Introduction
Human bladder cancer (BC) is the second most
frequently observed type of genitourinary cancer (1). Approximately 50% of patients diagnosed
with muscle-invasive bladder cancer (MIBC) develop distant
metastases in the lungs and liver, resulting in poor 5-year
survival rates (2,3). Currently, the advances in suitable
therapy for enhancing survival rate are limited since the
underlying mechanisms that result in tumor metastasis are not well
understood. Therefore, it is very important to clarify the key
factors mediating bladder cancer growth and metastasis and their
relative molecular mechanism for developing effective therapy.
MicroRNAs (miRNAs) are endogenous RNA molecules of
about 18–25 nucleotides in length that regulate gene expression in
a number of ways. In mammals, miRNAs are necessary for the
regulation of numerous processes, including normal development,
cell growth, differentiation, apoptosis (4). miRNAs are also known to serve
significant roles in tumorigenesis. A number of types of cancer are
associated with aberrantly expressed miRNAs. Both losses and gains
of miRNA function have been demonstrated to contribute to cancer
development and continued tumor growth (5).
miRNAs may act as both oncogenes or tumor
suppressors in different situations (6). MiR-128 is an miRNA that behaves
differently according to the tissue or cell background and has been
studied extensively in glioma. miR-128 inhibits glioma
proliferation and self-renewal by targeting Bmi-1, E2F3a and
mitogenic kinases (7–9). In addition, miR-128 inhibits tumor
growth and angiogenesis by targeting p70S6K1 (10), promotes cell-cell adhesion in U87
glioma cells via regulation of EphB2 (11) and modulates glioma progression by
regulating the SNAI1, miR-128 and SP1 axis (12). miR-128 has been demonstrated to target
polycomb repressor complexes in glioma stem cells, mediating cancer
stem cell maintenance and radioresistance (13). miR-128 has also been demonstrated to
serve a role in other types of cancer. In the majority of cases,
miR-128 acts as a tumor inhibitor and its expression is frequently
reduced in tumor cells; one of the proposed molecular mechanisms of
this reduced expression is methylation of the promotor region
(14). miR-128 reduces cell motility
and invasiveness in neuroblastoma (15) and prostate cancer (16). It has been proposed that miR-128
mediates cell apoptosis via inhibition of NTRK3 and Bax expression
and upregulation of BCL2 in neuroblastoma and embryonic kidney
cells (17,18). In ovarian cancer, glioblastoma and
breast tumor-initiating cells, overexpression of miR-128 may
promote chemosensitivity via different signaling (19–21). By
contrast, miR-128 was reported to act as an onco-miR in primary
osteosarcoma conferring metastatic potential and unfavorable
prognosis (22). However, to the best
of our knowledge, there have been no previous reports about the
role of miR-128 in bladder cancer.
Vascular endothelial growth factor-C (VEGF-C) has
been associated with angiogenesis, lymphangiogenesis and regional
lymph node metastasis and was reported to have an anti-apoptotic
and proliferative role (23). It was
reported that the involvement of VEGF-C expression in the promotion
of lymph node metastasis could be used as a decision-making
biomarker for selected patients with invasive bladder cancer who
underwent bladder-preserving radical surgery and were associated
with an anti-apoptotic phenotype (24–26). RNA
interference-mediated VEGF-C reduction suppresses malignant
progression and enhances mitomycin C sensitivity of bladder cancer
T24 cells (27). It has been observed
that miR-128 serves a critical role in human non-small cell lung
cancer tumorigenesis, angiogenesis and lymphangiogenesis by
directly targeting VEGF-C (28), but
the association between miR-128 and VEGF-C in BC remains
unknown.
In the present study, the expression levels of
miR-128 and VEGF-C were determined in BC tissues and T24 and 5637
BC cells, using reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). Subsequently, the role of miR-128 expression in
the migration and invasion of T24 and 5637 cells were investigated.
The present study aimed to investigate VEGF-C and miR-128 as novel
diagnostic or therapeutic targets for BC.
Materials and methods
Ethical statement
Prior written informed consent was obtained from all
patients and the study was approved by the Protection of Human
Subjects Committee of XiangYa Hospital of Central South University
(Changsha, China).
Patients and tissue samples
A total of 9 BC tissue samples and adjacent
non-tumorous kidney tissue counterparts were used for RT-qPCR and
western blot analysis and were collected at XiangYa Hospital of
Central South University. The hard and firm tumor tissues were
trimmed free of normal tissue and were immediately snap frozen in
liquid nitrogen. All BC cases were clinically and pathologically
confirmed to be bladder carcinoma.
Cell culture and reagents
The human bladder epithelial cell line, SV-HUC-1,
and the bladder tumor cell lines T24, 5637, 3-UM-UC-3 and RT4 were
obtained from the Cell Bank of Central South University (Changsha,
China). Cells were cultured in RPMI-1640 medium (Invitrogen Life
Technologies, Carlsbad, CA, USA) with 10% FBS (Invitrogen Life
Technologies), 50 U/ml of penicillin and 50 mg/ml of streptomycin
(Invitrogen Life Technologies). All cells were cultured in a
sterile incubator maintained at 37°C with 5% CO2.
RT-qPCR analysis
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen Life Technologies) following the manufacturer's
instructions. The relative expression level of miR-128 was
determined by RT-qPCR using mirVana™ qRT-PCR microRNA detection kit
(Ambion Life Technologies, Carlsbad, CA, USA) following the
manufacturer's instructions. Specific primer sets for miR-128
(HmiRQP3056) and U6 (HmiRQP9001) were obtained from Genecopoeia,
Inc. (Rockville, MD, USA). The expression levels of VEGF-C mRNA was
detected by RT-qPCR using the standard SYBR Green RT-PCR Kit
(Takara Bio, Inc., Otsu, Japan) following the manufacturer's
instructions. The specific primer pairs are as follows: VEGF-C,
sense 5′-CACGAGCTACCTCAGCAAGA-3 and antisense
5′-GCTGCCTGACACTGTGGTA-3′; and β-actin as an internal control,
sense 5′-AGGGGCCGGACTCGTCATACT-3′ and antisense
5′-GGCGGCACCACCATGTACCCT-3′. The relative expression levels of
VEGF-C mRNA or miR-128 were quantified using GraphPad Prism
software, version 4.0 (GraphPad Software, Inc., San Diego, CA, USA)
and the 2−ΔΔCt method (29).
