Introduction
Liposarcoma (LPS) contains three subtypes: i)
Well-differentiated LPS and dedifferentiated LPS; ii) myxoid LPS
(MLPS); and iii) pleomorphic LPS. MLPS accounts for ~30% of all LPS
cases and is characterized by the appearance of uniform, round- to
oval-shaped cells with myxoid stroma (1,2). For local
disease, the 5-year disease-specific survival (DSS) rate is 93%. By
multivariate analysis, an age of >45 years, the male gender and
locally recurrent disease are predictive of a poor 5-year DSS rate.
For metastatic disease, the outcome is poor, with a 5-year DSS rate
of 8.2%. In comparison with other sarcomas, MLPS often metastasizes
to the abdomen (49%), rather than the lungs (14%) and bones (23%)
(3). Understanding the metastasis
pattern and underlying mechanism should aid in improving the
survival of patients with this disease.
The current study presents a rare case of MLPS with
multiple metastases in fat-bearing areas, but no involvement of the
lungs. Unexpectedly, the presence of bone metastasis as diagnosed
by magnetic resonance imaging (MRI) and proven by histology, was
negative on bone scans. Written informed consent was obtained from
the patient's family.
Case report
A 53-year-old male presented with a slowly enlarging
mass in the right thigh in May 2008. The patient underwent a local
excision at Shanghai General Hospital (Shanghai, China). and the
histology revealed a diagnosis of MLPS. The patient subsequently
received adjuvant radiotherapy (details unknown) and was free from
recurrence for 34 months after the resection. The patient presented
to The Sixth People's Hospital of Shanghai Jiao Tong University
