Introduction
Papillary thyroid cancer (PTC) is the most prevalent
type of malignant tumor of the endocrine system and accounts for
70–80% of all diagnosed cancers of the thyroid gland (1). Histopathological diagnosis of PTC is
effective in the majority of cases. However, the diagnosis of rare
variants of PTC and of Hashimoto's thyroiditis (HT) with PTC-like
nuclear alterations is challenging.
Intercellular adhesion molecule 1 (ICAM-1) is a
transmembrane glycoprotein receptor and a member of the
immunoglobulin superfamily of adhesion molecules. It is expressed
on the surface of various cell types, including endothelial cells,
leukocytes (with the exception of basophilic granulocytes), T
cells, B cells and fibroblasts. ICAM-1 is responsible for the
arrest and transmigration of leukocytes out of blood vessels and
into tissue, as well as the formation of immunological synapses
during T cell activation (2).
Recently, a number of studies have reported that ICAM-1 is present
in several types of cancer, including prostate, breast and oral
cancers (3–5), and is involved (at least in part) in
their progression.
Few reports have found ICAM-1 expression to be
elevated in PTC (6,7), and the prognosis and clinical
significance of ICAM-1 remain unclear, particularly in certain
histological types of thyroid lesions (e.g., HT with PTC-like
alterations). The present study sought to validate ICAM-1 as a
sensitive immunohistochemical (IHC) marker to distinguish PTC from
different diseases of the thyroid gland, and to estimate the
predictive value of ICAM-1 by studying the aggressive behavior of
PTC.
Materials and methods
Ethical statement
The present study was approved by the Research
Ethics Committee of Shandong Provincial Hospital Affiliated to
Shandong University (Jinan, China) and written informed consent was
obtained from all patients.
Clinical data
The study cohort comprised 245 consecutive patients
(171 women and 74 men; age range, 28–75 years; mean age, 42 years).
Of these, 132 had primary PTC, 10 had follicular cancer, 15 had
follicular adenoma, 72 had HT and 16 had nodular goiter. In
addition, 8 normal thyroid tissue samples were taken from the
contralateral lobe of thyroid specimens containing cancer. None of
the patients received medication prior to surgery. All underwent
total thyroidectomy or lobectomy in the Department of Thyroid
Surgery, Shandong Provincial Hospital, Shandong University, between
January 2007 and December 2011. The pathological diagnoses of all
specimens were graded according to the classification of thyroid
malignancy by the World Health Organization (2004) (8) by two experienced pathologists.
For all patients, data were collected by
retrospective review of medical records for gender, age, tumor
size, extra-thyroidal invasion, pathological stage, lymph node
status, focality, recurrence and distant metastatic dissemination.
Tumors were staged according to the 7th edition of the
tumor-node-metastasis-based staging system recommended by the
American Joint Committee on Cancer and Union for International
Cancer Control (9).
Antibodies for IHC analyses
Rabbit polyclonal antibodies against ICAM-1 (cat.
no. sc-7891; dilution, 1:100) and galectin-3 (cat. no. sc-20157;
dilution, 1:100) were purchased from Santa Cruz Biotechnology,
Inc., (Santa Cruz, CA, USA). Mouse monoclonal antibodies against
cytokeratin 19 (CK-19; cat. no. IS615; dilution, 1:100), Hector
Battifora mesothelial-1 (HBME-1; cat. no. M3505; dilution, 1:100)
and thyroid peroxidase (TPO; cat. no. M7257; dilution, 1:200) were
obtained from Dako (Carpinteria, CA, USA).
IHC procedure
Sections (thickness, 4 mm) were cut from
formalin-fixed, paraffin-embedded blocks, and subsequently
deparaffinized in xylene and rehydrated using a series of graded
washes with ethanol. After inhibition of endogenous peroxidase and
antigen retrieval (microwave irradiation in 0.01 M citrate buffer
at pH 6.0), sections were incubated with each primary antibody at
4°C overnight, followed by incubation with horseradish peroxidase
(HRP)-conjugated secondary antibodies (dilution, 1:1,00; Dako) for
1 h at 4°C. Slides were developed for 5 min with the chromogen
3,3′-diaminobenzidine, counterstained with hematoxylin to
distinguish the nucleus from the cytoplasm, and evaluated under a
microscope (BX51; Olympus Corporation, Tokyo, Japan). Normal tonsil
tissues (which are known to express ICAM-1) were used as positive
and negative controls after being stained with or without primary
antibodies, respectively. All assessments were undertaken in three
separate experiments, and one representative assessment of three
different experiments is shown.
