Introduction
Prostate cancer is the most common malignancy among
males worldwide, and is the second leading cause of cancer-related
mortality among men in United States (1). Bone metastases occur in ~90% of the
patients presenting with advanced prostate cancer, and there is a
direct correlation between the burden of metastases and survival
(2,3).
Although numerous effective systematic agents for males with bone
metastatic prostate cancer exist, including endocrine therapy for
non-hormone-resistant prostate cancer (HRPC) and second-line
hormonal therapy and chemotherapy for HRPC and castration-resistant
prostate cancer, the clinicians must decide how to sequence these
options to maximise their benefit for the patients; what images
must be captured prior and post-treatment in order to assess
disease status; and how to interpret these images and use them to
guide management. However, identifying whether the selected agent
is effective for the treatment of prostate cancer with bone
metastases is challenging, often due to the uncertainty of
interpreting the post-therapy alterations observed on bone
scintigraphy (BS) and computed tomography (CT) images as a
consequence of the ‘flare’ phenomenon (4,5). This
paradoxical phenomenon refers to an improvement on the levels of
prostate-specific antigen (PSA) and pain in patients with bone
metastases, which may be accompanied by an initial apparent
deterioration of certain lesions or the detection of novel lesions
on the images (6).
According to previous reports, MRI has become a
promising method to assess the therapeutic response and guide
treatment decisions in patients affected by prostate cancer
(7). However, the ‘flare’ phenomenon
on MRI has not been reported thus far. In the present report, the
first case of a false-positive diagnosis of disease progression on
MRI follow-up during systematic therapy of HRPC with bone
metastases is described. This case showed that the images captured
during the follow-up of patients treated for prostate cancer, which
are aimed at assessing the tumor burden and response to therapy,
are to be interpreted with caution, in order to avoid a
false-positive diagnosis of disease progression and the consequent
inappropriate discontinuation of an efficacious therapy. In the
present report, the pitfalls of images of bone metastases in
patients with prostate cancer were reviewed, including the ‘flare’
phenomenon on BS and CT, and marrow reconversion on MRI. In
addition, potential mechanisms that account for these phenomena
were proposed, and subsequent treatment assessment was suggested.
Future studies require a more accurate assessment of treatment
response in cases of prostate cancer presenting with bone
metastases.
Case report
A 67-year-old male, diagnosed in February 2010 with
prostate adenocarcinoma with Gleason 9 (5+4), T4 (infiltration of
the posterior urethra), was admitted in June 2011 to the Department
of Radiation Oncology of the Shandong Cancer Hospital and Institute
at Shandong University (Jinan, Shandong, China) for biochemical
progression, following radical prostatectomy. The medical history
of the patient included hypercholesterolemia and moderate
hypertension treated with betaloc and nifedipine controlled-release
tablets (Adalat CC; Bayer China Ltd., Shanghai, China). The levels
of PSA in serum at the time of diagnosis were 9.56 ng/ml (normal
range, 0.0–4.0 ng/ml). Following radical prostatectomy, the patient
remained asymptomatic with low PSA levels for 15 months (PSA
minimum 0.004 ng/ml). Since the patient had not received any
androgen deprivation therapy, the risk of recurrence was high, and
at the time of admission, the patient was diagnosed with
biochemical progression and an increased PSA of 13.93 ng/ml. To
ascertain the patient's progress, a whole-body positron emission
tomography (PET)/CT was conducted that revealed metastases of the
T5 vertebral body, which was confirmed by MRI. In consequence,
palliative radiotherapy of the metastatic T5 vertebral body and
combined androgen blockade therapy were initiated, comprising
lutein-releasing hormone analog (Zoladex; AstraZeneca, Shanghai,
China) 3.6 mg/28 days and bicalutamide 50 mg/day. Additionally,
zoledronic acid was administered intravenously every 3–4 weeks.
Following treatment, the patient was at castration testosterone
level (<50 ng/dl) persistently, and his PSA levels reduced to a
minimum of 0.013 ng/ml.