(Shanghai, China) on April 26, 2011, with lower back pain that
radiated to the bilateral thighs for more than one month. Bone
scans showed no evidence of bone metastasis. However, MRI showed
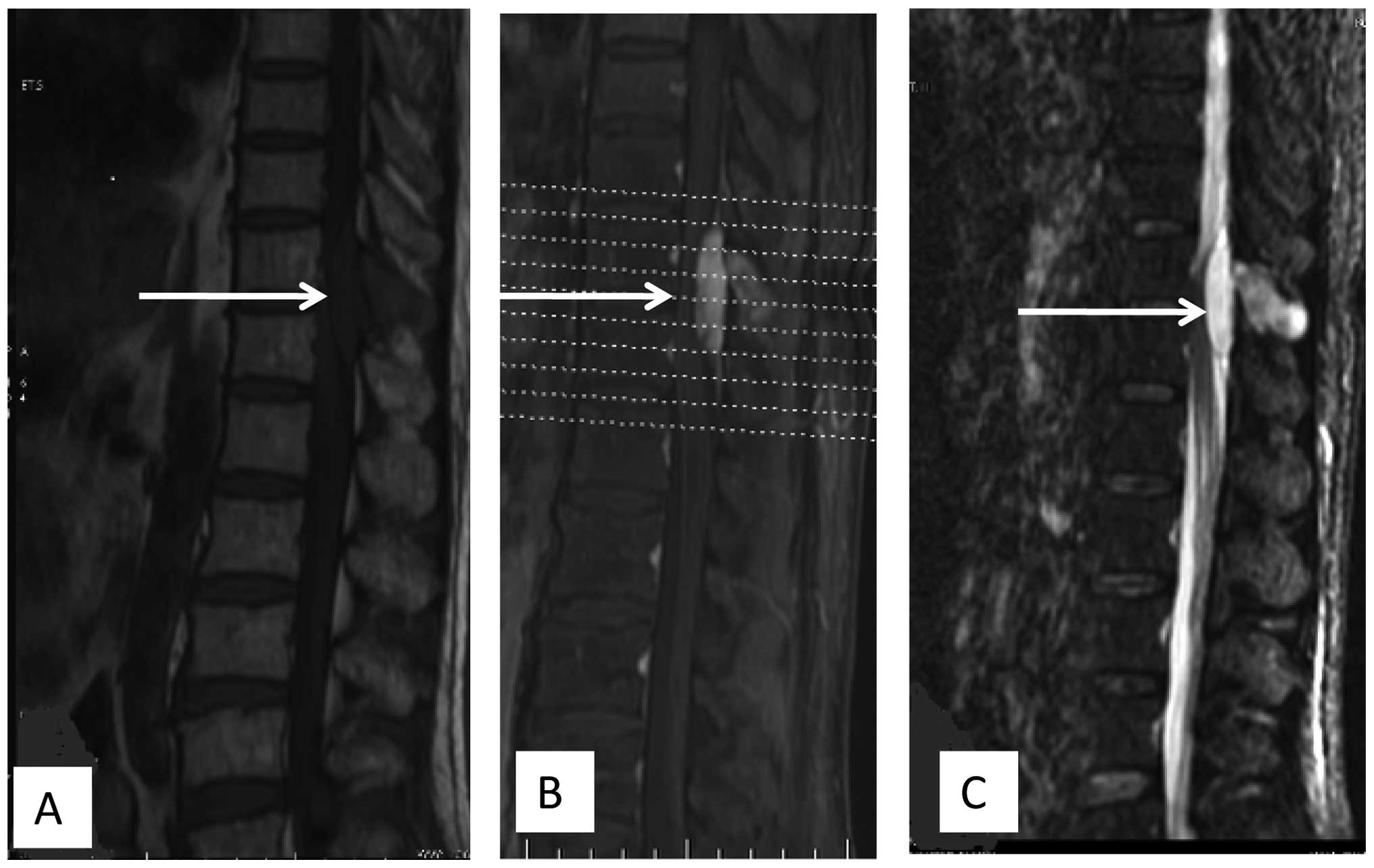
abnormal signals in the vertebral body of T11 and T12, and a mass
in the epidural region at the T11 and T12 vertebral level (Fig. 1). Angiography showed that the feeding
vessels were the T11 and T12 intercostal arteries. Transcatheter
arterial embolization was performed using polyvinylpyrrolidone and
gelfoam particles, and the contrast extravasation almost
disappeared in the two arteries. Next day, a laminectomy was
performed and the soft tumor in the epidural region was marginally
resected. Immunohistochemically, the tumor cells were positive for
vimentin, slightly positive for Ki-67 (2%), and negative for
cluster of differentiation (CD)31, CD34, S-100, smooth muscle
actin, glial fibrillary acidic protein and CD56. These findings
together with hematoxylin and eosin staining revealed a diagnosis
of metastatic MLPS for the involved bones and the mass in the
epidural region.
Following recovery from the surgery, radiotherapy
was administered at 48 Gy for 24 fractions over 5 weeks. At 1 week
after the initiation of radiotherapy, concurrent chemotherapy
consisting of gemcitabine (0.8 g/m2 on days 1 and 8) and docetaxel
(75 mg/m2 on day 8) was administered. The adverse events were mild,
such as leukopenia of World Health Organization grade I (4). At 16 days post-radiotherapy, a second
course of chemotherapy with the same regimen was administered.
Another 18 days later, computed tomography (CT) detected a
4.5×3.7-cm mass in the abdominal cavity below the gastric body.
