Introduction
As the most common thyroid malignancy worldwide,
papillary thyroid carcinoma (PTC) accounts for ~80% of all thyroid
cancers (1). The incidence rate of
PTC exhibits the most rapid increase of all cancers among women,
and the second most rapid increase among men (2). Despite the advances in surgery, ~5% of
patients with PTC experience recurrence within 5 years of the
initial treatment (3). Understanding
the molecular mechanism involved in the proliferation, apoptosis
and invasion of PTC is extremely important for the development of
more effective therapeutic strategies.
Recently, certain differentially-expressed genes
(DEGs) have been reported to exhibit important roles in PTC, and
identification of these may be useful in the investigation of the
molecular mechanisms of PTC (4,5). Previous
studies showed that ret proto-oncogene, neurotrophic tyrosine
kinase receptor type 1 and v-raf murine sarcoma viral oncogene
homolog B may be useful therapeutic targets for PTC (6–8). Ye et
al found that Krüppel-like factor 17 may serve as a candidate
tumor suppressor and a therapeutic target in PTC (9). Programmed cell death 4 was reported to
exhibit an inhibitory role in the cell proliferation, malignant
progression and invasion of PTC (10). microRNA (miRNA/miR)-199b-5p,
miR-30a-3p and miR-146b-5p may be associated with PTC invasiveness
(11). Although serious attempts have
been made to find novel targets for gastric cancer treatment, at
present, this knowledge is insufficient.
In the present study, DEGs between PTC patients and
normal individuals were identified. Modules were then screened from
the protein-protein interaction (PPI) network and the significant
target genes were selected from the miRNA regulatory network.
Through the identification of key genes, the possible molecular
mechanism and potential therapeutic targets for PTC were
investigated.
Materials and methods
Affymetrix microarray data
The gene expression profile, GSE53157, which was
deposited by Pita et al (12),
was obtained from the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/). Gene
expression profiling was based on the platform of GPL570
([HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array). The
array consists of 54,675 probesets that can be used to detect the
transcription level of 18,750 human genes. Only 10 chips, including
3 specimens of normal thyroid tissues and 7 specimens of
well-differentiated thyroid carcinomas, were analyzed in the
present study.
Identification of DEGs
The raw data were first preprocessed using the Affy
package (13) in R language. Next,
DEGs between normal thyroid tissues and well-differentiated thyroid
carcinomas were analyzed by limma package in R (14). Fold-change (FC) of the expression of
individual genes was also calculated for the differential
expression test. DEGs with an adjusted P-value (Adj.P.Val) of
<0.05 and |log FC| ≥1 were considered to be significant.
Adj.P.Val was the result of multiple testing corrections using the
Benjamini-Hochberg (HB) method (15).
Gene Ontology (GO) and pathway
enrichment analysis of DEGs
The GO analysis has become a commonly used approach
for functional studies of large-scale transcriptomic or genomic
data (16). The Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway database (17) contains information of how molecules or
genes are networked. The Database for Annotation Visualization and
Integrated Discovery (DAVID) (18)
was used to systematically extract biological meaning from large
gene or protein lists. The GO function and KEGG pathway of DEGs
were analyzed using DAVID 6.7 with FDR<0.05.
Construction of PPI network and
screening of module
Genes associated with PTC were downloaded from the
Malacards database (19). The
downloaded genes and the identifided DEGs were then combined; the
pooled dataset is referred to as PTC-associated genes in the
present study. The Search Tool for the Retrieval of Interacting
Genes (STRING) (20) database was
used to retrieve the predicted interactions for the PTC-associated
genes; version 9.1 of STRING covers 1,133 completely sequenced
species. All associations obtained in STRING are provided with a
confidence score, and each score represents a rough estimate of how
likely a given association describes a functional linkage between
two proteins (21). The
PTC-associated genes with a confidence score of >0.4 were
selected to construct the PPI network, using the Cytoscape software
(22).
Visualizing complex networks and integrating these
networks to any type of attribute data was allowed by Cytoscape
(http://cytoscape.org/). The clusterMaker 1.11
(23) plugin in Cytoscape and the
Markov cluster algorithm (24) were
used to divide the PPI into modules (granularity parameter, 2). GO
functional analysis of the genes in the modules was performed using
the BinGo 2.44 plugin in Cytoscape (25) with a threshold of P<0.05 using the
hypergeometric test.
Enrichment analysis of microRNA
targets
The PTC-associated microRNAs were screened from
genes that were obtained from the Malacards database (19). The identified DEGs were then submitted
into a web-based gene set analysis toolkit (WebGestalt) (26), and miRNA-target gene enrichment
analysis was performed. The enrichment significance of the
predicted target genes in the DEGs was tested using hypergeometric
distribution. The target genes with an adj.P.Val of <0.01 were
considered to be significant, which was the result of multiple
testing correction using the HB method (15). Next, miRNA regulatory networks were
built using Cytoscape (25).