However, 3 months later, increased levels of PSA
were measured, reaching a maximum of 10.73 ng/ml (similar to the
values presented prior to chemotherapy), and the patient also
suffered from bone pain. The PET/CT and MRI reevaluation revealed
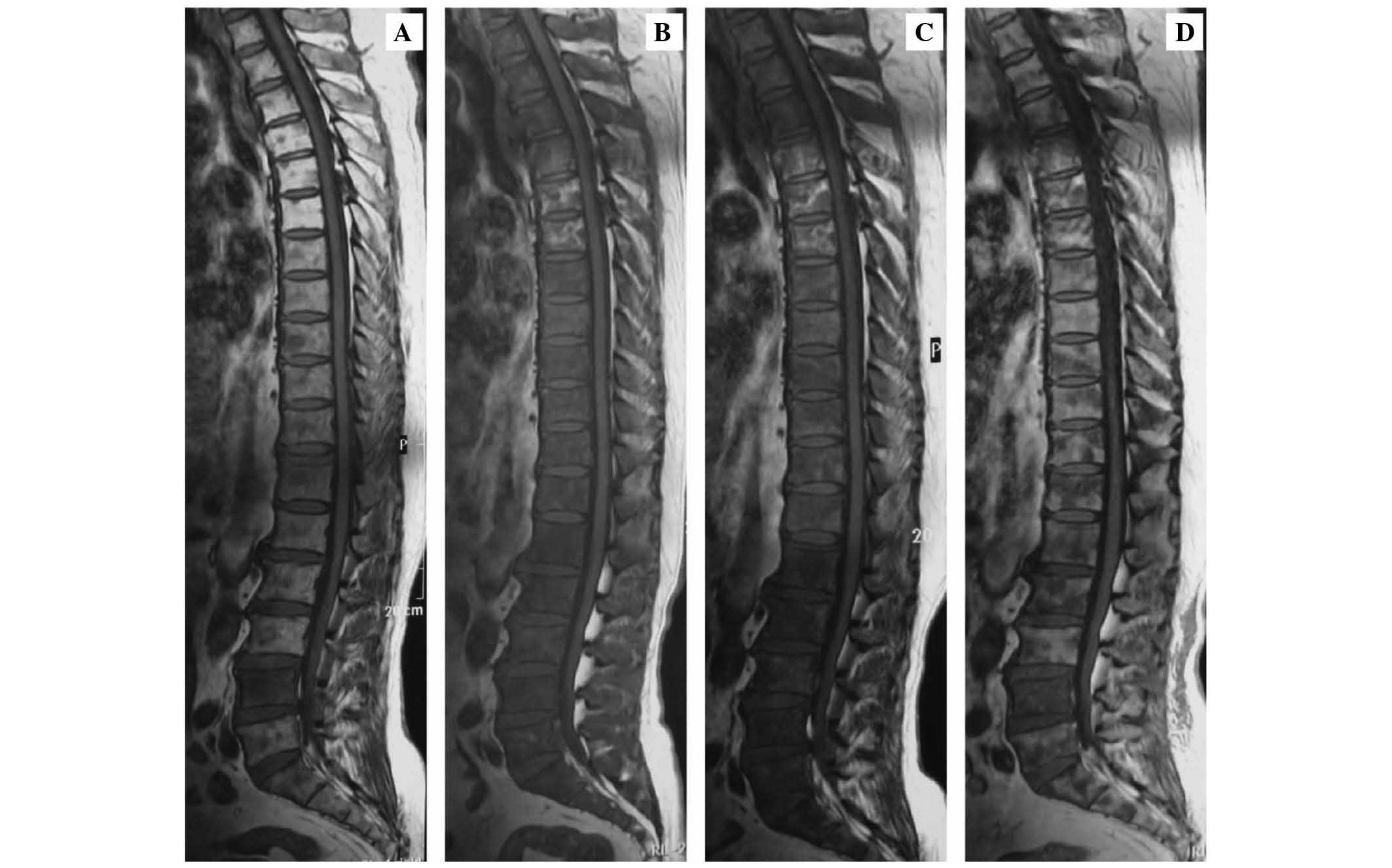
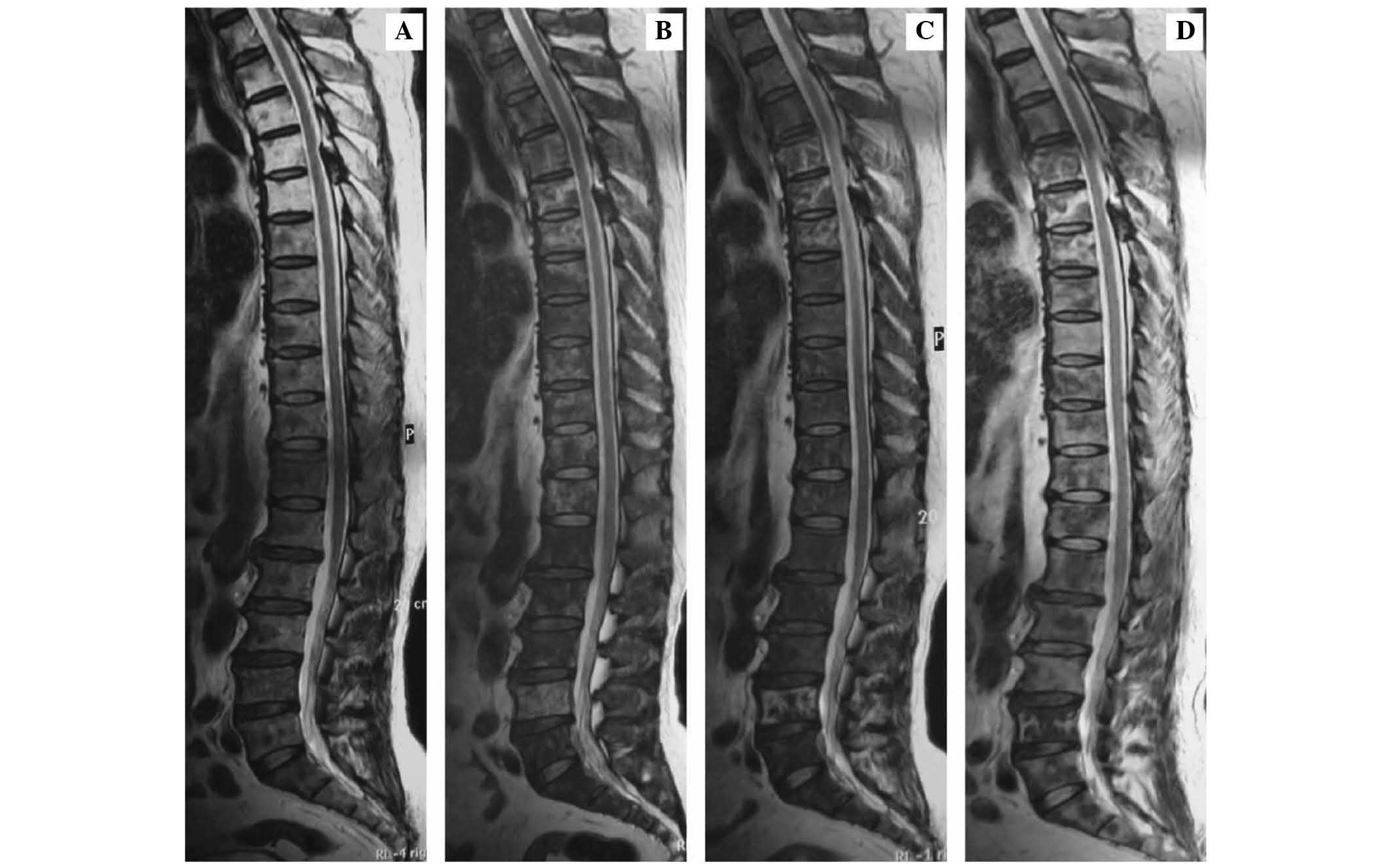
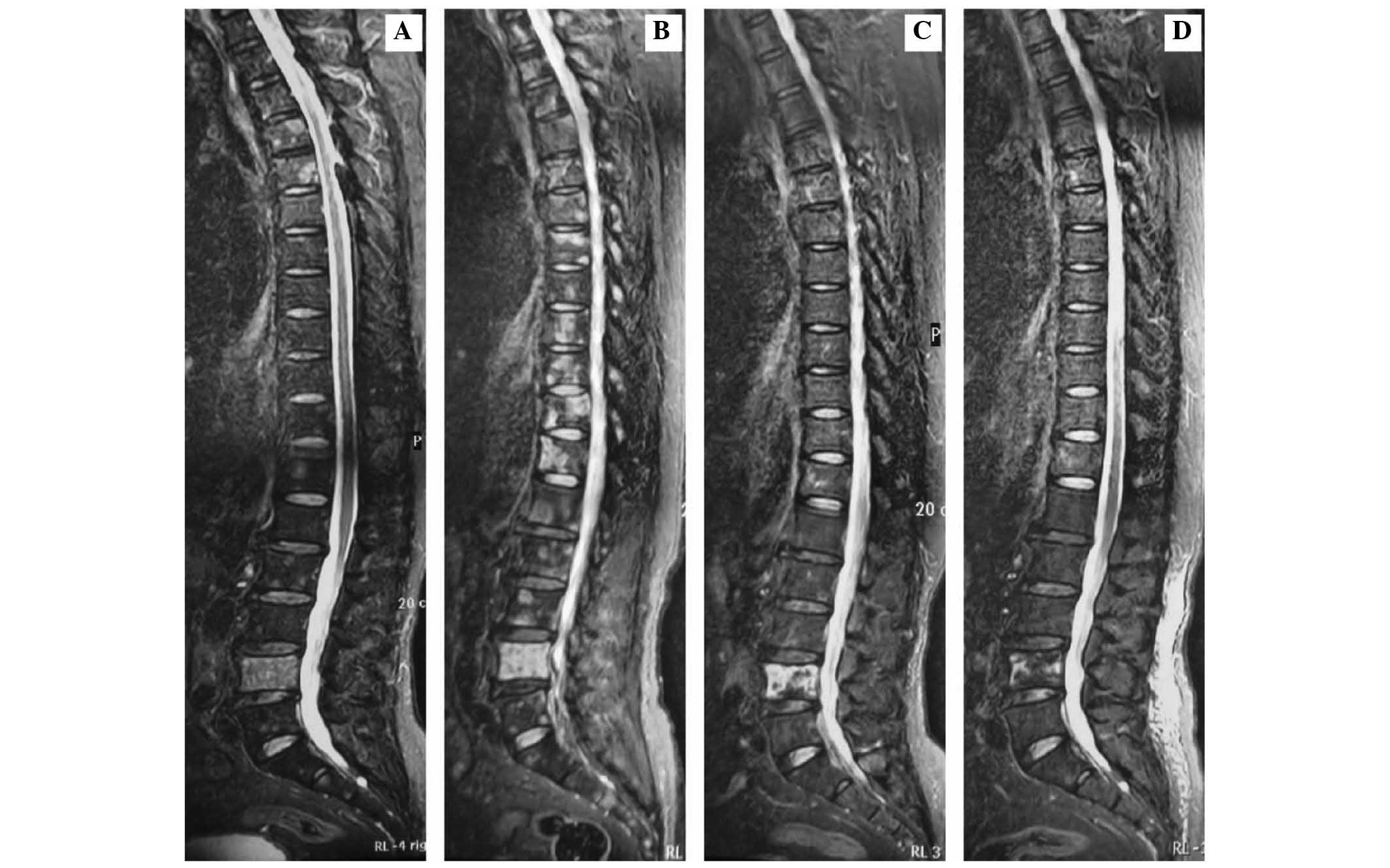