Protein extraction and western blot
analysis
Western blot analysis was performed as described
previously (13). Protein levels were
quantified by Bradford assay (30). A
total of 30 mg protein from each sample was fractionated by 10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(PVDF membranes; EMD Millipore, Billerica, MA, USA). The membrane
was blocked in 0.1% Triton X-100 (Invitrogen Life Technologies) and
5% low fat milk powder (Sigma-Aldrich, St. Louis, MO, USA) in
phosphate-buffered saline for 1 h at 4°C and then probed with
rabbit polyclonal primary anti-β-actin (1:500, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or rabbit polyclonal primary
anti-VEGF-C (1:500, Immunoway, USA). After washing 3 times with
Tris-buffered saline Tween-20 (Sigma-Aldrich, the membrane was
incubated in peroxidase-conjugated goat anti-mouse/rabbit IgG
antibody (1:1,000, ImmunoWay Biotechnology, Co., Newark, DE, USA).
The bands were visualized by an enhanced chemiluminescence
detection system (Thermo Fisher Scientific, Waltham, MA, USA) using
medical X-ray films (Kodak, Rochester, NY, USA) and quantified by
Photoshop software (Adobe Systems, San Jose, CA, USA). The
intensities of the bands of interest were expressed relative to the
β-actin intensities from the same sample.
Transfection
For the miR-128 functional analysis, the
pre-miR-128, pre-control [negative control (NC) of pre-miRNA],
anti-miR-128 or anti-control (NC inhibitor) (Genecopoeia, Inc.)
constructs were transfected into T24 and 5637 cell lines using
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
manufacturer's instructions.
Cell proliferation assay
Cells in exponential growth were seeded at a final
concentration of 2×103 cells/well in 96-well plates. The
viability of the cells was evaluated by MTT assay (Invitrogen Life
Technologies) after 24, 48 and 72 h of seeding. The optical density
at 570 nm (OD570) of each well was measured with an
ELISA reader (ELX-800 type, BioTek Instruments, Inc., Winooski, VT,
USA).
Colony-formation assay
For all groups, 3 ml complete medium containing 150
cells were added to each well of a 6-well plate. The plates were
incubated at 37°C, 5% CO2 for 14 days. The cells were
then gently washed and stained with Giemsa (Invitrogen Life
Technologies). Colonies containing ≥50 cells (0.3–1.0 mm) were
counted.
Cell invasion assay
The invasive ability of bladder cancer cells was
then studied in 24-well transwell chambers coated with matrigel
(EMD Millipore). For all groups, 200 µl of 1×106
cells/ml cell suspension was added in triplicate wells. After a
24-h incubation, the dye on the membrane was dissolved with 10%
acetic acid, dispensed into 96-well plates (150 µl/well), and the
OD570 of each well was measured with an ELISA reader
(ELX-800 type; BioTek Instruments, Inc.).
Cell migration assay
The cell migratory capability was estimated using a
wound healing assay as described previously (31). In brief, cells were cultured to
confluence. Wounds of approximately 1 mm width were created with a
plastic scriber and cells were washed and incubated in a serum-free
medium. After wounding, the cells were incubated for 24 h in a
medium containing 10% fetal bovine serum. Cultures at 0 and 48 h
were observed under an inverted microscope (TS100; Nikon
Corporation, Tokyo, Japan).
Dual luciferase reporter assay
The 3′-UTR of VEGF-C (NM_005429.4) containing the
miR-128 binding sites and its corresponding mutated sequence were
cloned into the psi-CHECK2 luciferase reporter vector (Promega
Corporation, Madison, WI, USA) downstream of Renilla
luciferase, named VEGF-C-3′-UTR and VEGF-C-Mut 3′-UTR,
respectively. Using Lipofectamine 2000 (Invitrogen Life
Technologies), T24 and 5637 cells were co-transfected with the
reporter constructs and miR-128 mimics, the miR-128 inhibitor, NC
or the NC inhibitor. Luciferase activity was determined after 48 h
using the Dual-Glo substrate system (Promega Corporation) and a
Beckman Coulter LD400 luminometer (Beckman Coulter, Inc., Brea, CA,
USA). Data are presented as the ratio of experimental (Renilla)
luciferase to control (Firefly) luciferase.
Statistical analysis
Data are expressed as the mean ± standard deviation
from >3 separate experiments. Statistical analysis was performed
using SPSS software, version 15.0 (SPSS, Inc., Chicago, IL, USA).
The difference between 2 groups was analyzed by the Student's
t-test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-128 and VEGF-C in BC
tissues and cell lines
The mRNA expression levels of miR-128 and VEGF-C in
clinical tissues or in BC cell lines were determined using RT-qPCR.