Furthermore, the integrated network was constructed using the miRNA
regulatory network and the PPI modules.
Results
GO and pathway enrichment analysis of
DEGs
In total, 668 DEGs, including 262 upregulated genes
and 406 downregulated genes, were selected. Results of GO analysis
showed that the upregulated DEGs were significantly enriched in
biological processes such as the regulation of protein kinase
activity, the regulation of transferase activity and the induction
of programmed cell death (Table I).
Results of pathway analysis showed that the upregulated DEGs were
significantly enriched in the p53 signaling pathway (Table I). However, there were no significant
GO biological processes and pathways in the downregulated DEGs.
 | Table I.GO and pathways analysis of the
upregulated DEGs. |
Table I.
GO and pathways analysis of the
upregulated DEGs.
| Category | Term | Count | FDR |
|---|
| GOTERM_BP_FAT |
GO:0045859-regulation of protein kinase
activity | 18 |
1.17×10−2 |
| GOTERM_BP_FAT | GO:0045860-positive
regulation of protein kinase activity | 14 |
1.22×10−2 |
| GOTERM_BP_FAT |
GO:0043549-regulation of kinase
activity | 18 |
1.36×10−2 |
| GOTERM_BP_FAT | GO:0033674-positive
regulation of kinase activity | 14 |
1.47×10−2 |
| GOTERM_BP_FAT | GO:0051347-positive
regulation of transferase activity | 15 |
1.51×10−2 |
| GOTERM_BP_FAT |
GO:0012502-induction of programmed cell
death | 16 |
1.96×10−2 |
| GOTERM_BP_FAT | GO:0010942-positive
regulation of cell death | 19 |
2.16×10−2 |
| GOTERM_BP_FAT |
GO:0006917-induction of apoptosis | 16 |
2.35×10−2 |
| GOTERM_BP_FAT |
GO:0051338-regulation of transferase
activity | 19 |
2.54×10−2 |
| GOTERM_BP_FAT | GO:0043068-positive
regulation of programmed cell death | 19 |
2.61×10−2 |
| GOTERM_BP_FAT | GO:0043627-response
to estrogen stimulus | 9 |
2.93×10−2 |
| GOTERM_BP_FAT | GO:0043065-positive
regulation of apoptosis | 18 |
4.20×10−2 |
| KEGG_PATHWAY | hsa04115:p53