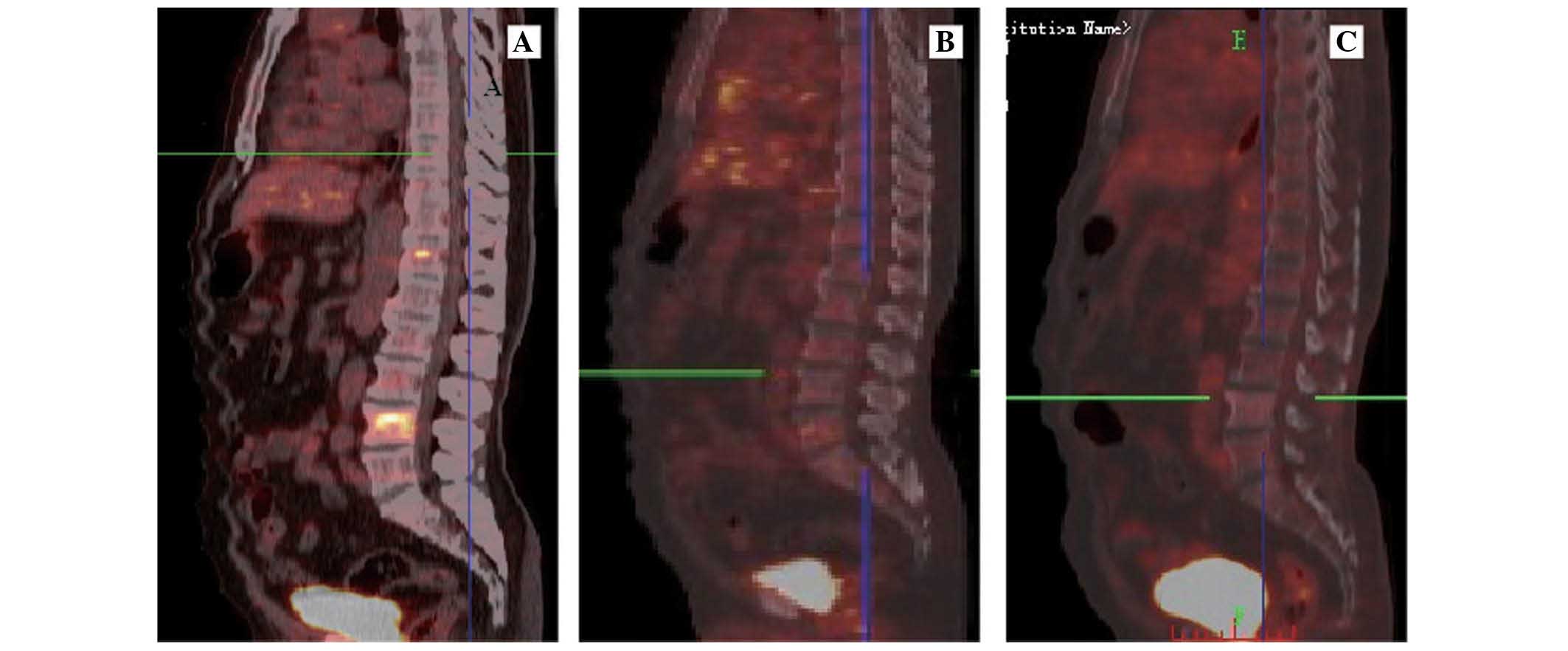
metastases in T4-12, L1–5 and S1–2 vertebral bodies (Figs. 1A, 2A,
3A and 4A), indicating disease progression.