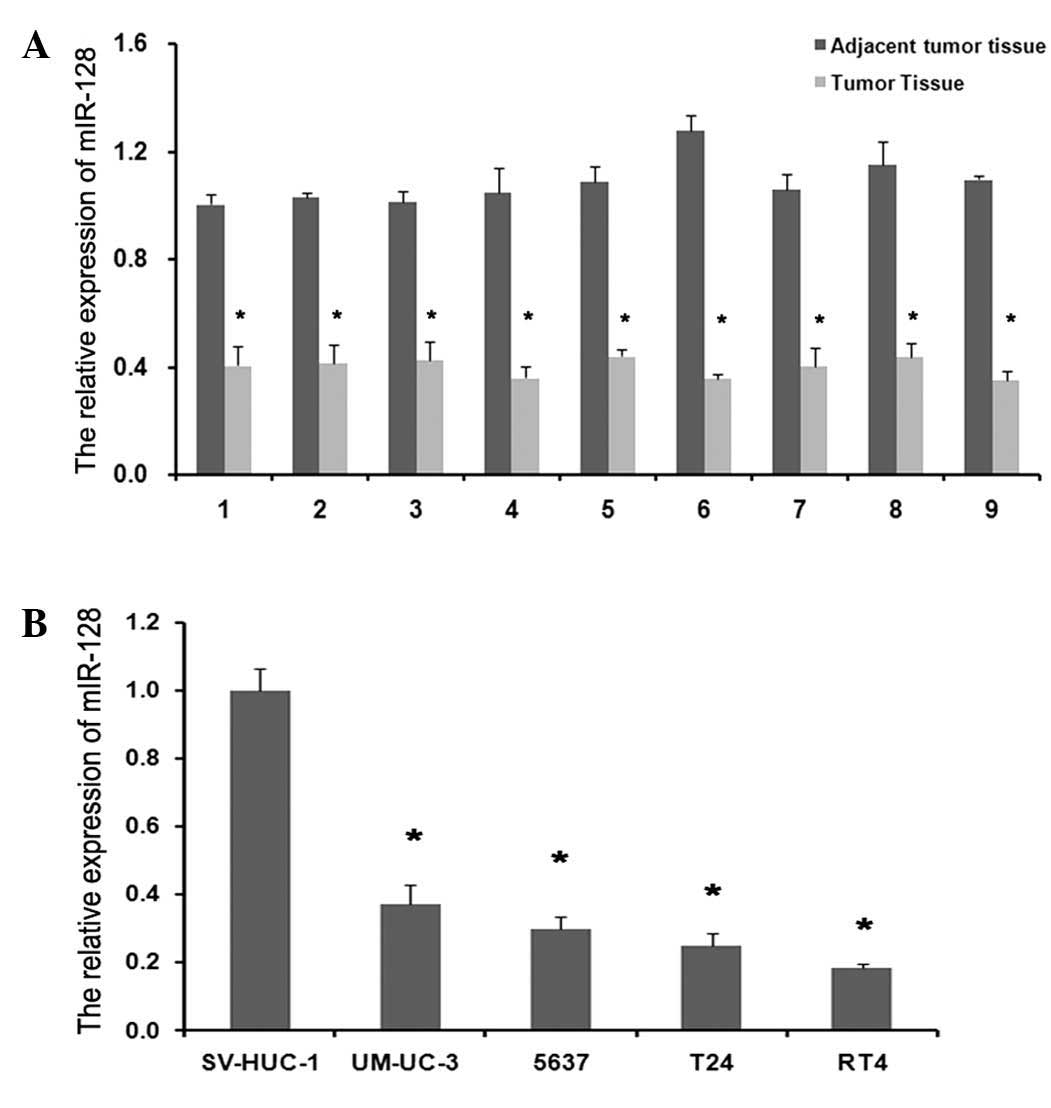
Compared with normal adjacent tissues, the mRNA expression levels
of miR-128 in tumor tissues were significantly reduced compared
with in normal tissues (Fig. 1A;
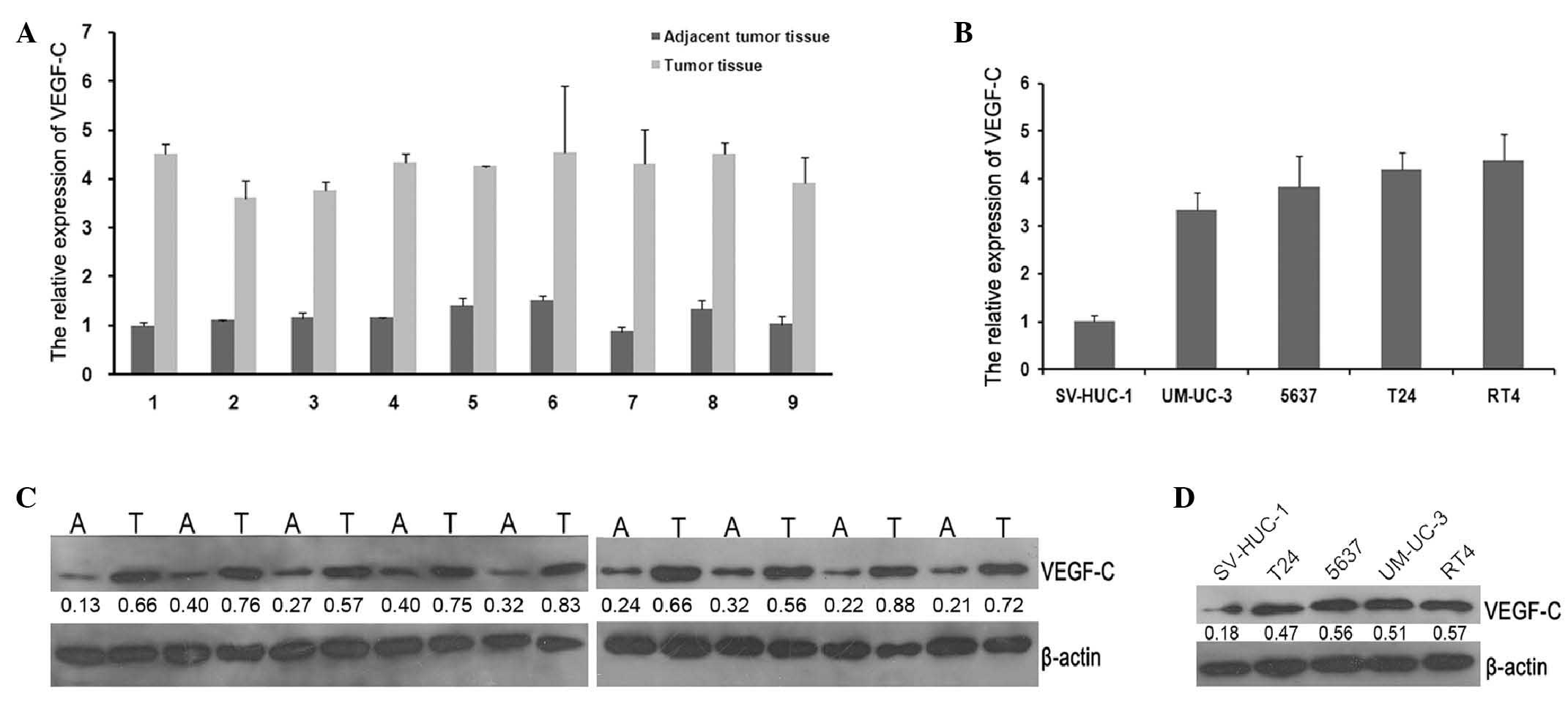
P=0.0017), while the mRNA expression levels of VEGF-C were reduced
(Fig. 2A; P=0.013). Meanwhile, the
downregulation of miR-128 and upregulation of VEGF-C were also
observed in BC cell lines compared with SV-HUC-1 (Figs. 1B and 2B; P=0.004 and P=0.003, respectively). The
VEGF-C protein expression was apparently increased in tumor tissues
or BC cell lines compared with adjacent normal tissues or SV-HUC-1
as assessed by western blot analysis (Fig. 2C and D; P=0.0023 and P=0.015,
respectively). These results indicate that miR-128 may serve an
important role in malignant progression of BC. Furthermore, the
coexistence of VEGF-C upregulation and miR-128 downregulation in BC
cells implies a potential regulatory association between the 2
molecules.
