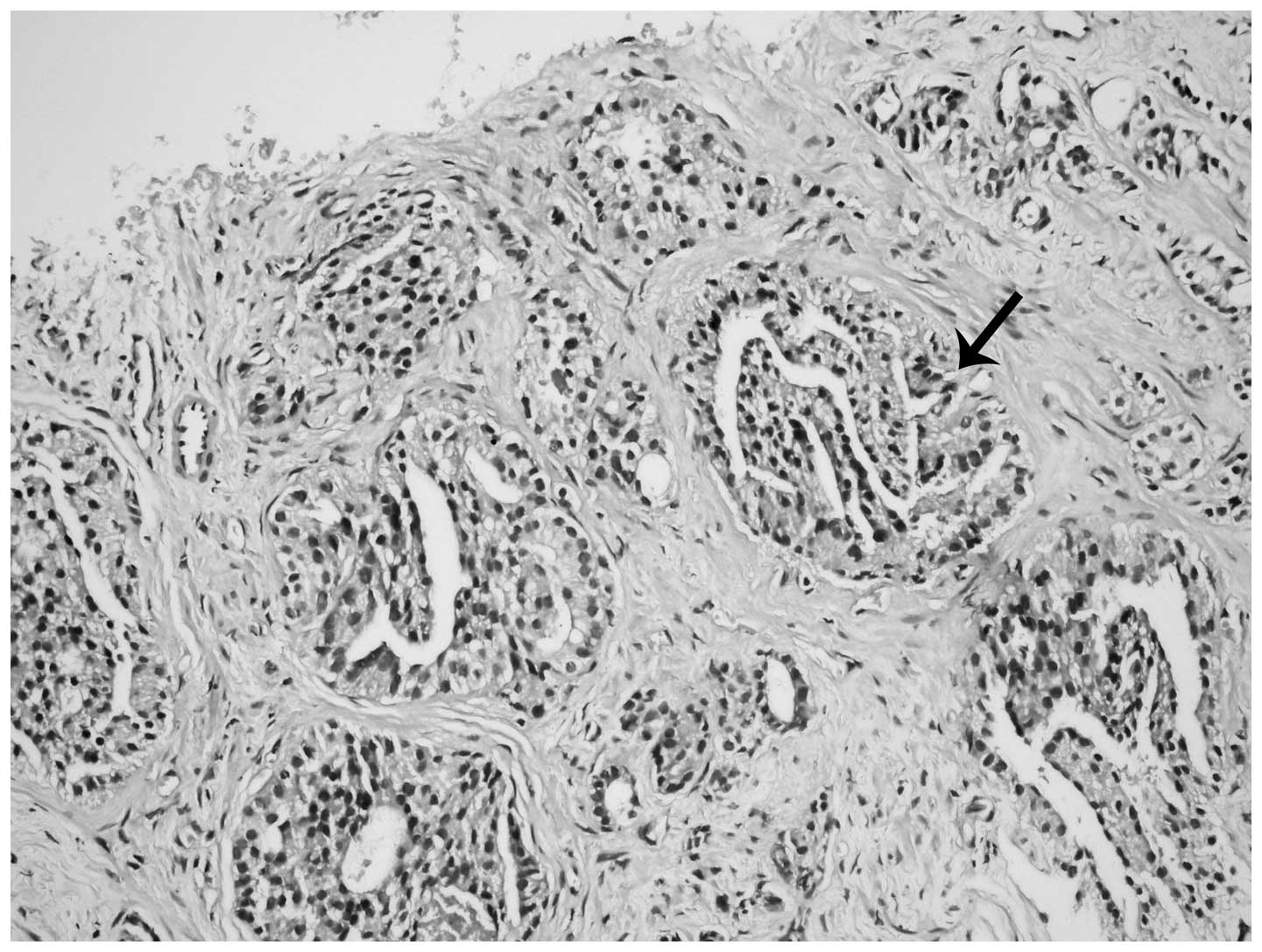
Histological examinations of hobnail
PIN
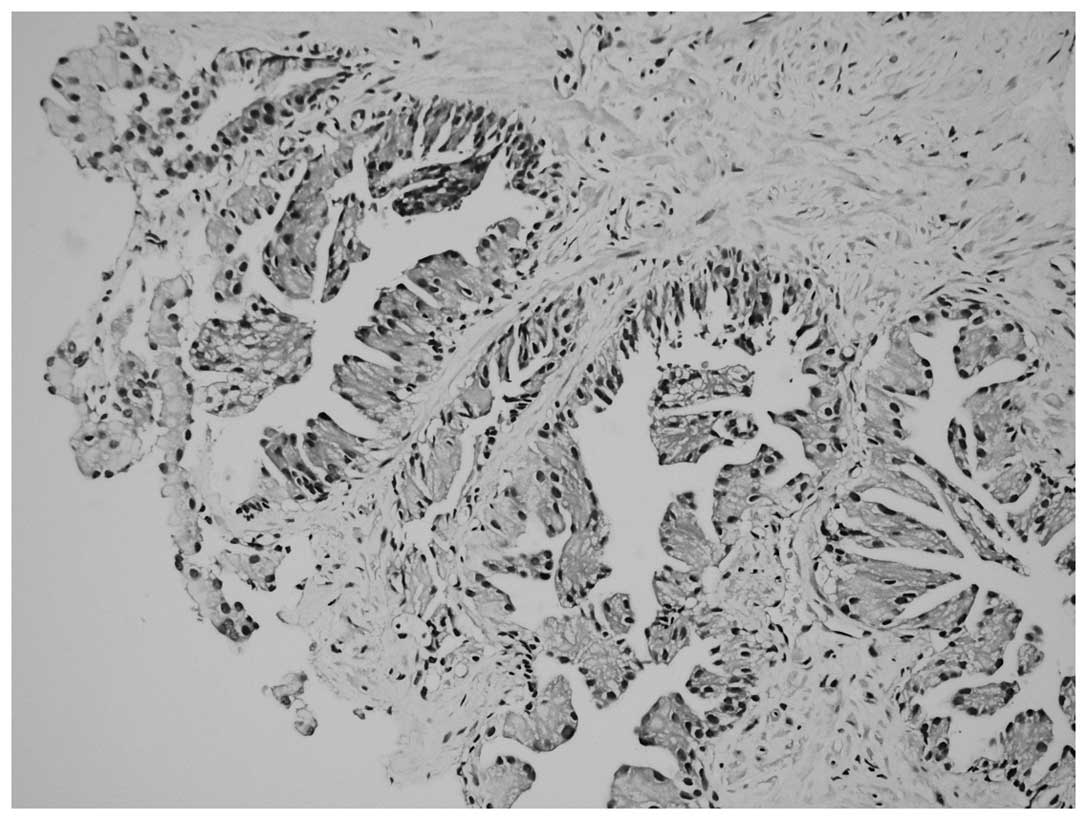
Proliferation in the form of micropapillary
projections and mounds (tufting), particularly in the acini, was