signaling pathway | 9 |
3.46×10−2 |
Module screening from the PPI
network
A total of 355 genes asscoiated with PTC were
downloaded from the Malacards database. These genes and the
identified DEGs were combined, and 983 PTC-associated genes were
obtained. The PPI network was constructed based on the predicted
interactions of the 983 PTC-associated genes. A total of 1,157
unique PPI pairs and 413 nodes were included. The PPI network was
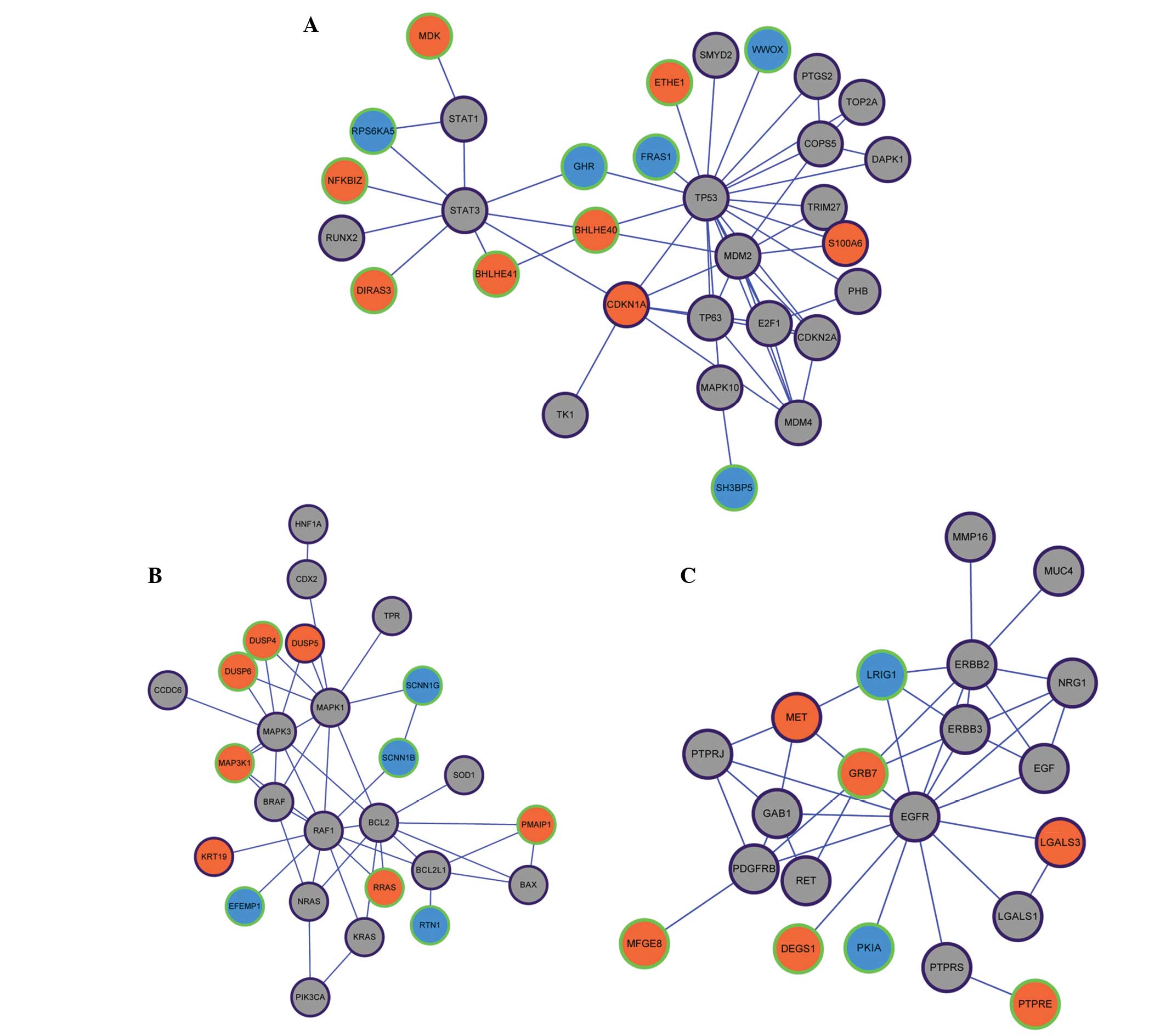
divided into three modules (Fig.
1A–C). Tumor protein p53 (TP53) was the core of module 1, which
included 55 unique PPI pairs and 31 nodes. In module 1, the
upregulated genes of cyclin-dependent kinase inhibitor 1A and S100
calcium binding protein A6 (S100A6) were included in the Malacards
database (Fig. 1A). V-raf-1 murine
leukemia viral oncogene homolog 1 was the core of module 2, and a
total of 45 unique PPI pairs and 26 nodes were included in module
2. In module 2, the upregulated genes of dual specificity
phosphatase 5 and keratin 19 were included in the Malacards
database (Fig. 1B). Epidermal growth
factor receptor was the core of module 3, and a total of 39 unique
PPI pairs and 21 nodes were included in this module. In module 3,
the upregulated genes of met proto-oncogene (MET) and lectin
galactoside-binding soluble 3 were included in the Malacards
database (Fig. 1B). Modules 1, 2 and
3 were found to be significantly enriched (P<0.05) for one GO
term each (Table II). The
significant GO term in module 1 was the negative regulation of
cellular metabolic process (P=8.16×10−6). The term
response to chemical stimulus was the most significantly enriched
function in module 2 (P=7.53×10−7). Notably, the
transmembrane receptor protein tyrosine kinase signaling was the
most significantly enriched pathway (P=6.50×10−11) in
module 3.
 | Table II.GO terms of genes in three
modules. |
Table II.
GO terms of genes in three
modules.