Several days later, the patient started complaining of blurred
vision and painless diplopia in the left eye. Proptosis of the left
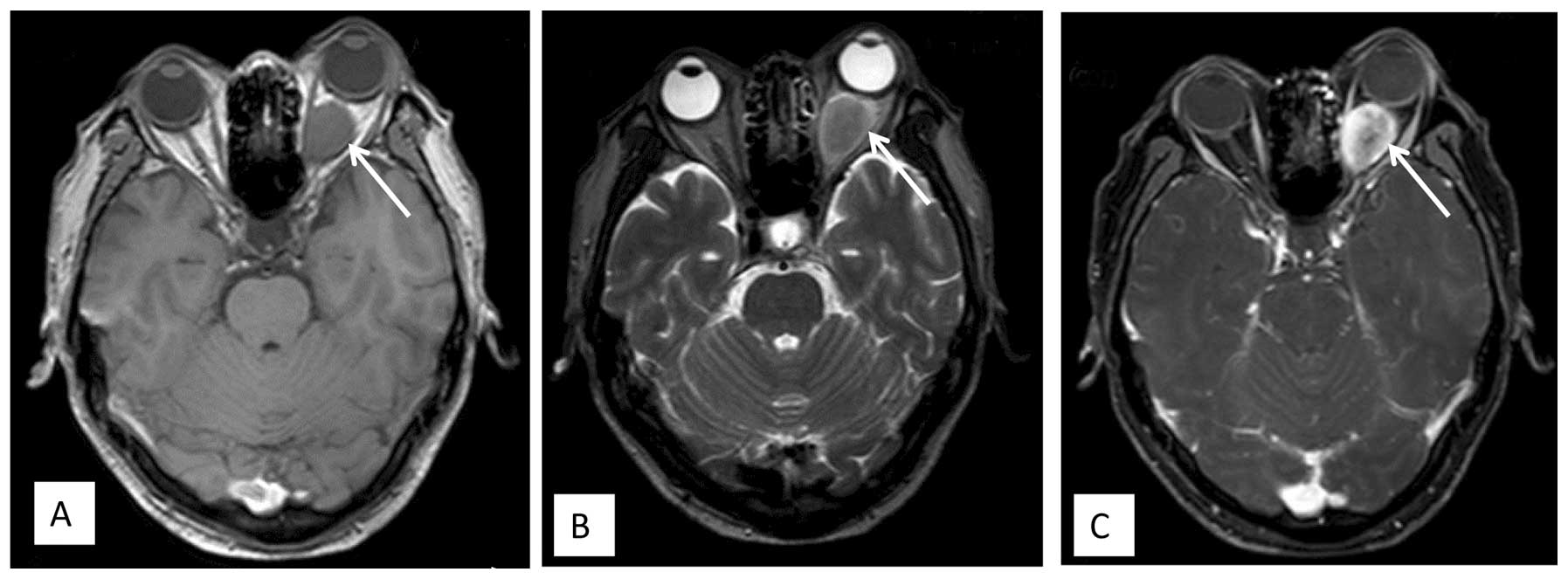
eye became progressively evident. MRI showed a retrobulbar mass,
which was isointense on T1-weighted images and hypo- to isointense
on T2-weighted images, with ring enhancement following
gadolinium-DTPA infusion (Fig. 2).
The mass slightly compressed the optic nerve. The diagnosis was of
an orbital metastasis. To alleviate the eye symptoms, the patient
received palliative radiotherapy (21 fractions of 51 Gy over 10
weeks) to the retrobulbar mass. During the radiotherapy, the
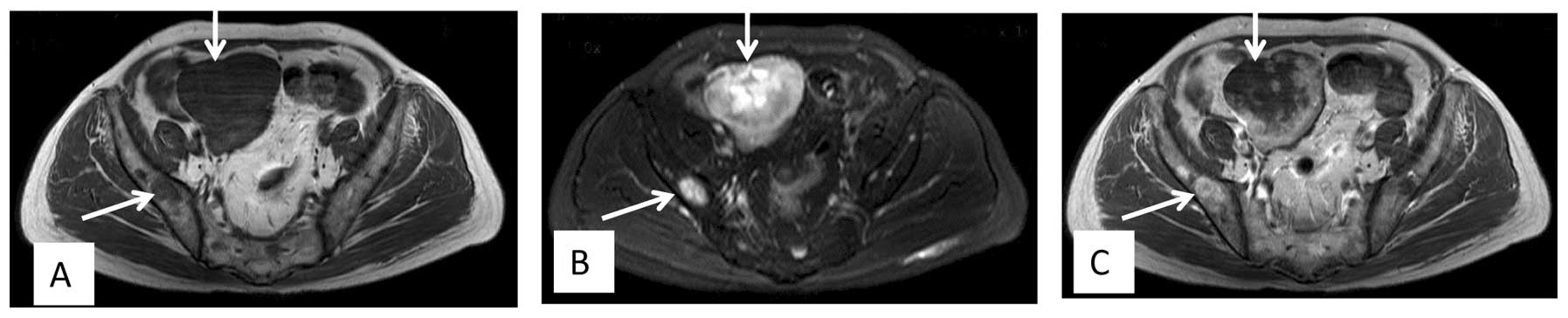
patient developed multiple metastases in regions that included the
pelvic cavity, the pelvis femur and the lumbar vertebrae, and
progression of the orbital mass was observed (Fig. 3). However, there was no involvement of
the lungs. Although the patient received second-line chemotherapy
with ifosfamide (1.8 g/m2 on days 1 to 5) and epirubicin (60 mg/m2
on days 1 and 2), the disease progressed and the patient's
performance state deteriorated. The patient succumbed to the
disease 3 months later.
Discussion
Metastasis to the orbit from LPS is rare and only
four previous reports can be found in a search of the literature
(5–8).
The primary tumor locations were the retroperitoneum, the thigh and
the abdominal cavity, and the pathological types were
low/intermediate-differentiated LPS, MLPS, spindle-cell LPS or
dedifferentiated LPS, respectively. Irrespective of the palliative
surgical and systemic treatment, the prognosis of patients with
orbit metastasis is poor.