observed via microscopy in all 13 patients. However, nuclei
localized on the luminal surface of the glands and not on the
periphery (reverse polarity) were the most notable features
(Fig. 1). In addition, inverted cell
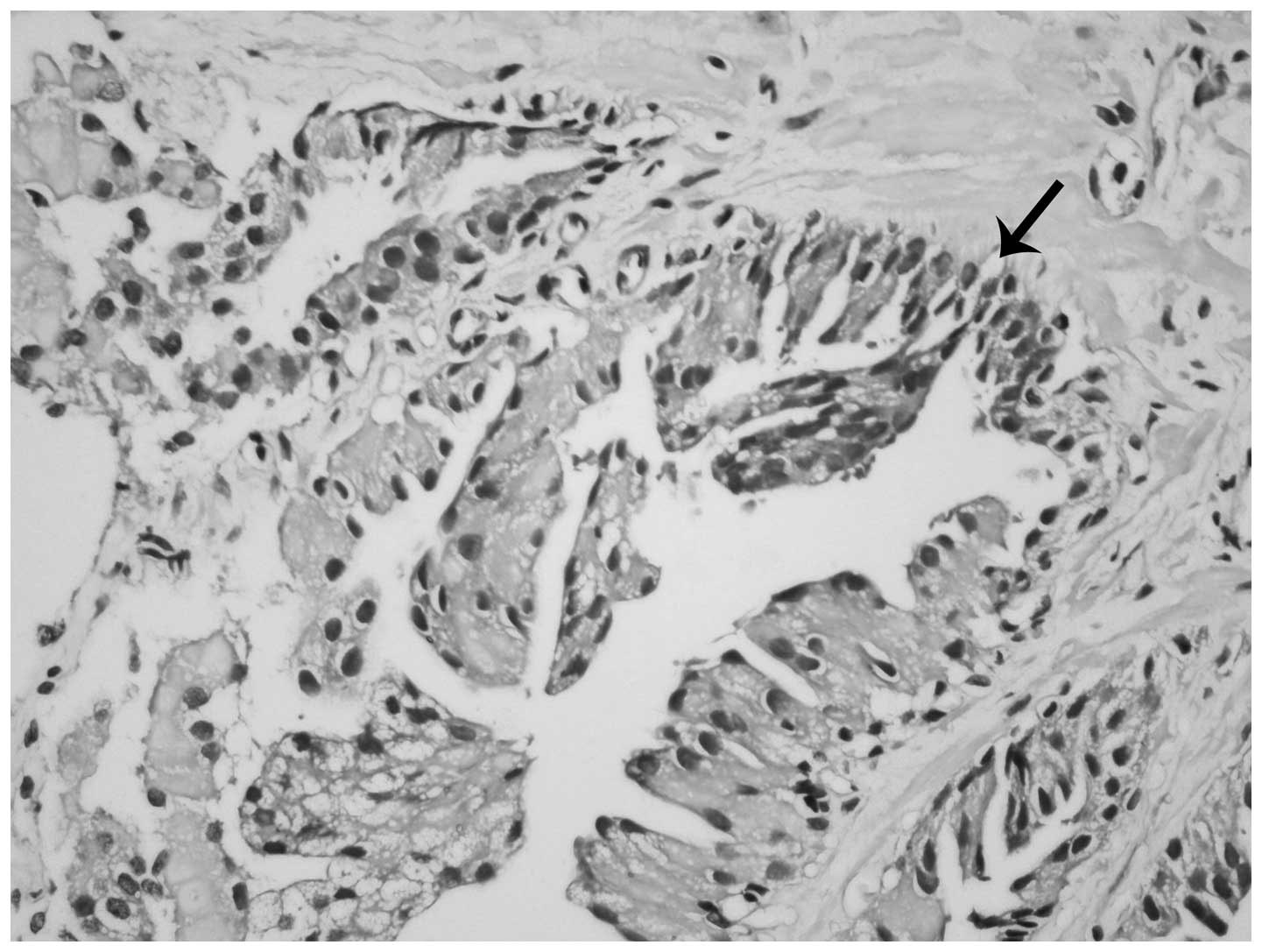
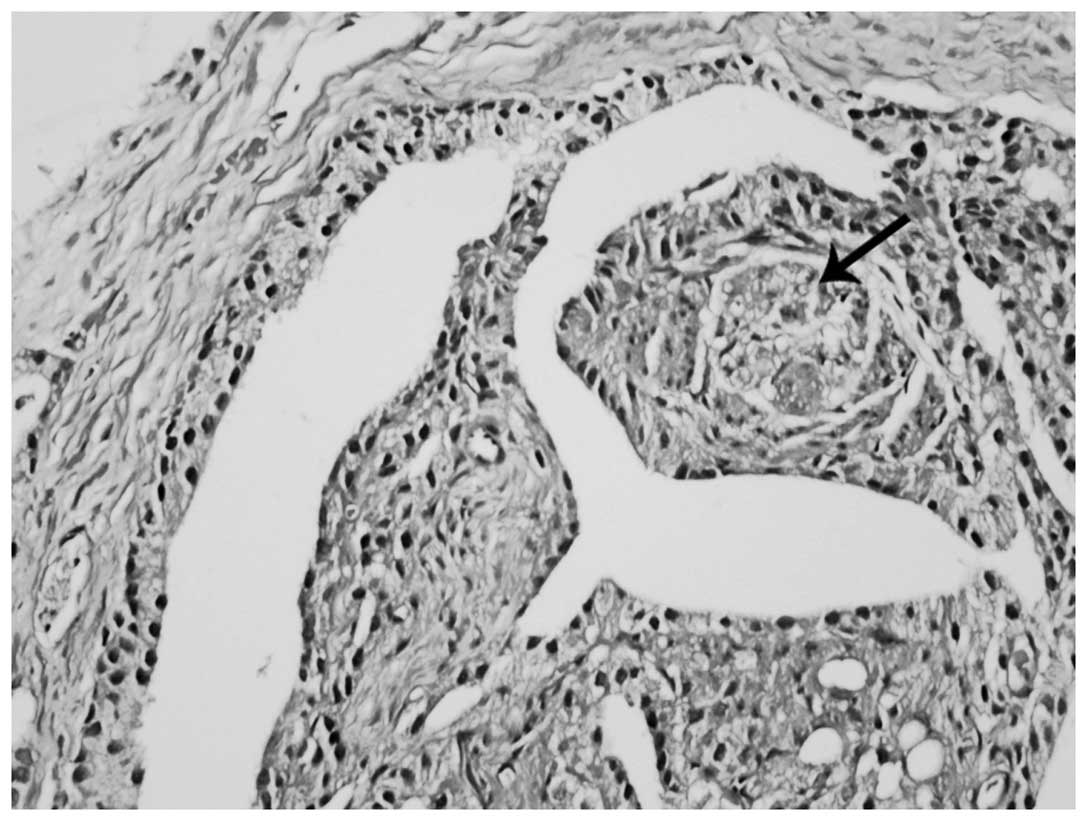
nuclei frequently demonstrated less prominent nucleoli compared
with adjacent non-inverted cell nuclei (Fig. 2). Certain acini demonstrated the
inverted feature in all parts, while others only partially
demonstrated this feature (Fig. 2).