Expression of miR-128 and VEGF-C in
gain-of-function or loss-of-function model cells
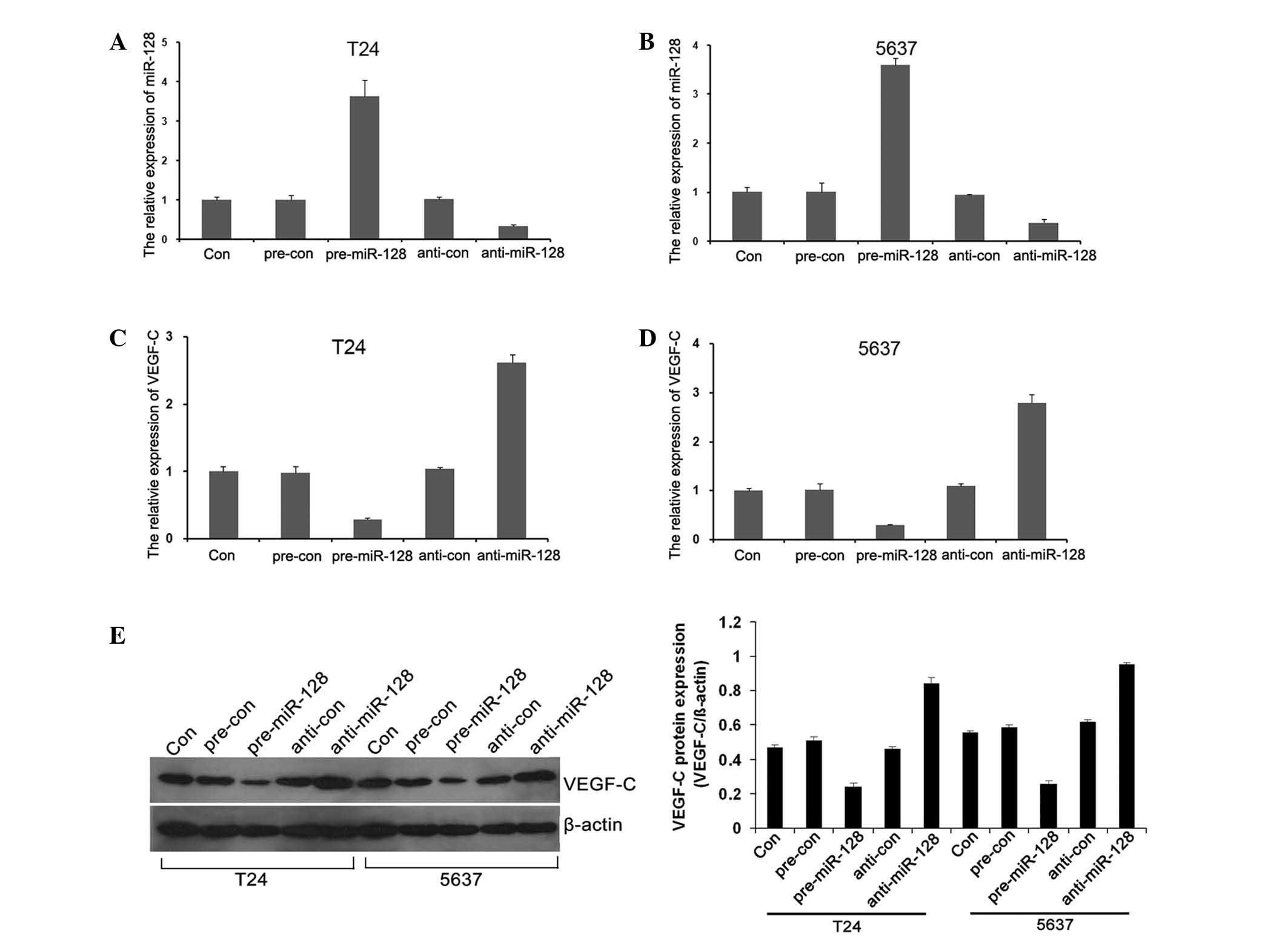
To clarify the functions of miR-128 in BC cells,
gain-of-function and loss-of-function cell models were constructed
by transfection with pre-miR-128 and anti-miR-128. The miR-128 mRNA
level was significantly upregulated (Fig.
3A and B; P=0.034 and P=0.037, respectively), while VEGF-C
level was downregulated when transfected with pre-miR-128 (Fig. 3C and D; P=0.042 and P=0.039,
respectively). By contrast, when transfected with anti-miR-128, the
mRNA level of miR-128 was significantly downregulated (Fig. 3A and B; P=0.034 and P=0.037,
respectively) and VEGF-C was significantly upregulated (Fig. 3C and D; P=0.042 and P=0.039,
respectively). The results indicated that overexpression of miR-128
reduced the VEGF-C expression in T24 and 5637 cells (Fig. 3E; P=0.044).