| Module | GO term | Corr. P | Genes in test
set |
|---|
| 1 | Negative regulation
of cellular metabolic process |
8.16×10−6 | E2F1,
CDKN1A, CDKN2A, PHB, TRIM27,
TP53, MDM2, TP63, MDM4, BHLHE40,
RUNX2, STAT3 |
| 2 | Response to
chemical stimulus |
7.53×10−7 | HNF1A,
BRAF, PMAIP1, BCL2L1, SOD1,
MAPK1, DUSP4, KRT19, KRAS, BAX,
BCL2, MAPK3, SCNN1G, SCNN1B,
DUSP6 |
| 3 | Transmembrane
receptor protein tyrosine kinase signaling pathway |
6.50×10−11 | PTPRJ,
EGFR, RET, ERBB3, ERBB2, GAB1,
PDGFRB, EGF, NRG1, GRB7 |
Enrichment analysis of microRNA target
genes
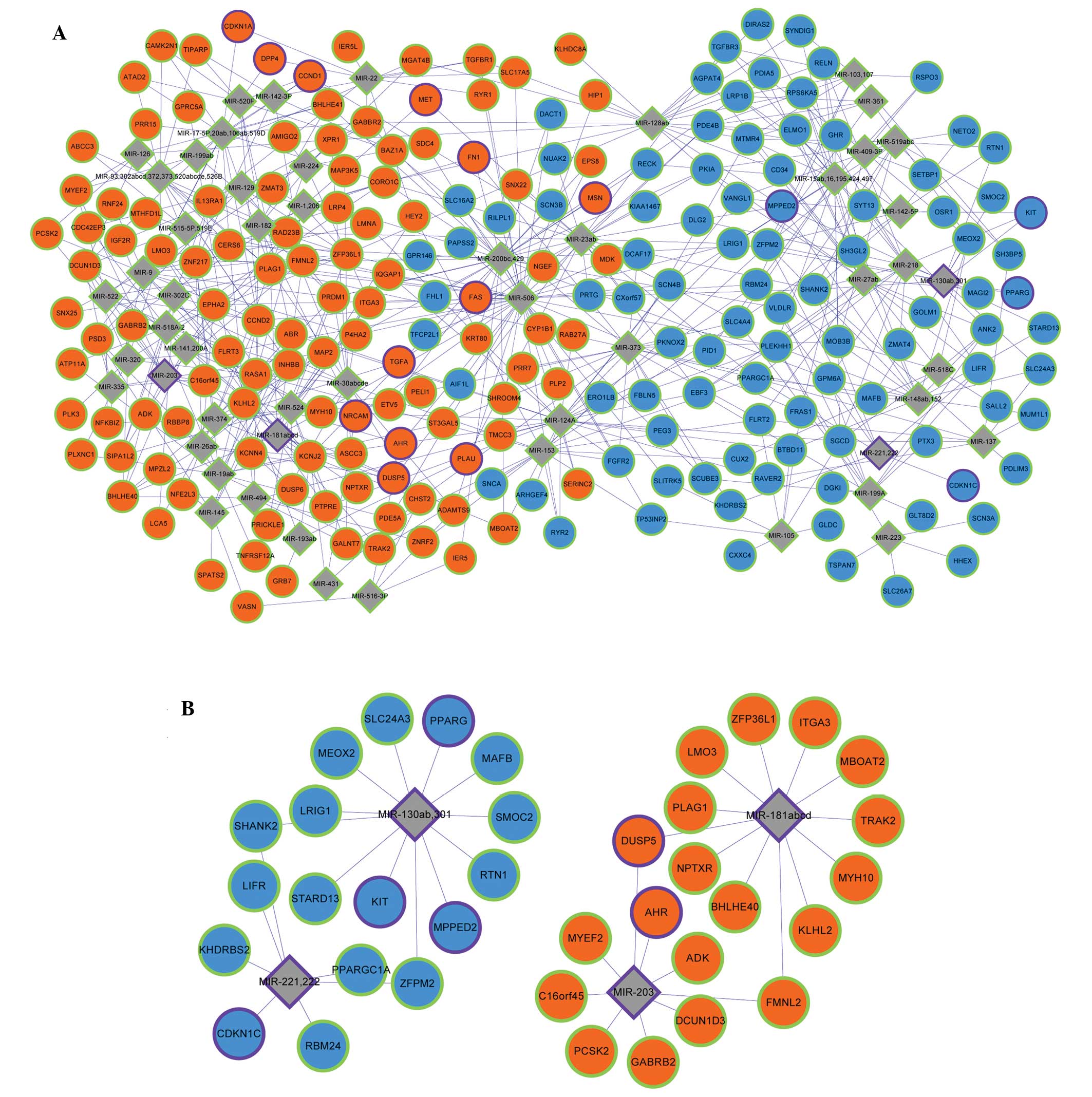
The PTC-associated microRNAs acquired from the 355
genes were miR203, miR34b, miR221, miR222, miR146a, miR146b, miR21,
miR181a1 and miR130b. Through miRNA-target gene enrichment
analysis, a miRNA regulatory network consisting of 54 miRNAs and
210 DEGs was constructed (Fig. 2A).
miR-221, miR-222, miR-181a, miR-203 and miR-130b, which were
included in the 355 genes, were involved in the miRNA regulatory
network. In addition, cyclin-dependent kinase inhibitor 1C (CDKN1C)
was the target of miRNA-221 and miRNA-222; v-kit Hardy-Zuckerman 4
feline sarcoma viral oncogene homolog, metallophosphoesterase
domain containing 2 and peroxisome proliferator-activated receptor
γ were the targets of miRNA-130b; aryl hydrocarbon receptor was the
target of miRNA-203; and dual specificity phosphatase 5 was the
target of miRNA-203 and miRNA-181a (Fig.