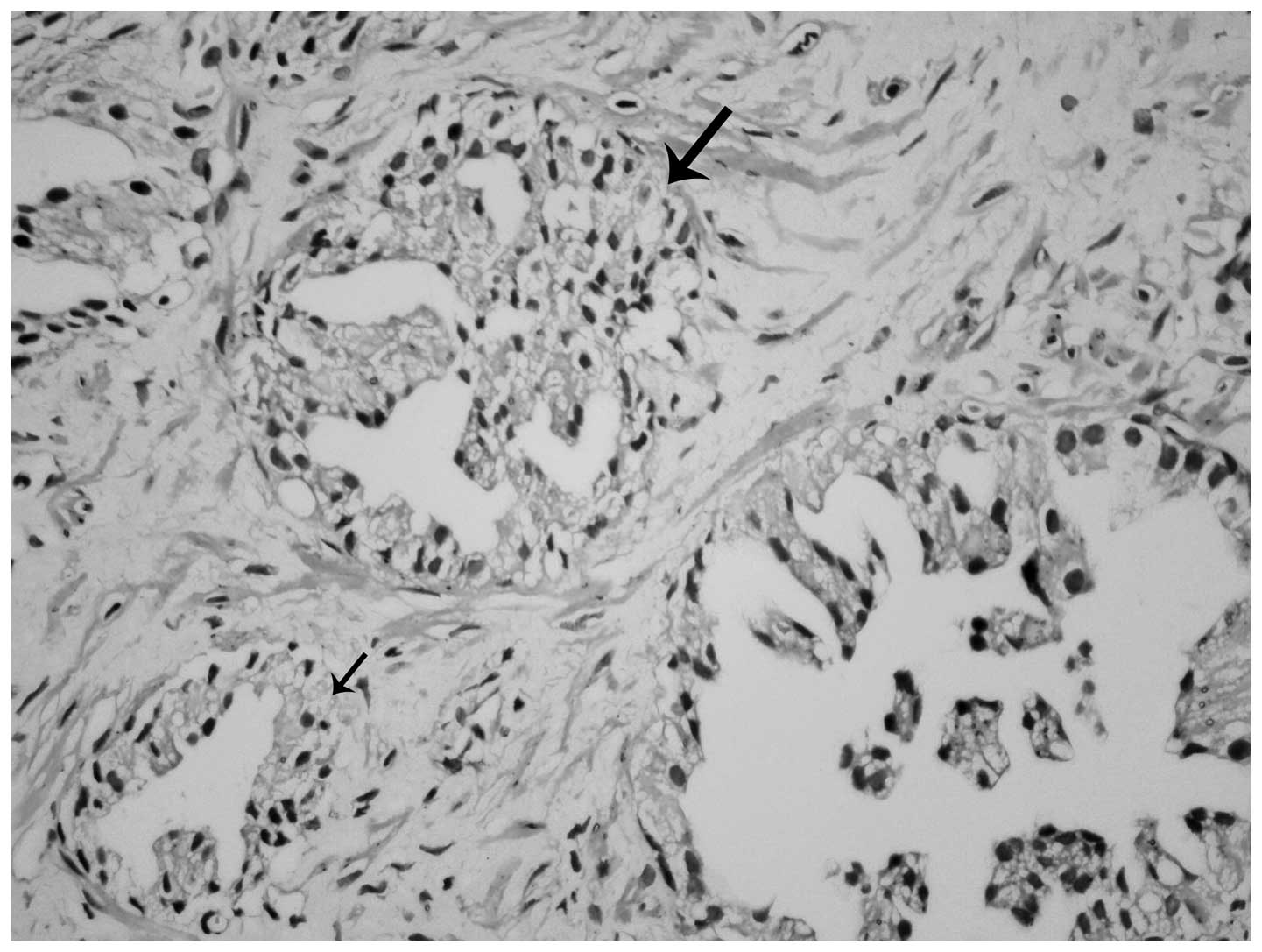
Micropapillary projections and tufting patterns were also
accompanied by cribriform and flat patterns in certain inverted PIN
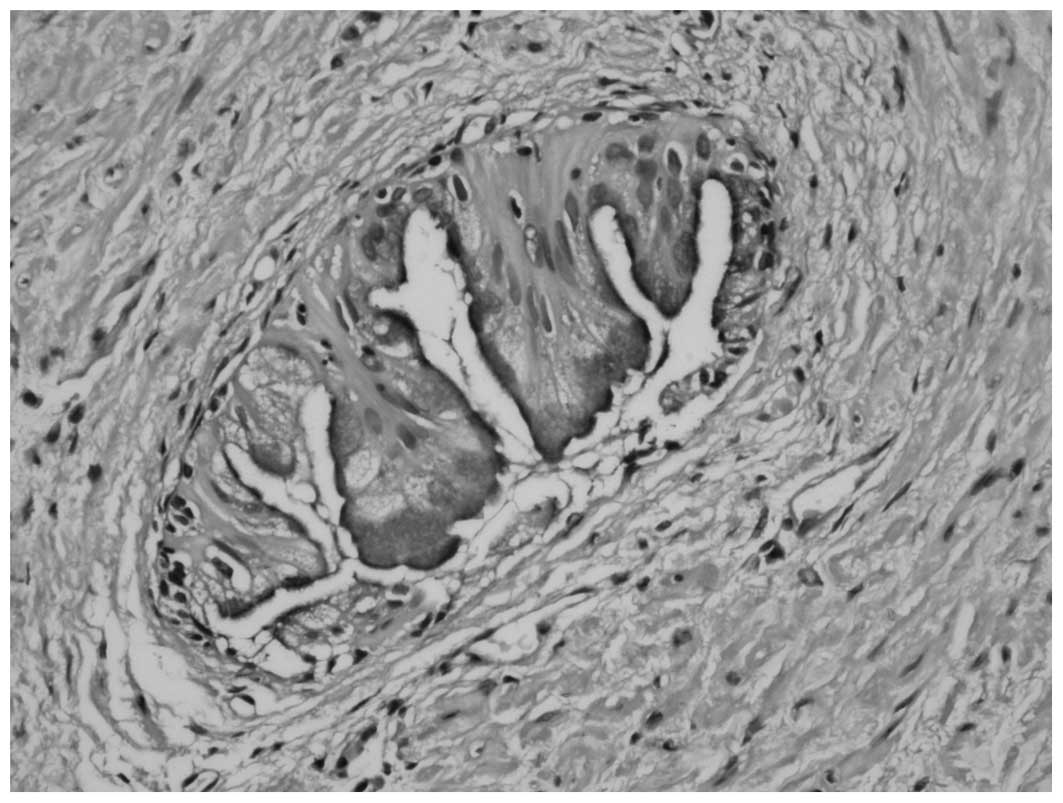
cases (Fig. 3). Furthermore, inverted
morphology and intracytoplasmic mucin were observed in certain
acini in one case (Fig. 4). Pure
inverted histology was present in 4 of the 13 cases and PIN with
other patterns was identified in 9 patients.
Gleason scores
All 7 carcinomas accompanied by inverted PIN were
conventional acinar adenocarcinoma cases with a Gleason score of 7.
These scores were of 3+4 in 6 cases and 4+3 in the other case.
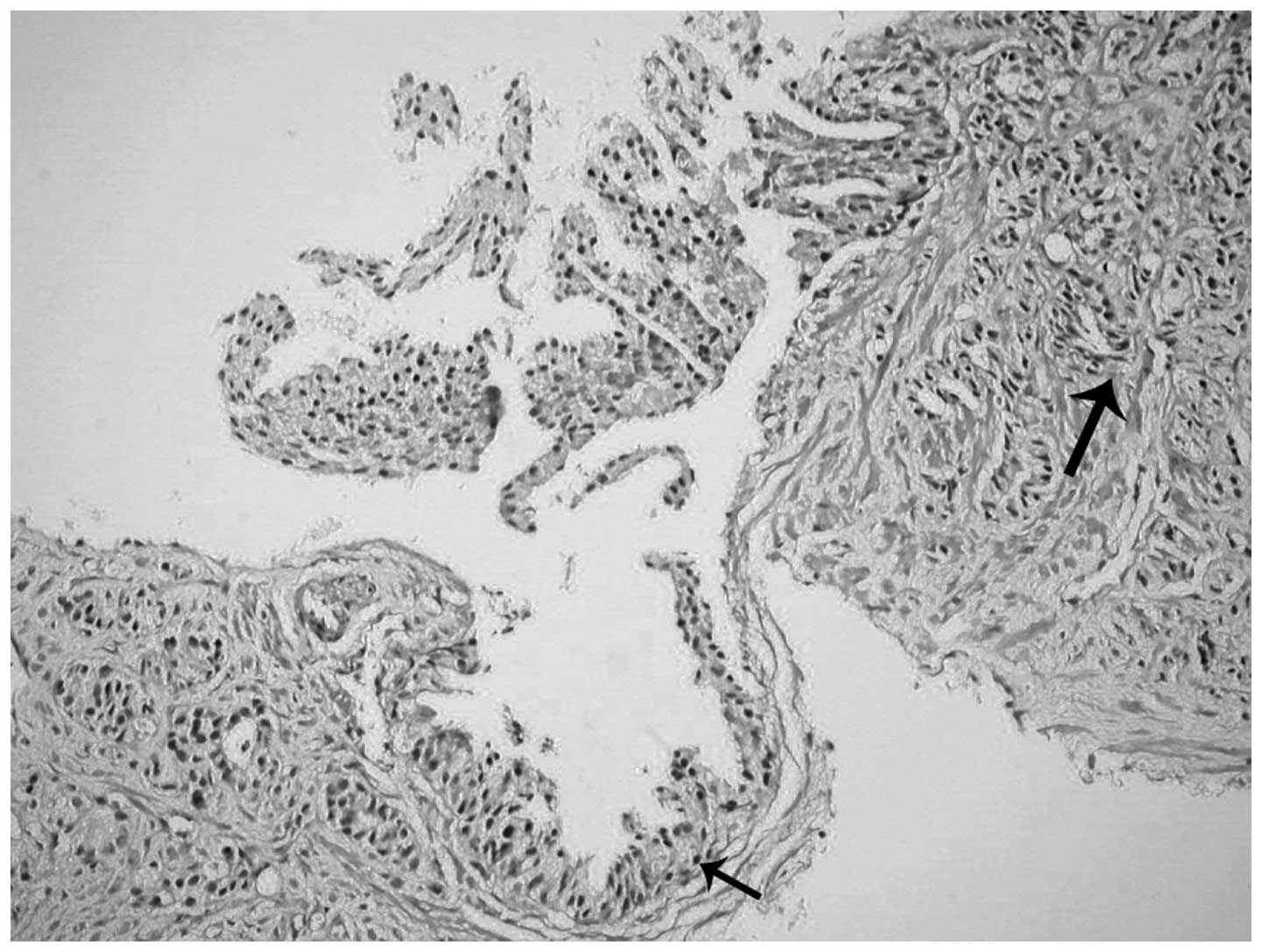
Invasive features of hobnail PIN
associated with invasive adenocarcinoma
One case of acinar adenocarcinoma accompanying
inverted PIN revealed large hobnail areas in the invasive
component. The nuclei in the invasive cribriform structures had
proliferated and were located on the luminal aspect of the
cytoplasm, which was comparable to that of the inverted HGPIN
sections. In addition, similar characteristics were observed in the
separate invasive glands as well (Figs.
5–8). A cribriform pattern and
single glandular structures were prominent in the invasive
component (Fig. 5). Similar patterns
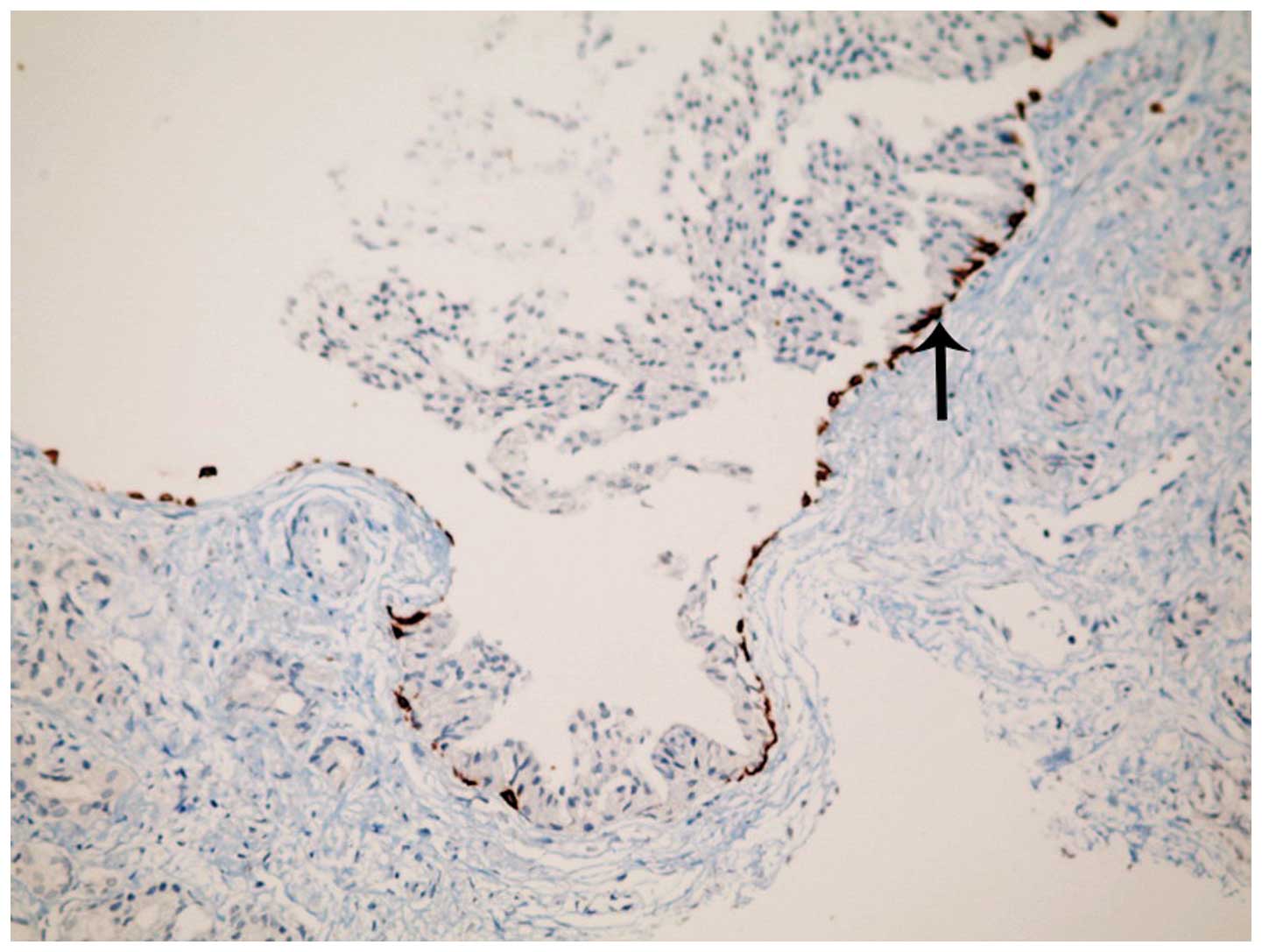