Evaluation of stained samples
Immunostains were examined independently by two
clinical pathologists. For ICAM-1, a staining value from 0 to 9 was
calculated as the intensity of positive staining of the membrane or
cytoplasm (negative, 0; weak, 1; moderate, 2; strong, 3) multiplied
by the percentage of immunostained tumor cells (≤10%, 1; 11–50%, 2;
≥51%, 3). The intensity of staining was assessed in 100 cells. The
final staining scores were classified according to the following
scale: Value 0, score 0; values 1–2, score 1+; values 3–6, score
2+; values 7–9, score 3+. All cases were divided into two groups of
negative (score 0 or 1+) and positive (score 2+ or 3+)
expression.
Expression of galectin-3 in tumor cells was defined
as ‘positive’ if cytoplasm was stained and occasional staining of
the nucleus was also observed (10).
Expression of CK-19 and HBME-1 was evaluated by staining of the
cell membrane, and TPO by staining of the cytoplasm, respectively.
The intensity of staining for each antibody was scored as follows:
Negative, 0; weak, 1+; moderate, 2+; strong, 3+. Positive
immunostaining was defined as a staining intensity of 2+ to 3+ in
>10% of cells. There were no differences in opinion between the
two pathologists. All assessments were conducted in three separate
experiments, and one representative assessment of three different
experiments is shown.
Western blotting
Frozen tissues (100 mg) were collected from each
sample and analyzed in modified radioimmunoprecipitation assay
buffer (0.05 M Tris-HCl, pH 7.4; 1% NP-40; 0.25% Na-deoxycholate;
0.15 M NaCl; 0.001 M Na3VO4; 0.001 M EDTA;
and 0.5% of a protease inhibitor cocktail; Roche Diagnostics
Operations, Indianapolis, IN, USA) chilled on ice for 30 min and
centrifuged at 10,000 × g for 5 min at 4°C. The resultant
supernatant was collected and stored at −70°C. Protein
concentration was determined using a Pierce™ bicinchoninic acid
protein assay (Thermo Fisher Scientific, Rockford, IL, USA).
Proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA).
Following incubation in blocking buffer for 2 h at room
temperature, incubation with rabbit anti-ICAM-1 polyclonal antibody
(cat. no. sc-7891; dilution, 1:1,000; Santa Cruz Biotechnology,
Inc.) and anti-β-actin antibody (cat. no. sc-130656; dilution,
1:1,000; Santa Cruz Biotechnology, Inc.) was conducted at 4°C
overnight, followed by incubation with HRP-conjugated goat
anti-rabbit secondary antibody (Universal LSAB2 kit/HRP; dilution,
1:100; Dako) for 1 h at 4°C. Western blot signals were visualized
using a Pierce™ enhanced chemiluminescent substrate (ECL Western
Blotting Substrate; substrate, HRP; cat. no. 32106; Thermo Fisher
Scientific) and quantified using a FluorChem Q Imaging System
(Protein Simple, San Jose, CA, USA).
Statistical analyses
Data were analyzed by SPSS software version 16.0
(SPSS, Inc., Chicago, IL, USA). The χ2 test was used to
calculate the statistical significance of the variables. P<0.05
was considered to a indicate statistically significant
difference.