VEGF-C is a direct target of miR-128
in T24 and 5637 cells
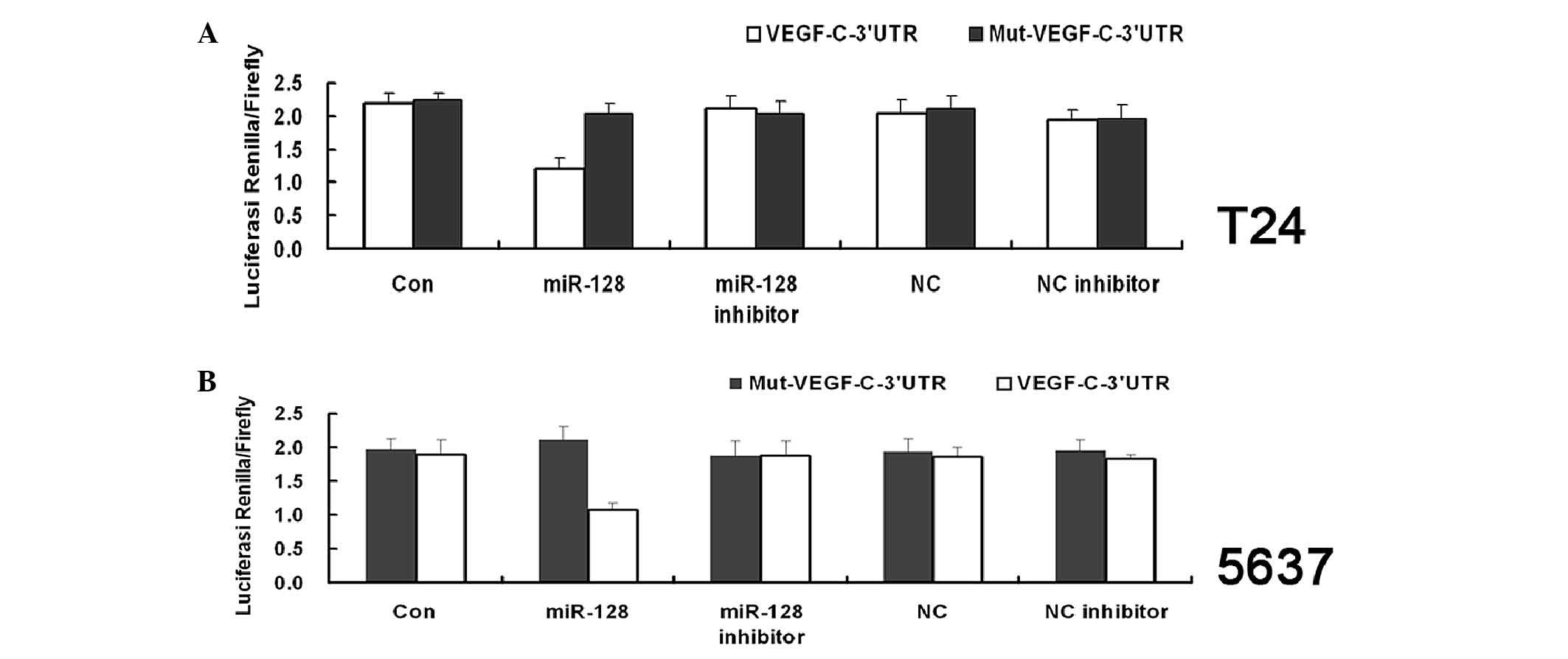
To assess whether VEGF-C is a direct target of
miR-128, luciferase reporter assays were applied. The VEGF-C 3′-UTR
fragment containing the miR-128 binding site and mutated targeting
sequence were cloned into psi-CHECK2 dual luciferase reporter
vectors. The miR-128 signifcantly inhibited the luciferase activity
in both T24 and 5637 cells transfected with the VEGF-C-3′-UTR.
However, miR-128 mimics did not suppress the luciferase activity
levels in the T24 and 5637 cells transfected with Mut-VEGF-C-3′-UTR
(Fig. 4A and B; P=0.024 and P=0.027,
respectively). These findings indicate that VEGF-C is a direct
target of miR-128.
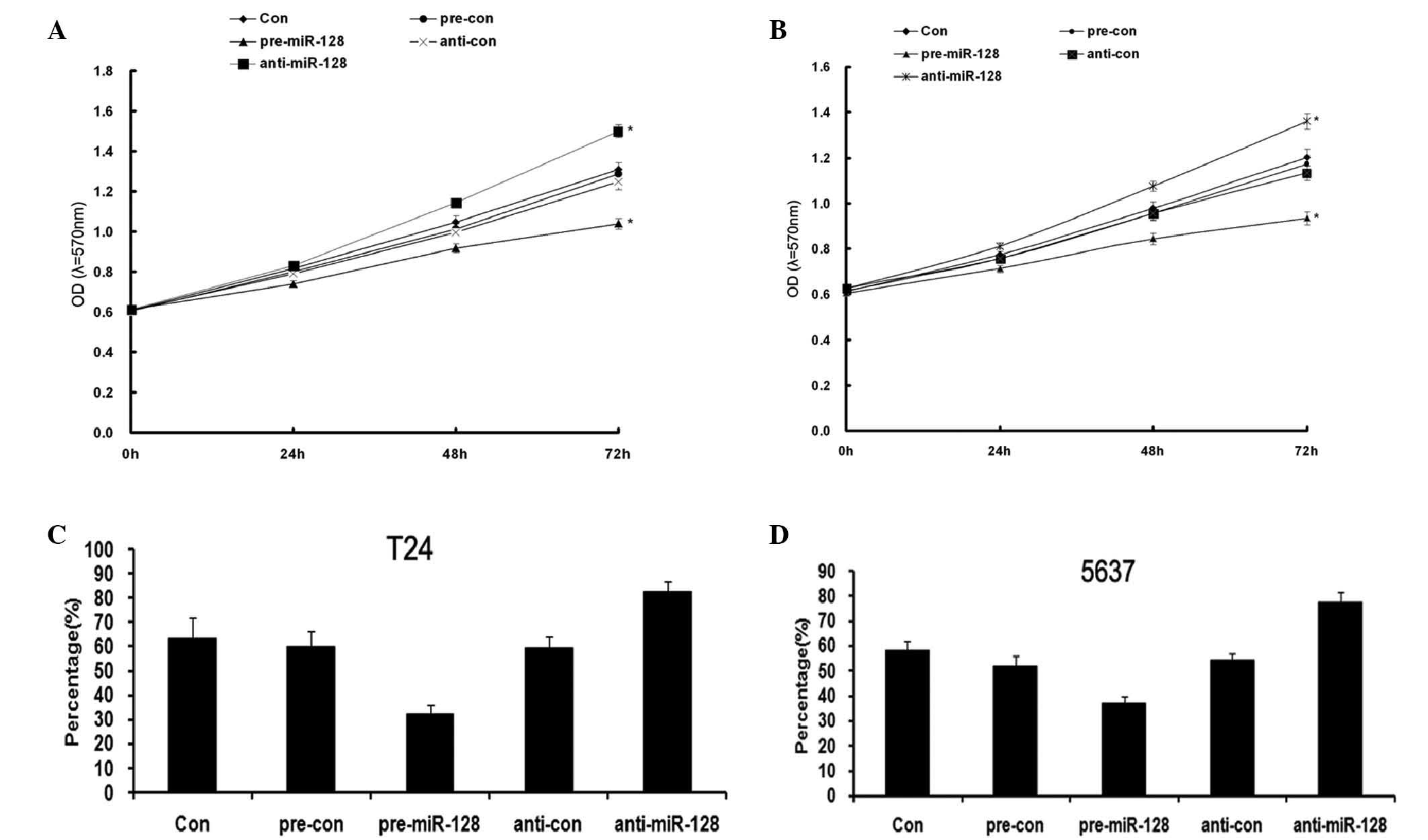
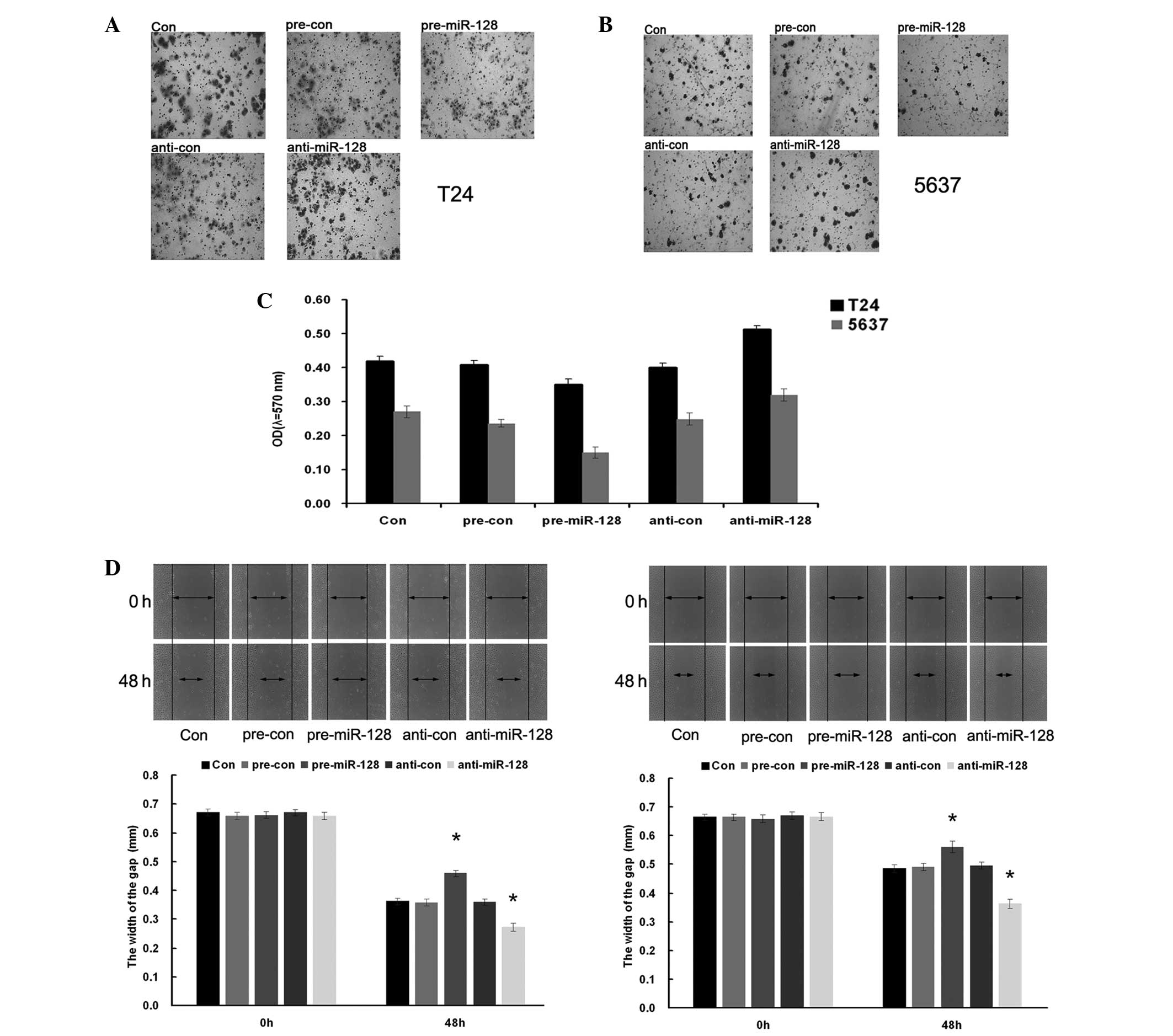
miR-128 inhibiting proliferation of
bladder cancer cells
To investigate the effects of miR-128 on
proliferation of BC cells, T24 and 5637 cells were transfected with
miR-128 mimics or its inhibitor. MTT and colony formation assays
were used to demonstrate that overexpression of miR-128 markedly
reduced the growth (Fig. 5A and B;
P=0.0017 and P=0.015, respectively) and clone-formation (Fig. 5C and D; P=0.035 and P=0.038,
respectively) rate of the 2 BC cell lines as compared with that of
negative control (NC) transfected cells. However, inhibition of
miR-128 increased the growth rate of the 2 BC cell lines as
compared with that of NC transfected cells. These results indicated
that miR-128 inhibited the proliferation of BC T24 and 5637
cells.
miR-128 suppressing migration and
invasion of bladder cancer cells
To explore the functional role of miR-128 on
migration and invasion in BC cells, T24 and 5637 cells were
transfected with pre-miR-28 and anti-miR-128, respectively. Wound
healing and transwell assays were performed to evaluate the cell
metastasis capacity. The results demonstrated that cell migration
and invasion capacity were significantly increased when the BC
cells were transfected with pre-miR-128, while cell migration and
invasion capacity were significantly reduced when cells were
transfected with anti-miR-128 (Fig.