were also observed surrounding inverted PIN areas (Fig. 6). Immunohistochemical 34βE12
expression was evident along the basal cell layer in glands with
inverted PIN (Fig. 7). Typical
inverted features were also identified in tumoral glandular
structures surrounding the peripheral nerves (Fig. 8).
Immunohistochemical analysis
Basal cell markers, p63 and 34βE12, were found to be
positively expressed in the hobnail PIN areas, indicating the
presence of a basal cell layer and the absence of carcinoma. In
addition, cytoplasmic staining with AMACR was observed in all 13
cases following immunohistochemical study, confirming the presence
of both PIN and carcinoma.
Discussion
HGPIN requires much consideration due to its
frequent association with prostate cancer in prostatic core
biopsies; increased risk of cancer has been associated with an
increased number of HGPIN-positive cores. In addition, at the
molecular level, HGPIN displays the characteristics of a precursor
cancerous lesion (5–12,15–17). PIN
has four well-defined primary architectural patterns, including
tufting, micropapillary, cribriform and flat, which do not carry
prognostic significance (12). In
addition, the signet-ring cell, small-cell neuroendocrine, foamy,
mucinous, squamous differentiation and inverted patterns are less
commonly observed (12).
The inverted (hobnail) pattern is rare and has only
been reported by one previous study (12). Argani and Epstein (12) reported the incidence rate of hobnail
PIN as 0.43% in their study. In the present study, an incidence
rate of 0.63% was determined; however, the present study was
prospective and actively searched for this entity in all TRUS
biopsies, which may account for the increase in reported incidence
rate.
A hobnail appearance is the typical feature of clear
cell adenocarcinoma, which is predominantly observed in the genital
system (18). The distinguishing
features of hobnail from other patterns include the polarization of
enlarged cell nuclei towards the lumen of the glands. This pattern
may be missed as it is rare in prostate biopsies and resembles the
central zone acini of the prostate; however, it may be
distinguished from the central zone acini with features such as
enlarged nuclei and with the known localization of the biopsy
(12).
An association with prostate adenocarcinoma was
identified in almost half of the cases in a previous study on
inverted PIN (12). In the present
study, it was observed that 7 of the 13 cases were associated with
acinar adenocarcinoma. The Gleason score was 7 in all the
adenocarcinoma-associated cases in the present study, which was
higher than those reported in the Argani and Epstein study: The
Gleason score was 6 (3+3) in 80% of the cases with adenocarcinoma
accompanying PIN in the Argani and Epstein (12) study of 15 cases. The high association
with carcinoma and high Gleason score may therefore have
interesting prognostic significance for inverted PIN.
In the present study, histological features of
conventional prostatic acinar adenocarcinoma were identified in 6
of the 7 cases with adenocarcinoma accompanying hobnail PIN. The
remaining case exhibited a different adenocarcinoma histology and
demonstrated features similar to that of the accompanying hobnail
PIN. Therefore, it was difficult to distinguish the invasive
carcinoma cribriform pattern areas with PIN morphology from the
actual hobnail PIN regions in certain areas. The negative
immunohistochemical results of the basal cell markers, p63 and
34βE12, in the carcinoma areas and positive results in the hobnail
PIN areas aided the differential diagnosis. In addition, most of
the individual invasive glands outside the cribriform pattern
demonstrated inverted features. The Gleason score of this case was
7 (4+3) and tumor was present in all 12 core biopsies. In a study
by Arkani and Epstein (12), no
adenocarcinoma cases with inverted features were reported. Thus, to
the best of our knowledge, the current study reports the first case
of prostatic adenocarcinoma with hobnail histological features in
the invasive component in the English literature.