Results
ICAM-1 expression in different
diseases of the thyroid gland
Expression of ICAM-1 in 245 thyroid samples from
patients who underwent surgery of the thyroid gland were
investigated by IHC analyses. This revealed that 85.6% (113/132) of
PTC samples and 18.1% (13/72) of HT samples were positive for
ICAM-1, whereas all samples of follicular cancer (n=10), follicular
adenoma (n=15), nodular goiter (n=16) and normal thyroid (n=8) were
negative (Table I). As shown in
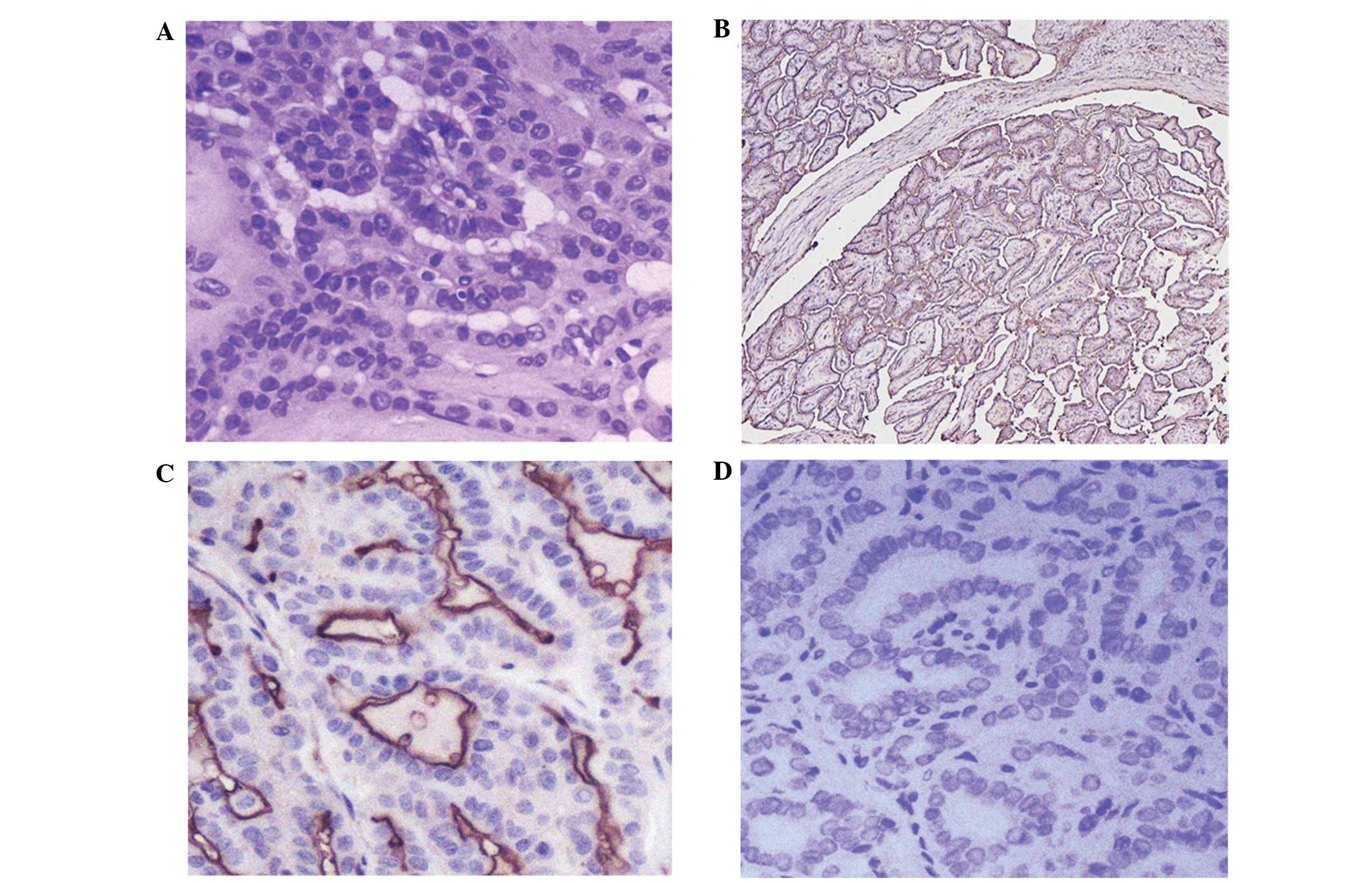
Fig. 1, ICAM-1 expression was
observed in the cell membrane and cytoplasm. Notably, in
well-differentiated PTC tissues, ICAM-1 expression was detected at
the apical surface of the papillary region and the thyroid gland
(Fig. 1B–C). Expression of ICAM-1 was
increased significantly in the PTC group compared with the other
groups (P<0.001), and was also markedly higher in the HT group
than in the other groups, with the exception of the PTC group
(P<0.001).
 | Table I.ICAM-1 expression in different thyroid
diseases by histopathology. |
Table I.