Metastasis to the epidural region from LPS is also
rare. Only three cases have been documented in the literature. The
histological type of these three cases was uniformly MLPS. One case
was in the cervical region and presented with liver, subcutaneous
tissue metastasis (9). The second
case was in the thoracic region and the patient had a history of
lymph node, left buttock and left thoracic wall metastases. The
patient succumbed to multiple lung metastases a few months later
(10). The third case was in the
thoracic region, and the patient developed abdominal metastasis and
succumbed 7 weeks after the laminectomy (10). In the present case, the patient
developed spinal metastases and progression with abdominal and
orbital metastases 4 months after laminectomy. Collectively, these
cases may suggest that epidural metastasis tends to arise from
MLPS. Since the disease progressed rapidly in the patients with
epidural metastasis, careful follow-up is strongly recommended to
rule out metastasis in other locations.
Metastases from sarcoma are found mainly in the
lungs and bones. LPS, which is the most common type of sarcoma, has
an extrapulmonary metastasis pattern. The data from a study of 45
patients with MLPS suggested that the most common sites of spread
were the bones, intraabdominal region, skin, mediastinum,
paraspinal region and pleura (11).
Cheng et al (12) also found
that LPS has a tendency towards metastases occurring in the
abdominal wall, axilla, breasts, subcutis, liver, dura mater,
thymus and intraabdominal region. This distinct metastasis pattern
was also proved by Hoffman et al (4) in a recent large-scale study of MLPS.
Collectively, these results suggest that LPS, particularly MLPS,
has a tendency to metastasize to fat-bearing areas (10). Concordant with this literature, the
present case exhibited metastases in fat-bearing areas, including
epidural, bone, abdominal and orbital metastases. However, the
reason for this tendency is not clear. Hoffman et al
(4) completed a pilot study
concerning the molecular characteristics of MLPS. The study showed
that MLPS expressed high levels of adipophilin, peroxisome
proliferator-activated receptor-γ (PPAR-γ), chemokine (C-X-C motif)
receptor 4 (CXCR4), AXL receptor tyrosine kinase and
platelet-derived growth factor receptor-β (PDGFR-β). The
aforementioned molecules were correlated with adipogenesis,
migration, invasion, angiogenesis and metastasis. Further
investigation is warranted to determine whether adipophilin and
CXCR4 molecules contributed to the unique metastatic pattern.
In the present case, spinal metastasis was detected
by MRI, although bone scans were negative. Bone scans normally
detect skeletal metastasis using technetium-99m methylene
diphosphonate binding to osteoblastic cells. For the majority of
other cancer types, it is a routine procedure for patients during
follow-up screening, as it is more sensitive than plain radiographs
and CT for finding skeletal metastasis. However, for MLPS, several
previous studies reported that skeletal metastasis was detectable
by MRI, but not by bone scans (13–17). In
one previous study, based on whole-body MRI findings, 33 MLPS
patients were identified with spinal metastases. These metastases
were detected by bone scans in 16% of patients and by positron
emission tomography scans in 14% of patients (1). The reason why bone scanning is not a
reliable method to detect skeletal metastasis remains unclear. We
hypothesize one possible reason for this may be that there are few
osteoblastic cells in skeletal metastasis. Recently, a variant
chromosomal translocation t(12;22)(q13;q12) was identified. This
translocation results in the production of the Ewing sarcoma
breakpoint region 1-DNA damage-inducible transcript 3 (EWSR1-DDIT3)
fusion protein. This fusion protein exerted a selective effect on
the transcriptional activity of cell lineage-specific marker genes
in multipotent mesenchymal C3H10T1/2 cells. The osteoblastic marker
Opn promoter was repressed, while the adipocytic marker PPAR-γ2
promoter was not affected. The study concluded that EWSR1-DDIT3 may
contribute to the phenotypic selection of mesenchymal cells during
MLPS initiation and development (18). We propose that by the effect of this
protein, mesenchymal cells in skeletal metastasis mainly
transformed to adipocyte-related cells, but few osteoblastic cells.
Therefore, this histological change may result in ineffective
detection of the skeletal metastasis by bone scans.
In summary, a case of MLPS with skeletal, epidural,
orbital and abdominal cavity metastases was reported. MRI clearly
showed an abnormal signal in the vertebral body, however, bone
scans were negative. Recent results have suggested that MLPS
expresses high levels of adipophilin, PPAR-γ, CXCR4, AXL receptor
tyrosine kinase, PDGFR-β and may present with the EWSR1-DDIT3
fusion transcript. We hypothesize that these genetic and molecular
changes may contribute to the distinct biological characteristics
of MLPS.