In conclusion, the present study identified the
histological features of the uncommonly diagnosed hobnail PIN. The
high rate of association with prostatic acinar adenocarcinoma (54%)
and the high Gleason score (7) of the
carcinomas were noteworthy. However, further studies are required
in order to determine whether the hobnail pattern is aggressive. In
addition, the present study identified inverted pattern
histological features in the invasive component of an associated
adenocarcinoma in one case of hobnail PIN.
References
|
1
|
Kurman RJ, Malkasian GD Jr, Sedlis A and
Solomon D: From Papanicolaou to Bethesda: The rationale for a new
cervical cytologic classification. Obstet Gynecol. 77:779–782.
1991.PubMed/NCBI
|
|
2
|
Hruban RH, Adsay NV, AlboresSaavedra J, et
al: Pancreatic intraepithelial neoplasia: A new nomenclature and
classification system for pancreatic duct lesions. Am J Surg
Pathol. 25:579–586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mutter GL: Histopathology of genetically
defined endometrial precancers. Int J Gynecol Pathol. 19:301–309.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosai J: Borderline epithelial lesions of
the breast. Am J Surg Pathol. 15:209–221. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bostwick DG: Progression of prostatic
intraepithelial neoplasia to early invasive adenocarcinoma. Eur
Urol. 30:145–152. 1996.PubMed/NCBI
|
|
6
|
Epstein JI and Herawi M: Prostate needle
biopsies containing prostatic intraepithelial neoplasia or atypical
foci suspicious for carcinoma: Implications for patient care. J
Urol. 175(3)Pt 1820–834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou M, Netto JG and Epstein JI:
Neoplastic disease of the prostateHigh-Yield Pathology:
Uropathology. Elsevier; Philadelphia, PA: pp. 52–54. 2012
|
|
8
|
Bostwick DG, Liu L, Brawer MK and Qian J:
High-grade prostatic intraepithelial neoplasia. Rev Urol.
6:171–179. 2004.PubMed/NCBI
|
|
9
|
Cheng L, Paterson RF, Beck SD and Parks J:
Prostatic intraepithelial neoplasia: An update. Clin Prostate
Cancer. 3:26–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheville JC, Reznicek MJ and Bostwick DG:
The focus of ‘atypical glands, suspicious for malignancy’ in
prostatic needle biopsy specimens: Incidence, histologic features,
and clinical follow-up of cases diagnosed in a community practice.
Am J Clin Pathol. 108:633–640. 1997.PubMed/NCBI
|
|
11
|
Kronz JD, Allan CH, Shaikh AA and Epstein
JI: Predicting cancer following a diagnosis of high-grade prostatic
intraepithelial neoplasia on needle biopsy: Data on men with more
than one follow-up biopsy. Am J Surg Pathol. 25:1079–1085. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Argani P and Epstein JI: Inverted
(Hobnail) high-grade prostatic intraepithelial neoplasia (PIN):
Report of 15 cases of a previously undescribed pattern of
high-grade PIN. Am J Surg Pathol. 25:1534–1539. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elbe JN, Sauter G, Epstein JI and
Sesterhenn IA: Tumours of the prostateWorld Health Organization
Classification of Tumours. Pathology and Genetics of Tumours of the
Urinary System and Male Genital Organs. IARC Press; Lyon, France:
pp. 159–214. 2004
|
|
14
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) Consensus Conference on Gleason
Grading of Prostatic Carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hughes C, Murphy A, Martin C, Sheils O and
O'Leary J: Molecular pathology of prostate cancer. J Clin Pathol.
58:673–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Godoy G and Taneja SS: Contemporary
clinical management of isolated high-grade prostatic
intraepithelial neoplasia. Prostate Cancer Prostatic Dis. 11:20–31.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Merrimen JL, Evans AJ and Srigley JR:
Preneoplasia in the prostate gland with emphasis on high grade
prostatic intraepithelial neoplasia. Pathology. 45:251–263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montag AG, Jenison EL, Griffiths CT, Welch
WR, Lavin PT and Knapp RC: Ovarian clear cell carcinoma. A
clinicopathologic analysis of 44 cases. Int J Gynecol Pathol.
8:85–96. 1989. View Article : Google Scholar : PubMed/NCBI
|