ICAM-1 expression in different thyroid
diseases by histopathology.
|
|
| ICAM-1 expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Disease group | Patients, n | Positive | Negative | P-value |
|---|
| PTC | 132 | 113
(85.6) | 19
(14.4) |
<0.001a |
| Classic
PTC | 107 |
95 (72.0) | 12 (9.1) |
|
|
Follicular variant of PTC | 14 | 10
(7.6) | 4
(3.0) |
|
| Other
variants of PTC | 11 |
8 (6.0) | 3
(2.3) |
|
| Hashimoto's
thyroiditis | 72 |
22 (30.1) | 50
(69.9) |
<0.001b |
| Follicular
carcinoma | 10 |
0 (0.0) | 10 (100) |
|
| Follicular
adenoma | 15 |
0 (0.0) | 6
(100) |
|
| Nodular goiter | 16 |
0 (0.0) | 16 (100) |
|
| Normal thyroid |
8 |
0 (0.0) | 8
(100) |
|
ICAM-1 expression in PTC assessed
using western blotting
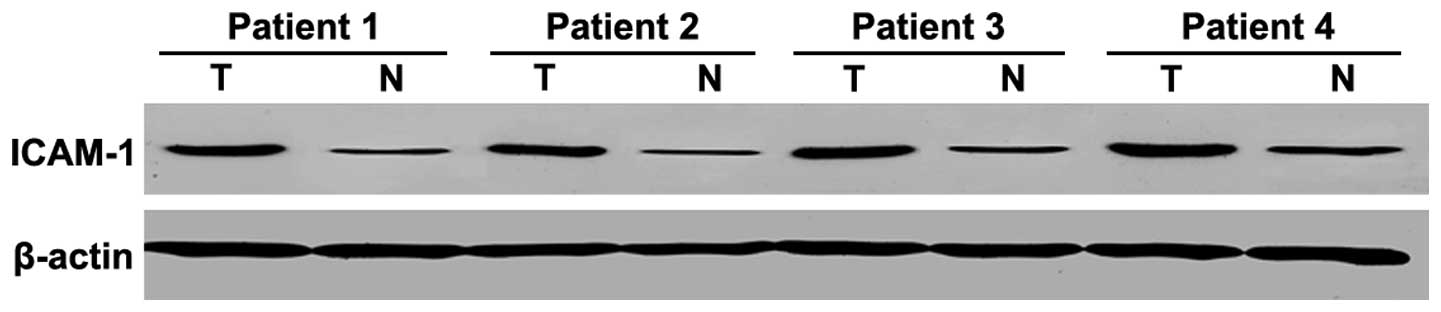
In 42 PTC samples and paired non-tumorous thyroid
tissue obtained from the same patients, ICAM-1 overexpression was
found in 36 PTC samples in comparison with non-tumorous tissues
(P<0.001) (Fig. 2). Western
blotting results for ICAM-1 corroborated the immunostaining data
(in which 34 of the corresponding 42 samples demonstrated positive
immunostaining), and the agreement with IHC results was 94.4% (data
not shown).
Association between ICAM-1 expression
and clinicopathologic features of PTC
The associations between ICAM-1 expression and
clinicopathological features of patients are shown in Table II. ICAM-1 expression was not
correlated with age, gender, tumor size, multifocality,
pathological stage, recurrence or distant metastasis (P>0.05).
However, ICAM-1 expression was associated with extra-thyroidal
invasion (P=0.015) and lymph node metastasis (LNM; P=0.027). Of the
132 PTC patients, 47 had metastasis in the cervical lymph nodes, 35
had local recurrence, and 17 had distant metastasis (8 with lung
metastasis, 4 with brain metastasis and 5 with bone metastasis).