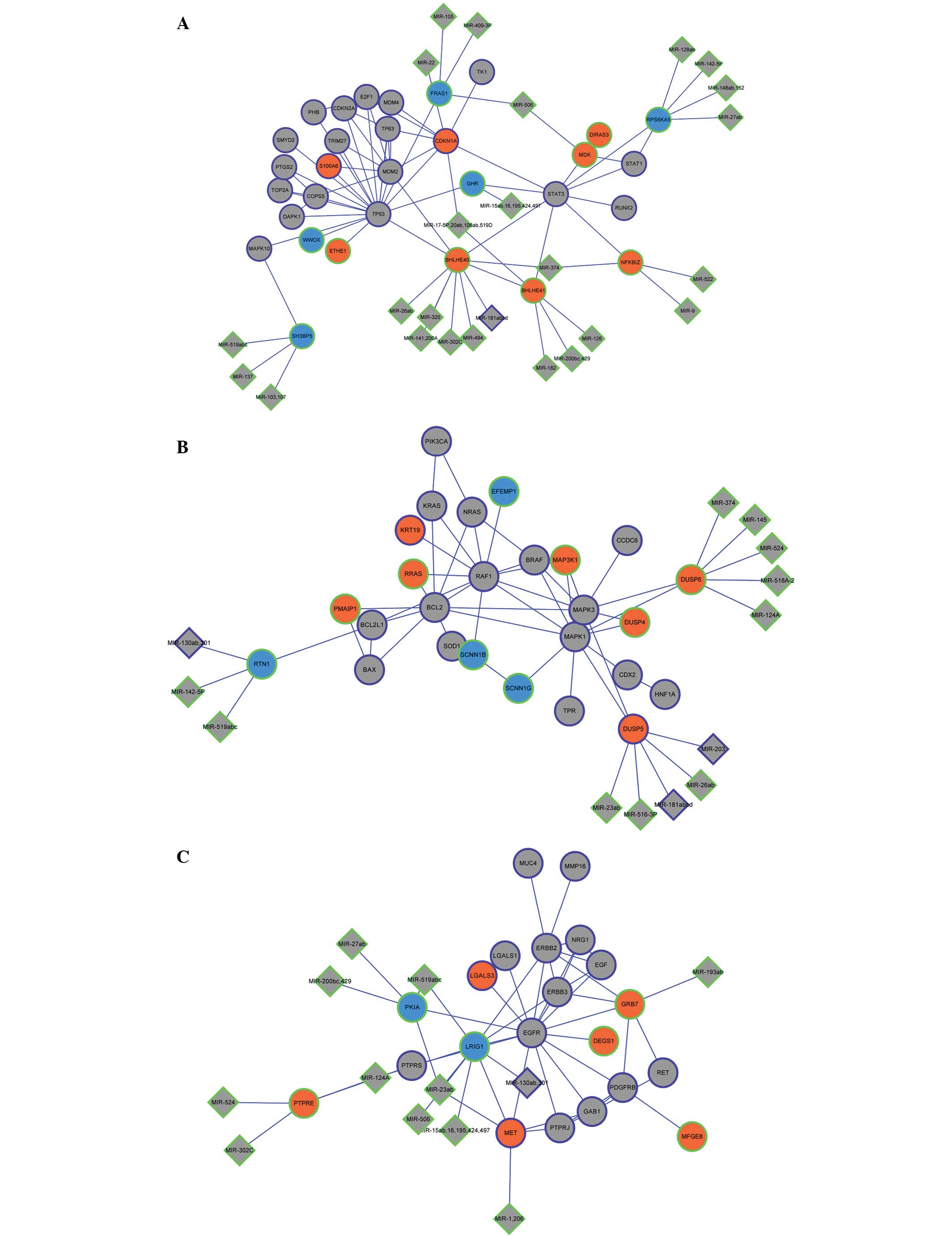
2B). By combining the miRNA regulatory network with the PPI
network modules, the integrated network was constructed (Fig. 3A–C). The upregulated gene basic
helix-loop-helix family member e40 in module 1 was the target of
miR-181a (Fig. 3A); and the
downregulated gene reticulon 1 in module 2 was the target of
miR-130b (Fig. 3B).
Discussion
PTC accounts for nearly 80% of human thyroid
cancers, worldwide (27). Now, the
burden of thyroid disease in the global population is enormous
(28). Thus, the potential use of
therapeutic targets appears to be the most promising area of
research. In the present study, a bioinformatics approach was used
to predict the potential therapeutic targets for PTC. The study
identified 668 DEGs between normal thyroid tissues and
well-differentiated thyroid carcinomas, among which, 406 genes were
downregulated and 262 were upregulated. By constructing the PPI and
miRNA regulatory networks, key genes were found, including
S100A6, MET and CDKN1C.
S100A6 encodes a member of the S100A family
of calcium binding proteins, and it is reported to be involved in
the regulation of a wide range of cellular processes, including
cell proliferation, differentiation and apoptosis (29,30).