The patient was then suggested the possibility of
receiving second-line hormone therapy for HRPC. However, the
patient refused this option, and in consequence, was then treated
every 3 weeks with docetaxel (75 mg/m2, day 1) and
prednisone (5 mg bid, days 1–21). The patient also received
bilateral orchiectomy of his own accord, and discontinued the
combined androgen blockade therapy following the second cycle of
therapy. Since the patient suffered severe neutropenia during this
period, granulocyte colony-stimulating factor (G-CSF) was used as
an adjunct to the systematical therapy following every cycle of
chemotherapy. Additionally, moderate anemia (hemoglobin, 78 g/l)
was observed subsequently to the second cycle of chemotherapy;
thus, erythropoietin was used during this interval.
Tumor re-evaluation was performed prior to the
administration of the third cycle of chemotherapy. The MRI revealed
diffuse abnormal signal in almost all the vertebral bodies on T1-,
T2-weighted and short TI inversion recovery (STIR) sequence images
(Figs. 1B, 2B and 3B).
Concomitantly, bone pain relieved, and the PSA levels reduced
>50%, to 3.54 ng/ml.
These parameters were combined to assess the
therapeutic response. Since the treatment was beneficial to the
patient, it was continued, but the dose of docetaxel was reduced to
60 mg/m2 due to the severe neutropenia, and the use of
G-CSF as an adjunct following each cycle of the chemotherapy was
maintained. At the second re-evaluation following 6 cycles of
chemotherapy, the bone lesions on PET/CT had apparently improved
(Fig. 4B), compared with the PET-CT
results prior to the chemotherapy. The MRI examinations also
revealed diffuse signal abnormality (Fig.
1C), which was stable, compared to the T1-weighted images prior
to chemotherapy (Fig. 1A); diffuse
signal abnormality on T2-weighted images (Fig. 2C); and focal signal abnormality on
STIR sequence images (Fig. 3C),
demonstrating a marked improvement, compared to the images captured
prior to the treatment with chemotherapy (Figs. 1A and 3A).
Based on results derived from the TAX327 study
(8,9),
the chemotherapy was discontinued following the tenth cycle.
Subsequently, MRI was conducted, which revealed focal abnormal
signal (Figs. 1D, 2D and 3D),
indicating an improvement, compared to the MRI images captured
prior to the chemotherapy treatment (Figs. 1A, 2A
and 3A).
Zoledronic acid was then administered to the patient
intermittently every 3–4 weeks, and 1 year later, the follow-up
PET/CT revealed no abnormality on the previous bone metastatic
position (Fig. 4C). Currently, the
patient is alive without any osseous symptoms.
Discussion
The accurate assessment of treatment response
regarding prostate cancer with bone metastases is crucial. However,
unexpected findings on clinical images may occasionally be
encountered, which may complicate the diagnosis when the
radiologists attempt to interpret these examinations. The present
article reviewed the pitfalls of various images aimed to assess the
treatment response in patients with prostate cancer presenting with
bone metastases, in the context of their mechanisms, and explores
how to recognize the false positive images from the true positive
ones, in order to accurately assess the therapeutic response.
‘Flare’ phenomenon
Since the ‘flare’ phenomenon was first observed in
1972 (10), other studies reporting
similar observations have emerged (4,5). The
‘flare’ phenomenon was defined as an early successful treatment of
patients with bone metastases that may be accompanied by an initial
apparent deterioration of certain lesions or the appearance of
novel lesions on the clinical images, followed by improvement
(6). This phenomenon is frequently
observed in patients with breast and prostate cancer with bone
metastases, following systematic therapy such as endocrine therapy
and chemotherapy. It is important to highlight the ‘flare’
phenomenon in order to avoid a false decision on the basis of a
potentially erroneous interpretation of the images in clinical
practice.
The ‘flare’ phenomenon would usually emerge between
2 weeks and 3 months subsequent to the initiation of the
efficacious therapy, with reported frequencies of 6–25% in patients
with prostate cancer metastases, and 33% in patients with treated
breast metastases (11). Thus,
consensus criterion such as that provided by the Prostate Cancer
Working Group 2 (12)indicates that
disease progression of bone metastatic patients requires a
confirmatory scan that reveals additional lesions, compared to the
first follow-up scan, which must be performed ≥6 weeks later,
whereas the first follow-up scan is not recommended to be conducted
until 12 weeks since the initial date of the treatment, due to the
‘flare’ phenomenon (12).
Conventional radiography, BS and CT rely on the
activation of bone cells (osteoblasts and osteoclast) to detect
modifications in the bone trabeculae as a result of neoplastic
lesions (13). A possible mechanism
for the ‘flare’ phenomenon is the osteoblastic healing of the bone
metastases (14), which has been
demonstrated by Messiou et al (11) and Hashisako et al (15). This mechanism may also explain the
‘flare’ phenomenon on CT, which is capable of differentiating
osteoblastic alterations by itself (5). Another mechanism proposed by Cook et
al (16) suggests that the
‘flare’ phenomenon would amplify the signal and improve the
sensitivity and specificity to detect the occult lesions existing
prior to the initiation of the treatment. In their studies, the
bones of patients thought not to suffer of bone metastasis on BS
were demonstrated to be affected, following an efficacious
treatment (16). This possible
mechanism of ‘flare’ phenomenon may be explained by the fact that
the occult lesions need time to become visible on BS and CT images.