High expression of ICAM-1 was detected in the tumor cells of the 4
patients who died of distant metastasis.
 | Table II.Correlation between
clinicopathological features and ICAM-1 expression in papillary
thyroid cancer patients (n=132). |
Table II.
Correlation between
clinicopathological features and ICAM-1 expression in papillary
thyroid cancer patients (n=132).
|
|
| ICAM-1 expression,
n (%) |
|
|---|
|
|
|
|
|
|---|
| Variable | Patients, n
(%) | Positive | Negative | P-value |
|---|
| Age, years |
|
|
| 0.185 |
|
≤40 | 74
(56.1) | 66
(50.0) | 8
(6.1) |
|
|
>40 | 58
(43.9) | 47
(35.6) | 11 (8.3) |
|
| Gender |
|
|
| 0.402 |
|
Male | 38
(28.8) | 31
(23.5) | 7
(5.3) |
|
|
Female | 94
(71.2) | 82
(62.1) | 12 (9.1) |
|
| Tumor size, cm |
|
|
| 0.226 |
| ≤1 | 40
(30.3) | 32
(24.2) | 8
(6.1) |
|
|
>1 | 92
(69.7) | 81
(61.4) | 11 (8.3) |
|
| pT stage |
|
|
| 0.311 |
| I | 38
(28.8) | 34
(25.8) | 4
(3.0) |
|
| II | 74
(56.1) | 64
(48.5) | 10 (7.6) |
|
|
III | 20
(15.1) | 15
(11.4) | 5
(3.8) |
|
| Extra-thyroid
invasion |
|
|
| 0.008a |
|
Yes | 72
(54.5) | 67
(50.8) | 5
(3.8) |
|
| No | 60
(45.5) | 46
(34.8) | 14
(10.6) |
|
| Multifocality |
|
|
| 0.261 |
|
Yes | 41
(31.1) | 33
(25.0) | 8
(6.1) |
|
| No | 91
(68.9) | 80
(60.6) | 11 (8.3) |
|
| Lymph node
metastasis |
|
|
| 0.010a |
|
Yes | 47
(35.6) | 45
(34.1) | 2
(1.5) |
|
| No | 85
(64.4) | 68
(51.5) | 17
(12.9) |
|
| Recurrence |
|
|
| 0.589 |
|
Yes | 35
(26.5) | 29
(22.0) | 6
(4.5) |
|
| No | 97
(73.5) | 84
(63.6) | 13 (9.8) |
|
| Distance |
|
|
| 0.250 |
|
Yes | 17
(12.9) | 13 (9.8) | 4
(3.0) |
|
| No | 115 (87.1) | 100 (75.8) | 15
(11.4) |
|
ICAM-1 expression in HT patients
Of the 72 HT cases, 22 exhibited ICAM-1 expression.
Contralateral thyroid cancer was diagnosed in 5 of these cases at
8–63 months after surgery, and LNM was detected in 2 of these 5
patients. Of 50 ICAM-1-negative patients, only 4 had contralateral
thyroid cancer, and LNM was detected in 1 patient. Among the HT
cases, the HT-to-PTC progression rate of ICAM-1 positive patients
was significantly higher than that of ICAM-1 negative patients
(22.7 vs. 8%). Of 72 HT cases, 21 exhibited PTC-like features, 13
of which were positive for ICAM-1. Only 9 patients expressed ICAM-1
out of 51 with non-PTC-like HT (Table
III). Thus, ICAM-1 expression in patients with PTC-like HT was
significantly higher than that of cases with non-PTC-like HT
(P<0.001).
 | Table III.ICAM-1 expression in Hashimoto's
thyroiditis patients (n=72). |
Table III.