6C, P=0.037 and P=0.026, T24 and 5637 cells, respectively;
Fig. 6D, P=0.031; Fig 6E, P=0.035). The results indicated that
miR-128 supressed the migration and invasion of bladder cancer T24
and 5637 cells.
Discussion
miR-128 is an miR that behaves differently according
to the tissue or cell background. In the majority of cases, miR-128
acts as a tumor inhibitor and its biological functions range from
mediating cell proliferation to migration, apoptosis and
chemoresistance. However, there is a lack of studies investigating
the deregulation of miR-128 and functional role in BC is very
limited.
In the present study, the mRNA level of miR-128 was
investigated in 9 cases of BC and their adjacent tissues. The
results demonstrated that miR-128 was reduced significantly in BC
tumor tissues compared with their adjacent tissues. by contrast,
miR-128 was also downregulated in the 4 examined BC cell lines
compared with normal bladder epithelial cells. The results indicate
that miR-128 may be a tumor inhibitor in BC.
To investigate the biological function of miR-128 in
BC, gain-of-function and loss-of-function cell models were
constructed via transfection of pre-miR-128 and anti-miR-128 into
T24 and 5637 BC cell lines, respectively. Using MTT, invasion,
wound healing and colony-formation assays, it was demonstrated that
overexpression of miR-128 inhibited growth rate, proliferation,
migration and invasion capacities. These results were consistent
with the results of previous studies (9,15,32,33). The
involvement in cell apoptosis and chemosensitivity of miR-128 was
also investigated, however no significant differences were observed
between parental cells and the functional model cells.
VEGF-C has been associated with angiogenesis,
lymphangiogenesis and regional lymph node metastasis and has also
been reported to serve an anti-apoptotic and proliferative role
(23). Bioinformatics software has
been used to predict that VEGF-C is one of the target genes of
miR-128 and it has been verified that miR-128 serves a critical
role in human non-small cell lung cancer tumorigenesis,
angiogenesis and lymphangiogenesis by directly targeting VEGF-C
(28), however the association
between miR-128 and VEGF-C in BC remains unknown. Using the Dual
Luciferase Reporter Assay, a direct target relationship between
miR-128 and VEGF-C was revealed in both T24 and 5637 cell lines.
How does miR-128 exert its mediating effect on cell metastasis and
proliferation? Is VEGF-C is a direct downstream factor in mediating
BC cell proliferation and metastasis? To answer these questions,
construction of VEGF-C knockout stable cell lines may be an
effective method for future experiments. If overexpression of
miR-128 in VEGF-C knockout stable cell lines results in less of an
inhibitory effect on cell proliferation and metastasis than in T24
and 5637 cell lines, then one may conclude that miR-128 serves a
critical role in BC proliferation, migration and invasion by
directly targeting VEGF-C. This hypothesis should be investigated
in future studies.
In conclusion, the present study provides evidence
demonstrating that miR-128 is significantly downregulated and
VEGF-C is significantly upregulated in BC tissues and cells. In
addition, it was demonstrated that miR-128 directly targets VEGF-C
in BC cells. The results of the present study support
miR-128/VEGF-C pairs as novel diagnostic or therapeutic target for
BC. Although the study provides a possible molecular mechanism for
miR-128 mediated VEGF-C signaling pathway affecting BC progression,
further studies are required to elucidate the details of this
process.
Acknowledgements
The present study was supported by Fundamental
Research Funds for the Central Universities of Central South
University (grant no. 2013zzts087).
References
|
1
|
Hussain SA and James ND: The systemic
treatment of advanced and metastatic bladder cancer. Lancet Oncol.
4:489–497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Overdevest JB, Thomas S, Kristiansen G,
Hansel DE, Smith SC and Theodorescu D: CD24 offers a therapeutic
target for control of bladder cancer metastasis based on a
requirement for lung colonization. Cancer Res. 71:3802–3811. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Huang Z, Zhu Z, Zheng X, Liu J,
Han Z, Ma X and Zhang Y: Upregulated UHRF1 promotes bladder cancer
cell invasion by epigenetic silencing of KiSS1. PLoS One.
9:e1042522014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kusenda B, Mraz M, Mayer J and Pospisilova
S: MicroRNA biogenesis, functionality and cancer relevance. Biomed
Pap Med Fac Univ Palacky Olomouc Czech Repub. 150:205–215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zabolotneva AA, Zhavoronkov A, Garazha AV,
Roumiantsev SA and Buzdin AA: Characteristic patterns of microRNA
expression in human bladder cancer. Front Genet. 3:3102013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca
EA and Lawler S: Targeting of the Bmi-1 oncogene/stem cell renewal
factor by microRNA-128 inhibits glioma proliferation and
self-renewal. Cancer Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papagiannakopoulos T, Friedmann-Morvinski
D, Neveu P, Dugas JC, Gill RM, Huillard E, Liu C, Zong H, Rowitch
DH, Barres BA, et al: Pro-neural miR-128 is a glioma tumor
suppressor that targets mitogenic kinases. Oncogene. 31:1884–1895.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Chao T, Li R, Liu W, Chen Y, Yan
X, Gong Y, Yin B, Liu W, Qiang B, et al: MicroRNA-128 inhibits
glioma cells proliferation by targeting transcription factor E2F3a.
J Mol Med (Berl). 87:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi ZM, Wang J, Yan Z, You YP, Li CY, Qian
X, Yin Y, Zhao P, Wang YY, Wang XF, et al: MiR-128 inhibits tumor
growth and angiogenesis by targeting p70S6K1. PLoS One.