In this regard, the prognostic significance of the ‘flare’
phenomenon must be considered, since certain undetected lesions,
which may have been present prior to the treatment, may respond to
the treatment. Janicek et al (17) highlighted that the ‘flare’ phenomenon
on BS is a favorable response to therapy not associated with
overall survival. Nonetheless, future studies are required to
evaluate the prognostic significance of the ‘flare’ phenomenon.
Marrow reconversion on MRI
MRI is sensitive to the early modifications in bone
marrow that precede the osteoclastic/osteoblastic response of the
bone matrix to tumor infiltration, prior to bone trabeculae or
cortices being affected by the disease (18). A prospective study has determined the
sensitivity and specificity to detect the metastatic lesions to be
100 and 88% for MRI, and 46 and 32% for BS, respectively (18). Thus, MRI has become a superior tool
than BS and CT for the detection and characterization of numerous
neoplastic lesions involving the skeleton.
However, on MRI, marrow reconversion would mimic
malignancy, since the malignancy and the red marrow exhibit similar
signal variations on MRI (19). There
are two main types of bone marrow, red and yellow. Yellow marrow is
mainly composed of fat cells with few hematopoietic cells, while
red marrow is mainly composed of hematopoietic cells. Yellow marrow
appears hyperintense on T1-weighted imaging, and hypointense on
T2-weighted imaging, whereas red marrow exhibits an intermediate
signal intensity on T1- and T2-weighted images, and exhibits a T1
signal of relatively lower intensity, compared to yellow marrow. On
STIR, red marrow displays an intermediate signal that is more
intense than fatty marrow and subcutaneous fat, and similar in
signal intensity to muscle (20).
Bone metastases are hypointense on T1-weighted images due to their
high sensitivity in detecting fatty marrow replacement by
neoplastic elements, with a high contrast between the low signal
intensity of the lesions and the high signal intensity of the
surrounding tissues. In addition, bone metastases usually exhibit
T2 and STIR hyperintensity (19).
Therefore, it is easy to confound marrow reconversion with bone
metastasis on MRI.
As the healthy human skeleton matures, a red-yellow
marrow conversion begins in childhood, and is usually completed at
25 years of age (21). Generally,
red-yellow marrow conversion proceeds from distal to proximal areas
in the limbs. In adults, the largest areas of red marrow remain in
the vertebrae, pelvis, ribs and sternum, with visible red marrow in
the proximal shafts of the femora and humeri (22). Marrow reconversion refers to the
process whereby mature yellow marrow is replaced by infantile
hematopoietic marrow when the existing marrow can no longer meet
the needs for hematopoiesis (20).
Demand for increased hematopoiesis occurs in a number of
situations, including i) consumption of marrow-stimulating
medications such as G-CSF and erythropoietin; ii) anemia; iii)
marrow replacement disorders; iv) high altitudes; v) smoking; and
vi) obesity. In patients experiencing marrow reconversion, the
sites in which red marrow first appears are those areas that last
converted to yellow marrow, and this process then continues in
reverse physiologic order (22).
Therefore, hematopoietic marrow hyperplasia initially affects the
axial skeleton, followed by the appendicular skeleton. Previous
reports regarding red marrow reconversion mimicking malignancy on
MRI were limited to primary musculoskeletal neoplasm (19).
In the present case report, when reviewing the
patient's clinical and radiographic course of disease, it is
possible to infer that the false positive pitfall was due to the
marrow reconversion. In order to avoid such situations in the
future, an adequate acquaintance of history of the current disease
is essential. It is generally accepted by radiologists and
clinicians that elevated bone marrow uptake on PET is induced by
G-CSF therapy, and therefore, 18 fluorodeoxyglucose-PET
examination should be delayed in patients receiving G-CSF (23). However the use of G-CSF is often
ignored when MRI is performed on the patient. The patient in the
present case report received chemotherapy against HRPC, and when
subjected to complete blood count, anemia and neutropenia were
revealed, as a result of the endocrine therapy and chemotherapy
administered. In addition, erythropoietin and G-CSF were used as
adjuvants for the anti-tumor therapy. However, the incidence of
bone reconversion increases due to anemia, chemotherapy and
marrow-stimulating medications such as G-CSF and erythropoietin
(20). Previous studies have reported
that the time interval from the last dose of GSF to the follow-up
MRI in the case of red marrow reconversion should be 0–42 days
(mean, 9 days) (19). By identifying
the signal variations on MRI, the pre- and post-GSF images should
be evaluated in combination with the history of G-CSF application
and the corresponding white blood cells response. The pre-and
post-GSF scans should be obtained with parameters matched as
closely as possible to facilitate comparison. Furthermore, if the
radiologist or clinician is uncertain whether the scans reveal red
marrow reconversion or tumor, it would be preferable to reimage the
area on the opposed-phase images. Seiderer et al (24) demonstrated that the signal in the
opposed-phase images correlates with the fat/water fraction. Since
normal red marrow exhibits low signal in opposed-phase images,
pathological processes such as neoplastic lesions that lead to an
increase of water are indicated by a high intensity signal
(24,25). These opposed-phase images proved to be
useful in the evaluation of hematopoietic hyperplasia as a result
of therapy with G-CSF in healthy blood stem cell donors at
low-field strength (26).