ICAM-1 expression in Hashimoto's
thyroiditis patients (n=72).
|
| ICAM-1 expression,
n (%) |
|
|---|
|
|
|
|
|---|
| Group | Positive | Negative | P-value |
|---|
| PTC-like | 13 (18.1) | 8
(11.1) |
<0.001a |
| Non-PTC-like | 9
(12.5) | 42 (58.3) |
|
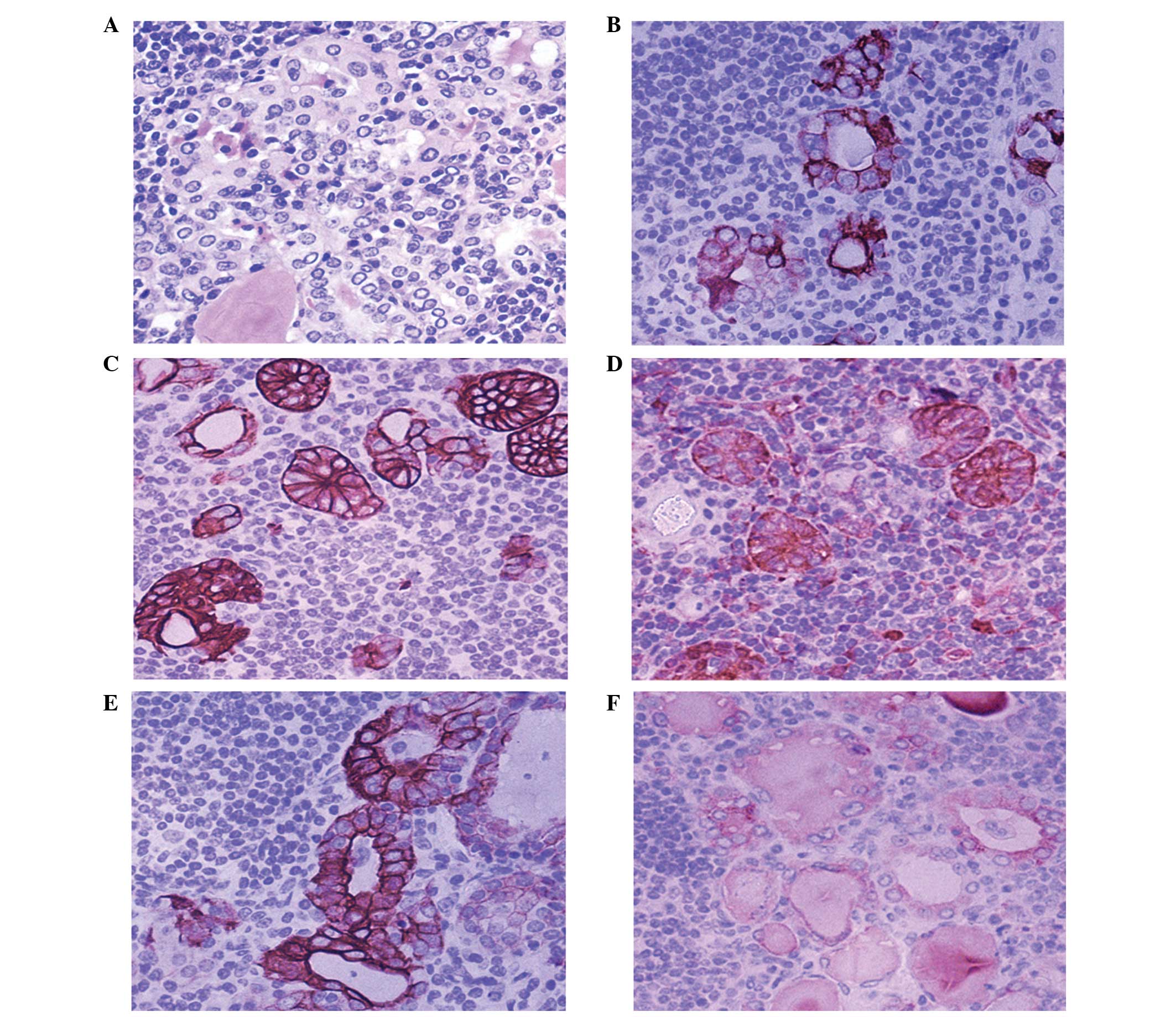
To evaluate the diagnostic value of ICAM-1
expression for the detection of PTC-like alterations, antibodies
against CK-19, galectin-3, HBME-1 and TPO were employed. The HT was
set to be investigated for PTC-nuclear change, and was confirmed on
the hematoxylin and eosin slides as the ‘golden criteria’. The
specificity and sensitivity of different antibodies and antibody
combinations were calculated (Fig.