Furthermore, S100A6 has been shown to be upregulated in
numerous cancers, including thyroid carcinoma (31,32). Li
et al showed that increased S100A6 expression
promoted the proliferation and migration of cells in human
hepatocellular carcinoma (HCC), and that S100A6 may function
as a potential therapeutic target in HCC (33). Komatsu et al showed that
S100A6 was involved in the invasion and metastasis processes
of human colorectal adenocarcinomas (34). S100A6 has been reported to be
overexpressed in PTC and may contribute to certain events in this
disease (35,36). In the present study, S100A6 was
overexpressed in PTC, and the PPI network showed that it had direct
interaction with TP53, which was associated with PTC
(37,38). Overall, S100A6 may be a
therapeutic target in PTC.
MET encodes the hepatocyte growth factor
receptor and could regulate a number of physiological processes,
including proliferation, scattering (cell dissociation and
motility), morphogenesis and survival (39). MET could trigger tumor growth,
angiogenesis and metastasis (40).
Rong et al found that MET was overexpressed in a
number of tumors, and suggested that MET activation
contributed to tumor progression (41). Fujita and Sugano found that MET
was overexpressed in primary colorectal cancer and liver
metastases, and suggested that MET played an important role
in the development of colorectal cancer liver metastases (42). In one study, Di Renzo et al
showed that the overexpression of MET in human thyroid
carcinomas may contribute to the progression of thyroid tumors
(43), while in a second study, it
was suggested that the upregulation of MET may add a
selective growth advantage to differentiated ovarian cancers
(44). In the present study,
MET was upregulated in PTC, and GO analysis showed that it
was involved in the biological process of transferase activity.
Combined with the aforementioned studies, this data shows that
MET may be a therapeutic target in PTC.
The putative tumor suppressor CDKN1C is a
negative regulator of cell proliferation and mutations. Soejima
et al showed that CDKN1C was downregulated in
esophageal cancer and contributed to the tumor (45), and Hoffmann et al found that
numerous advanced urothelial cancers displayed the downregulation
of CDKN1C (46). Larson et
al found that CDKN1C was a candidate tumor suppressor in
breast cancer (47), while Algar
et al found that it was a tumor suppressor in rhabdoid
tumors (48). However, there have
been no studies with regard to the association between
CDKN1C and PTC. In the present study, CDKN1C was
downregulated in PTC, and the miRNA regulatory network showed that
CDKN1C was the target of miRNA221 and miRNA222. One previous
study showed that miRNA221 and miRNA222 were upregulated in PTC,
and that they contributed to the procession of the disease
(49). Furthermore, Fornari et
al indicated that miR-221 controlled the expression of
CDKN1C in human hepatocellular carcinoma (50). Thus, CDKN1C may be a potential
therapeutic target in PTC.
In conclusion, S100A6, MET and
CDKN1C may exhibit key roles in the progression and
development of PTC, and may be used as specific therapeutic targets
in the treatment of PTC. However, further experiments are required
to confirm these results.
Acknowledgements
The authors wish to express gratitude to Fenghe
(Shanghai) Information Technology Co., Ltd. (Shanhai, China) for
ideas and assistance that provided a valuable added dimension to
the present study.
References
|
1
|
Zhang J, Yang Y, Liu Y, Fan Y, Liu Z, Wang
X, Yuan Q, Yin Y, Yu J, Zhu M, et al: MicroRNA-21 regulates
biological behaviors in papillary thyroid carcinoma by targeting
programmed cell death 4. J Surg Res. 189:68–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grant CS: Recurrence of papillary thyroid
cancer after optimized surgery. Gland Surg. 4:52–62.
2015.PubMed/NCBI
|
|
4
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer-the diagnostic
potential of urinary miR-205 and miR-214. PloS One. 8:e769942013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hodge LS, Elsawa SF, Grote DM,
Price-Troska TL, Asmann YW, Fonseca R, Gertz MA, Witzig TE, Novak
AJ and Ansell SM: MicroRNA expression in tumor cells from
Waldenstrom's macroglobulinemia reflects both their normal and
malignant cell counterparts. Blood Cancer J. 1:e242011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adeniran AJ, Zhu Z, Gandhi M, Steward DL,
Fidler JP, Giordano TJ, Biddinger PW and Nikiforov YE: Correlation
between genetic alterations and microscopic features, clinical
manifestations and prognostic characteristics of thyroid papillary
carcinomas. Am J Surg Pathol. 30:216–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Espinosa AV, Porchia L and Ringel MD:
Targeting BRAF in thyroid cancer. Br J Cancer. 96:16–20. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pelizzo MR, Boschin IM, Barollo S,
Pennelli G, Toniato A, Zambonin L, Vianello F, Piotto A, Ide EC,
Pagetta C, et al: BRAF analysis by fine needle aspiration biopsy of
thyroid nodules improves preoperative identification of papillary
thyroid carcinoma and represents a prognostic factor. A
mono-institutional experience. Clin Chem Lab Med. 49:325–329. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye WC, Gao L, Huang J, Fang XM and Xie G:
Suppressed Kruppellike factor 17 expression induces tumor
proliferation, metastasis and a poor prognosis in papillary thyroid
carcinoma. Mol Med Rep. 2014. View Article : Google Scholar
|
|
10
|
Pennelli G, Fassan M, Mian C, Pizzi M,
Balistreri M, Barollo S, Galuppini F, Guzzardo V, Pelizzo M and
Rugge M: PDCD4 expression in thyroid neoplasia. Virchows Arch.