Additionally, in-and out-of-phase gradient-echo MRI of bone marrow
signal intensity abnormalities may aid to predict the likelihood of
neoplastic or non-neoplastic lesions (25).
Other parameters for treatment
assessment
The present review aims to provide suggestions about
the assessment of therapeutic response in prostate cancer with bone
metastases by parameters other than imaging, since it is unwise to
affirm disease progression solely depending on images. Thus, a
potential prognostic factor is required in order to avoid the
selection of erroneous treatments. The pitfalls of images may be
potentially recognized by the evaluation of the patient symptoms,
the levels of the tumor marker PSA and the presence of lesions.
These parameters may provide useful clinical information to
oncologists and aid a wise decision. The Prostate Cancer Working
Group 2 defined a PSA partial response as >50% decline from
baseline, measured twice, 3–4 weeks apart (12). The use of a decline >50% from
baseline as a response measure was derived in part from prognostic
factor analyses that associated the degree of decline with survival
(27). The PSA response and the
relief of bone pain may aid the recognition of these pitfalls in
the clinical images, and in consequence, support the continuation
of the chemotherapy treatment. Similarly, cases of PSA flare
phenomenon (28) and pain flare
phenomenon (29) have been previously
reported. Therefore, it has been proposed that the pain and PSA
response are associated with survival, but are not adequate to use
as surrogate end points, according to the TAX-327 study, which
developed a prognostic model and nomogram using baseline clinical
variables to predict mortality among males diagnosed with
castration resistant prostate cancer (8,9). The
guidelines of the Prostate Cancer Working Group 2 also emphasize
that disease progression should not be solely defined by PSA
levels, pain or bone metastases on BS (12). Therefore, individual parameters,
including the presence of lesions, levels of PSA, clinical images
and pain response, are often combined together in order to assess
the therapeutic response and decide accordingly which is the best
treatment for the patient.
In conclusion, in patients affected by
castration-resistant prostate cancer, it is difficult to assess the
therapeutic response and decide which metrics to use when trying to
select the most convenient treatment for the patient and the most
suitable time for administration, due to the ‘flare’ phenomenon
observed on clinical images and the process of marrow reconversion
exhibited on MRI. Therefore, a better understanding of the pitfalls
on images, and a more accurate judgment of the treatment response
may aid the selection of the most beneficial treatment for patients
with prostate cancer.
References
|
1
|
Bashir MN: Epidemiology of Prostate
Cancer. Asian Pac J Cancer Prev. 16:5137–5141. 2015.PubMed/NCBI
|
|
2
|
Cooper CR, Chay CH, Gendernalik JD, Lee
HL, Bhatia J, Taichman RS, McCauley LK, Keller ET and Pienta KJ:
Stromal factors involved in prostate carcinoma metastasis to bone.
Cancer. 97(Suppl): 739–747. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlin BI and Andriole GL: The natural
history, skeletal complications and management of bone metastases
in patients with prostate carcinoma. Cancer. 88(Suppl): 2989–2994.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pollen JJ, Witztum KF and Ashburn WL: The
flare phenomenon on radionuclide bone scan in metastatic prostate
cancer. AJR Am J Roentgenol. 142:773–776. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Messiou C, Cook G, Reid AH, Attard G,
Dearnaley D, de Bono JS and de Souza NM: The CT flare response of
metastatic bone disease in prostate cancer. Acta Radiol.
52:557–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryan CJ, Shah S, Efstathiou E, Smith MR,
Taplin ME, Bubley GJ, Logothetis CJ, Kheoh T, Kilian C, Haqq CM, et
al: Phase II study of abiraterone acetate in chemotherapy-naive
metastatic castration-resistant prostate cancer displaying bone
flare discordant with serologic response. Clin Cancer Res.
17:4854–4861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tombal B, Rezazadeh A, Therasse P, Van
Cangh PJ, Van de Berg B and Lecouvet FE: Magnetic resonance imaging
of the axial skeleton enables objective measurement of tumor
response on prostate cancer bone metastases. Prostate. 65:178–187.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berthold DR, Pond G, De Wit R, Eisenberger
MA and Tannock IF: Association of pain and quality of life (QOL)
response with PSA response and survival of patients (pts) with
metastatic hormone refractory prostate cancer (mHRPC) treated with
docetaxel or mitoxantrone in the TAX-327 study. J Clin Oncol.
24:45162006.PubMed/NCBI
|
|
9
|
Berthold DR, Pond GR, de Wit R,
Eisenberger MA and Tannock IF: TAX 327 Investigators: Survival and
PSA response of patients in the TAX 327 study who crossed over to
receive docetaxel after mitoxantrone or vice versa. Ann Oncol.