References
|
1
|
Schwab JH, Boland PJ, Antonescu C, Bilsky
MH and Healey JH: Spinal metastases from myxoid liposarcoma warrant
screening with magnetic resonance imaging. Cancer. 110:1815–1822.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanfilippo R, Dei Tos AP and Casali PG:
Myxoid liposarcoma and the mammalian target of rapamycin pathway.
Curr Opin Oncol. 25:379–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman A, Ghadimi MP, Demicco EG,
Creighton CJ, Torres K, Colombo C, Peng T, Lusby K, Ingram D,
Hornick JL, et al: Localized and metastatic myxoid/round cell
liposarcoma: Clinical and molecular observations. Cancer.
119:1868–1877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fabi A, Salesi N, Vidiri A, Mirri A,
Ferraresi V and Cognetti F: Retroperitoneal liposarcoma with
metastasis to both orbits: An unusual metastatic site. Anticancer
Res. 25:4769–4771. 2005.PubMed/NCBI
|
|
6
|
Abdalla MI, Ghaly AF and Hosni F:
Liposarcoma with orbital metastases. Case report. Br J Ophthalmol.
50:426–428. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tehrani AH, Heegaard S, Prause JU,
Fledelius HC and Daugaard S: Liposarcoma metastatic to the orbit.
Eur J Ophthalmol. 13:108–112. 2003.PubMed/NCBI
|
|
8
|
Fezza J and Sinard J: Metastatic
liposarcoma to the orbit. Am J Ophthalmol. 123:271–272. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SY, Kim HJ, Park SY, Park YH and Chung
SK: Myxoid liposarcoma involving the liver, subcutaneous tissue and
epidural space in a polycystic disease patient. Clin Nucl Med.
33:507–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogose A, Hotta T, Inoue Y, Sakata S,
Takano R and Yamamura S: Myxoid liposarcoma metastatic to the
thoracic epidural space without bone involvement: Report of two
cases. Jpn J Clin Oncol. 31:447–449. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuglø HM, Maretty-Nielsen K, Hovgaard D,
Keller JØ, Safwat AA and Petersen MM: Metastatic pattern, local
relapse and survival of patients with myxoid liposarcoma: A
retrospective study of 45 patients. Sarcoma. 2013:548–628. 2013.
View Article : Google Scholar
|
|
12
|
Cheng EY, Springfield DS and Mankin HJ:
Frequent incidence of extrapulmonary sites of initial metastasis in
patients with liposarcoma. Cancer. 75:1120–1127. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakamoto A, Fukutoku Y, Matsumoto Y,
Harimaya K, Oda Y and Iwamoto Y: Myxoid liposarcoma with negative
features on bone scan and 18F-2-fluoro-2-deoxy-D-glucose-positron
emission tomography. World J Surg Oncol. 10:2142012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conill C, Setoain X, Colomo L, Palacín A,
Combalia-Aleu A, Pomés J, Marruecos J, Vargas M and Maurel J:
Diagnostic efficacy of bone scintigraphy, magnetic resonance
imaging and positron emission tomography in bone metastases of
myxoid liposarcoma. J Magn Reson Imaging. 27:625–628. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khurana JS, Rosenthal DI, Rosenberg AE and
Mankin HJ: Skeletal metastases in liposarcoma detectable only by
magnetic resonance imaging. Clin Orthop Relat Res. 204–207.
1989.PubMed/NCBI
|
|
16
|
Ishii T, Ueda T, Myoui A, Tamai N, Hosono
N and Yoshikawa H: Unusual skeletal metastases from myxoid
liposarcoma only detectable by MR imaging. Eur Radiol. 4:L185–L191.
2003. View Article : Google Scholar
|
|
17
|
Kato S, Kawahara N, Murakami H, Demura S,
Shirai T, Tsuchiya H and Tomita K: Multi-level total en bloc
spondylectomy for solitary lumbar metastasis of myxoid liposarcoma.
Orthopedics. 33:4462010.PubMed/NCBI
|
|
18
|
Suzuki K, Matsui Y, Higashimoto M,
Kawaguchi Y, Seki S, Motomura H, Hori T, Yahara Y, Kanamori M and
Kimura T: Myxoid liposarcoma-associated EWSR1-DDIT3 selectively
represses osteoblastic and chondrocytic transcription in
multipotent mesenchymal cells. PLoS One. 7:e366822012. View Article : Google Scholar : PubMed/NCBI
|