462:95–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng Y, Li C, Luo DC, Ding JW, Zhang W and
Pan G: Expression profile and clinical significance of microRNAs in
papillary thyroid carcinoma. Molecules. 19:11586–11599. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pita JM, Banito A, Cavaco BM and Leite V:
Gene expression profiling associated with the progression to poorly
differentiated thyroid carcinomas. Br J Cancer. 101:1782–1791.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smyth GK: Limma: Linear models for
microarray data. Bioinformatics and Computational Biology Solutions
Using {R} and Bioconductor. Gentleman R, Carey VJ, Huber W,
Irizarry RA and Dudoit S: (New York). Springer. 397–420. 2005.
View Article : Google Scholar
|
|
15
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Statist Soc B. 57:289–300. 1995.
|
|
16
|
Hulsegge I, Kommadath A and Smits MA:
Globaltest and GOEAST: Two different approaches for gene ontology
analysis. BMC Proc. 3(Suppl 4): S102009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogata H, Goto S, Sato K, Fujibuchi W, Bono
H and Kanehisa M: KEGG: Kyoto encyclopedia of genes and genomes.
Nucleic Acids Res. 27:29–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rappaport N, Nativ N, Stelzer G, Twik M,
Guan-Golan Y, Stein TI, Bahir I, Belinky F, Morrey CP, Safran M and
Lancet D: Mala Cards: An integrated compendium for diseases and
their annotation. Database (Oxford). 2013:bat0182013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:D561–D568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morris JH, Apeltsin L, Newman AM, Baumbach
J, Wittkop T, Su G, Bader GD and Ferrin TE: ClusterMaker: A
multi-algorithm clustering plugin for Cytoscape. BMC
Bioinformatics. 12:4362011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Enright AJ, Van Dongen S and Ouzounis CA:
An efficient algorithm for large-scale detection of protein
families. Nucleic Acids Res. 30:1575–1584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Duncan D, Shi Z and Zhang B:
WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013.
Nucleic Acids Res. 41:W77–W83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C and Crapo LM: The epidemiology of
thyroid disease and implications for screening. Endocrinol Metab
Clin North Am. 26:189–218. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Breen EC and Tang K: Calcyclin
(S100A6) regulates pulmonary fibroblast proliferation,
morphology and cytoskeletal organization in vitro. J Cell Biochem.
88:848–854. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsoporis JN, Izhar S and Parker TG:
Expression of S100A6 in cardiac myocytes limits apoptosis
induced by tumor necrosis factor-alpha. J Biol Chem.
283:30174–30183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cross SS, Hamdy FC, Deloulme JC and Rehman
I: Expression of S100 proteins in normal human tissues and common
cancers using tissue microarrays: S100A6, S100A8, S100A9 and
S100A11 are all overexpressed in common cancers. Histopathology.
46:256–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito Y, Yoshida H, Tomoda C, Uruno T, Miya
A, Kobayashi K, Matsuzuka F, Kakudo K, Kuma K and Miyauchi A:
Expression of S100A2 and S100A6 in thyroid carcinomas.
Histopathology. 46:569–575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Tang M, Ling B, Liu S, Zheng Y, Nie
C, Yuan Z, Zhou L, Guo G, Tong A and Wei Y: Increased expression of
S100A6 promotes cell proliferation and migration in human
hepatocellular carcinoma. J Mol Med (Berl). 92:291–303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komatsu K, Kobune-Fujiwara Y, Andoh A,
Ishiguro S, Hunai H, Suzuki N, Kameyama M, Murata K, Miyoshi J,
Akedo H, et al: Increased expression of S100A6 at the
invading fronts of the primary lesion and liver metastasis in
patients witholorectal adenocarcinoma. Br J Cancer. 83:7692000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ito Y, Yoshida H, Tomoda C, Uruno T, Miya
A, Kobayashi K, Matsuzuka F, Kakudo K, Kuma K and Miyauchi A:
Expression of S100A2 and S100A6 in thyroid carcinomas.