19:1749–1753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greenburg EJ, Chu FC, Dwyer AJ, Ziminski
EM, Dimich AB and Laughlin JS: Effects of radiation therapy on bone
lesions as measured by 47 Ca and 85 Sr local kinetics. J Nucl Med.
13:747–751. 1972.PubMed/NCBI
|
|
11
|
Messiou C, Cook G and de Souza NM: Imaging
metastatic bone disease from carcinoma of the prostate. Br J
Cancer. 101:1225–1232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Prostate Cancer Clinical Trials Working Group:
Design and end points of clinical trials for patients with
progressive prostate cancer and castrate levels of testosterone:
Recommendations of the Prostate Cancer Clinical Trials Working
Group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamaoka T, Madewell JE, Podoloff DA,
Hortobagyi GN and Ueno NT: Bone imaging in metastatic breast
cancer. J Clin Oncol. 22:2942–2953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pollen JJ and Shlaer WJ: Osteoblastic
response to successful treatment of metastatic cancer of the
prostate. AJR Am J Roentgenol. 132:927–931. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashisako M, Wakamatsu K, Ikegame S,
Kumazoe H, Nagata N and Kajiki A: Flare phenomenon following
gefitinib treatment of lung adenocarcinoma with bone metastasis. J
Exp Med. 228:163–168. 2012.
|
|
16
|
Cook GJ, Venkitaraman R, Sohaib AS,
Lewington VJ, Chua SC, Huddart RA, Parker CC, Dearnaley DD and
Horwich A: The diagnostic utility of the flare phenomenon on bone
scintigraphy in staging prostate cancer. Eur J Nucl Med Mol
Imaging. 38:7–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janicek MJ, Hayes DF and Kaplan WD:
Healing flare in skeletal metastases from breast cancer. Radiology.
192:201–204. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lecouvet FE, Geukens D, Stainier A, Jamar
F, Jamart J, d'Othée BJ, Therasse P, Van de Berg B and Tombal B:
Magnetic resonance imaging of the axial skeleton for detecting bone
metastases in patients with high-risk prostate cancer: Diagnostic
and cost-effectiveness and comparison with current detection
strategies. J Clin Oncol. 25:3281–3287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hartman RP, Sundaram M, Okuno SH and Sim
FH: Effect of granulocyte-stimulating factors on marrow of adult
patients with musculoskeletal malignancies: Incidence and MRI
findings. AJR Am J Roentgenol. 183:645–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Long SS, Yablon CM and Eisenberg RL: Bone
marrow signal alteration in the spine and sacrum. AJR Am J
Roentgenol. 195:W178–W200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogler JB III and Murphy WA: Bone marrow
imaging. Radiology. 68:679–693. 1988. View Article : Google Scholar
|
|
22
|
Kricun ME: Red-yellow marrow conversion:
Its effect on the location of some solitary bone lesions. Skeletal
Radiol. 14:10–19. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnston KL, Farnen JP, Manske BR and Go
RS: Abnormal positron emission tomography (PET) scan secondary to
the use of hematopoietic growth factors. Haematologica.
90:EIM032005.PubMed/NCBI
|
|
24
|
Seiderer M, Staebler A and Wagner H: MRI
of bone marrow: Opposed-phase gradient-echo sequences with long
repetition time. Eur Radiol. 9:652–661. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Disler DG, McCauley TR, Ratner LM, Kesack
CD and Cooper JA: In-phase and out-of-phase MR imaging of bone
marrow: Prediction of neoplasia based on the detection of
coexistent fat and water. AJR Am J Roentgenol. 69:1439–1447. 1997.
View Article : Google Scholar
|
|
26
|
Altehoefer C, Bertz H, Ghanem NA and
Langer M: Extent and time course of morphological changes of bone
marrow induced by granulocyte-colony stimulating factor as assessed
by magnetic resonance imaging of healthy blood stem cell donors. J
Magn Reson Imaging. 14:141–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scher HI, Kelly WM, Zhang ZF, Ouyang P,
Sun M, Schwartz M, Ding C, Wang W, Horak ID and Kremer AB:
Post-therapy serum prostate-specific antigen level and survival in
patients with androgen-independent prostate cancer. J Natl Cancer
Inst. 91:244–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nelius T, Klatte T, de Riese W and Filleur
S: Impact of PSA flare-up in patients with hormone-refractory
prostate cancer undergoing chemotherapy. Int Urol Nephrol.
40:97–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sartor O, Reid RH, Hoskin PJ, Quick DP,
Ell PJ, Coleman RE, Kotler JA, Freeman LM and Olivier P: Quadramet
424Sm10/11 Study Group: Samarium-153-Lexidronam complex for
treatment of painful bone metastases in hormone-refractory prostate
cancer. Urology. 63:940–945. 2004. View Article : Google Scholar : PubMed/NCBI
|