7:e327092012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin L, Chen X, Peng X, Zhou J, Kung HF,
Lin MC and Jiang S: MicroRNA-128 promotes cell-cell adhesion in U87
glioma cells via regulation of EphB2. Oncol Rep. 30:1239–1248.
2013.PubMed/NCBI
|
|
12
|
Dong Q, Cai N, Tao T, Zhang R, Yan W, Li
R, Zhang J, Luo H, Shi Y, Luan W, et al: An axis involving SNAI1,
microRNA-128 and SP1 modulates glioma progression. PLoS One.
9:e986512014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peruzzi P, Bronisz A, Nowicki MO, Wang Y,
Ogawa D, Price R, Nakano I, Kwon CH, Hayes J, Lawler SE, et al:
MicroRNA-128 coordinately targets polycomb repressor complexes in
glioma stem cells. Neuro Oncol. 15:1212–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi Y, Iwaya T, Sawada G, Kurashige
J, Matsumura T, Uchi R, Ueo H, Takano Y, Eguchi H, Sudo T, et al:
Up-regulation of NEK2 by microRNA-128 methylation is associated
with poor prognosis in colorectal cancer. Ann Surg Oncol.
21:205–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evangelisti C, Florian MC, Massimi I,
Dominici C, Giannini G, Galardi S, Buè MC, Massalini S, McDowell
HP, Messi E, et al: MiR-128 up-regulation inhibits Reelin and DCX
expression and reduces neuroblastoma cell motility and
invasiveness. FASEB J. 23:4276–4287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan AP, Poisson LM, Bhat VB, Fermin D,
Zhao R, Kalyana-Sundaram S, Michailidis G, Nesvizhskii AI, Omenn
GS, Chinnaiyan AM and Sreekumar A: Quantitative proteomic profiling
of prostate cancer reveals a role for miR-128 in prostate cancer.
Mol Cell Proteomics. 9:298–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adlakha YK and Saini N: MicroRNA-128
downregulates Bax and induces apoptosis in human embryonic kidney
cells. Cell Mol Life Sci. 68:1415–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guidi M, Muiños-Gimeno M, Kagerbauer B,
Martí E, Estivill X and Espinosa-Parrilla Y: Overexpression of
miR-128 specifically inhibits the truncated isoform of NTRK3 and
upregulates BCL2 in SH-SY5Y neuroblastoma cells. BMC Mol Biol.
11:952010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Chen H, Wu N, Zhang WJ and Shang LX:
Deregulation of miR-128 in ovarian cancer promotes cisplatin
resistance. Int J Gynecol Cancer. 24:1381–1388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
She X, Yu Z, Cui Y, Lei Q, Wang Z, Xu G,
Xiang J, Wu M and Li G: MiR-128 and miR-149 enhance the
chemosensitivity of temozolomide by Rap1B-mediated cytoskeletal
remodeling in glioblastoma. Oncol Rep. 32:957–964. 2014.PubMed/NCBI
|
|
21
|
Zhu Y, Yu F, Jiao Y, Feng J, Tang W, Yao
H, Gong C, Chen J, Su F, Zhang Y and Song E: Reduced miR-128 in
breast tumor-initiating cells induces chemotherapeutic resistance
via Bmi-1 and ABCC5. Clin Cancer Res. 17:7105–7115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian Z, Guo B, Yu M, Wang C, Zhang H,
Liang Q, Jiang K and Cao L: Upregulation of micro-ribonucleic
acid-128 cooperating with downregulation of PTEN confers metastatic
potential and unfavorable prognosis in patients with primary
osteosarcoma. Onco Targets Ther. 7:1601–1608. 2014.PubMed/NCBI
|
|
23
|
Mylona E, Magkou C, Gorantonakis G,
Giannopoulou I, Nomikos A, Zarogiannos A, Zervas A and Nakopoulou
L: Evaluation of the vascular endothelial growth factor (VEGF)-C
role in urothelial carcinomas of the bladder. Anticancer Res.
26:3567–3571. 2006.PubMed/NCBI
|
|
24
|
Li Z, Qi F, Qi L, Zhang H, Chen M, Wang L
and Zu X: VEGF-C as a decision-making biomarker for selected
patients with invasive bladder cancer who underwent
bladder-preserving radical surgery. Arch Med Res. 42:405–411. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki K, Morita T and Tokue A: Vascular
endothelial growth factor-C (VEGF-C) expression predicts lymph node
metastasis of transitional cell carcinoma of the bladder. Int J
Urol. 12:152–158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zu X, Tang Z, Li Y, Gao N, Ding J and Qi
L: Vascular endothelial growth factor-C expression in bladder
transitional cell cancer and its relationship to lymph node
metastasis. BJU Int. 98:1090–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HH, Qi F, Shi YR, Miao JG, Zhou M,
He W, Chen MF, Li Y, Zu XB and Qi L: RNA interference-mediated
vascular endothelial growth factor-C reduction suppresses malignant
progression and enhances mitomycin C sensitivity of bladder cancer
T24 cells. Cancer Biother Radiopharm. 27:291–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G,
Fu S, Zhang Y, Feng K and Feng Y: microRNA-128 plays a critical
role in human non-small cell lung cancer tumourigenesis,
angiogenesis and lymphangiogenesis by directly targeting vascular
endothelial growth factor-C. Eur J Cancer. 50:2336–2350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barbosa H, Slater NK and Marcos JC:
Protein quantification in the presence of poly(ethylene glycol) and
dextran using the Bradford method. Anal Biochem. 395:108–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen L, Chen XD and Zhang YH: MicroRNA-128
promotes proliferation in osteosarcoma cells by downregulating
PTEN. Tumour Biol. 35:2069–2074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tao T, Li G, Dong Q, Liu D, Liu C, Han D,
Huang Y, Chen S, Xu B and Chen M: Loss of SNAIL inhibits cellular
growth and metabolism through the miR-128-mediated
RPS6KB1/HIF-1α/PKM2 signaling pathway in prostate cancer cells.
Tumour Biol. 35:8543–8550. 2014. View Article : Google Scholar : PubMed/NCBI
|