Histopathology. 46:569–575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sofiadis A, Dinets A, Orre LM, Branca RM,
Juhlin CC, Foukakis T, Wallin G, Höög A, Hulchiy M, Zedenius J, et
al: Proteomic study of thyroid tumors reveals frequent
up-regulation of the Ca2+-binding protein S100A6 in
papillary thyroid carcinoma. Thyroid. 20:1067–1076. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pita JM, Figueiredo IF, Moura MM, Leite V
and Cavaco BM: Cell cycle deregulation and TP53 and RAS mutations
are major events in poorly differentiated and undifferentiated
thyroid carcinomas. J Clin Endocrinol Metab. 99:E497–E507.
2014.PubMed/NCBI
|
|
38
|
Quiros RM, Ding HG, Gattuso P, Prinz RA
and Xu X: Evidence that one subset of anaplastic thyroid carcinomas
are derived from papillary carcinomas due to BRAF and p53
mutations. Cancer. 103:2261–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naldini L, Vigna E, Narsimhan RP, Gaudino
G, Zarnegar R, Michalopoulos GK and Comoglio PM: Hepatocyte growth
factor (HGF) stimulates the tyrosine kinase activity of the
receptor encoded by the proto-oncogene c-MET. Oncogene. 6:501–504.
1991.PubMed/NCBI
|
|
40
|
Schmidt L, Junker K, Weirich G, Glenn G,
Choyke P, Lubensky I, Zhuang Z, Jeffers M, Van de Woude G, Neumann
H, et al: Two North American families with hereditary papillary
renal carcinoma and identical novel mutations in the MET
proto-oncogene. Cancer Rre. 58:1719–1722. 1998.
|
|
41
|
Rong S, Donehower LA, Hansen MF, Strong L,
Tainsky M, Jeffers M, Resau JH, Hudson E, Tsarfaty I and Van de
Woude GF: Met proto-oncogene product is overexpressed in tumors of
p53-deficient mice and tumors of Li-Fraumeni patients. Cancer Res.
55:1963–1970. 1995.PubMed/NCBI
|
|
42
|
Fujita S and Sugano K: Expression of c-met
proto-oncogene in primary colorectal cancer and liver metastases.
Jpn J Clin Oncol. 27:378–383. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Di Renzo M, Olivero M, Ferro S, Prat M,
Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R,
Pierotti MA, et al: Overexpression of the c-MET/HGF receptor gene
in human thyroid carcinomas. Oncogene. 7:2549–2553. 1992.PubMed/NCBI
|
|
44
|
Di Renzo MF, Olivero M, Katsaros D,
Crepaldi T, Gaglia P, Zola P, Sismondi P and Comoglio PM:
Overexpression of the Met/HGF receptor in ovarian cancer. Int J
Cancer. 58:658–662. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Soejima H, Nakagawachi T, Zhao W,
Higashimoto K, Urano T, Matsukura S, Kitajima Y, Takeuchi M,
Nakayama M, Oshimura M, et al: Silencing of imprinted CDKN1C gene
expression is associated with loss of CpG and histone H3 lysine 9
methylation at DMR-LIT1 in esophageal cancer. Oncogene.
23:4380–4388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoffmann MJ, Florl AR, Seifert HH and
Schulz WA: Multiple mechanisms downregulate CDKN1C in human bladder
cancer. Internat J Cancer. 114:406–413. 2005. View Article : Google Scholar
|
|
47
|
Larson PS, Schlechter BL, King CL, Yang Q,
Glass CN, Mack C, Pistey R, de Las Morenas A and Rosenberg CL:
CDKN1C/p57kip2 is a candidate tumor suppressor gene in human breast
cancer. BMC Cancer. 8:682008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Algar EM, Muscat A, Dagar V, Rickert C,
Chow CW, Biegel JA, Ekert PG, Saffery R, Craig J, Johnstone RW and
Ashley DM: Imprinted CDKN1C is a tumor suppressor in rhabdoid tumor
and activated by restoration of SMARCB1 and histone deacetylase
inhibitors. PLoS One. 4:e44822009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tetzlaff MT, Liu A, Xu X, Master SR,
Baldwin DA, Tobias JW, Livolsi VA and Baloch ZW: Differential
expression of miRNAs in papillary thyroid carcinoma compared to
multinodular goiter using formalin fixed paraffin embedded tissues.
Endocr Pathol. 18:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fornari F, Gramantieri L, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM,
Bolondi L and Negrini M: MiR-221 controls CDKN1C/p57 and CDKN1B/p27
expression in human hepatocellular carcinoma. Oncogene.
27:5651–5661. 2008. View Article : Google Scholar : PubMed/NCBI
|