3). Galectin-3 was the most specific (71.4%) single antibody,
whilst HBME-1 had the lowest specificity (38.1%). ICAM-1 was the
most sensitive marker for the diagnosis of PTC-like features
(82.4%). With respect to antibody combinations, co-expression of
any two or three antibodies increased the specificity of the
diagnosis of PTC-like features to >70% (range, 71.4–85.7%), with
three panels being 85.7% specific (CK-19/galectin-3;
ICAM-1/CK-19/galectin-3; and CK-19/galectin-3/HBME-1); however, the
sensitivity of the three antibody combinations was significantly
lower than for a single antibody (Table
IV).
 | Table IV.Expression of four
immunohistochemical markers in Hashimoto's thyroiditis patients
(n=72). |
Table IV.
Expression of four
immunohistochemical markers in Hashimoto's thyroiditis patients
(n=72).
|
|
| Golden criteria,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Markers | Patients, n | Positive | Negative | P-value | Specificity, % | Sensitivity, % |
|---|
| ICAM-1 |
|
|
|
1.000 |
|
|
|
Positive | 22 | 13 | 9 |
| 61.9 | 82.4 |
|
Negative | 50 | 8 | 42 |
|
|
|
| CK-19 |
|
|
|
0.071 |
|
|
|
Positive | 32 | 11 | 21 |
| 52.4 | 58.8 |
|
Negative | 40 | 10 | 30 |
|
|
|
| GAL-3 |
|
|
|
0.009 |
|
|
|
Positive | 35 | 15 | 20 |
| 71.4 | 60.8 |
|
Negative | 37 | 6 | 31 |
|
|
|
| HBME-1 |
|
|
|
1.000 |
|
|
|
Positive | 20 | 8 | 12 |
| 38.1 | 76.5 |
|
Negative | 52 | 13 | 39 |
|
|
|
| ICAM-1/CK-19 |
|
|
| <0.001 |
|
|
|
Positive | 43 | 16 | 27 |
| 76.2 | 47.1 |
|
Negative | 29 | 5 | 24 |
|
|
|
| ICAM-1/GAL-3 |
|
|
| <0.001 |
|
|
|
Positive | 44 | 17 | 27 |
| 81.0 | 47.1 |
|
Negative | 28 | 4 | 24 |
|
|
|
| ICAM-1/HBME-1 |
|
|
|
0.009 |
|
|
|
Positive | 35 | 15 | 20 |
| 71.4 | 60.8 |
|
Negative | 37 | 6 | 31 |
|
|
|
| CK-19/GAL-3 |
|
|
| <0.001 |
|
|
|
Positive | 51 | 18 | 33 |
| 85.7 | 35.3 |
|
Negative | 21 | 3 | 18 |
|
|
|
| CK-19/HBME-1 |
|
|
|
0.001 |
|
|
|
Positive | 39 | 15 | 24 |
| 71.4 | 52.9 |
|
Negative | 33 | 6 | 27 |
|
|
|
| GAL-3/HBME-1 |
|
|
|
0.001 |
|
|
|
Positive | 41 | 15 | 26 |
| 71.4 | 49.0 |
|
Negative | 31 | 6 | 25 |
|
|
|
|
ICAM-1/CK-19/GAL-3 |
|
|
| <0.001 |
|
|
|
Positive | 55 | 18 | 37 |
| 85.7 | 27.5 |
|
Negative | 17 | 3 | 14 |
|
|
|
|
ICAM-1/CK-19/HBME-1 |
|
|
| <0.001 |
|
|
|
Positive | 46 | 17 | 29 |
| 81.0 | 43.1 |
|
Negative | 26 | 4 | 22 |
|
|
|
|
ICAM-1/GAL-3/HBME-1 |
|
|
| <0.001 |
|
|
|
Positive | 49 | 17 | 32 |
| 81.0 | 37.3 |
|
Negative | 23 | 4 | 19 |
|
|
|
|
CK-19/GAL-3/HBME-1 |
|
|
| <0.001 |
|
|
|
Positive | 52 | 18 | 34 |
| 85.7 | 33.3 |
|
Negative | 20 | 3 | 17 |
|
|
|
Discussion
PTC is the most prevalent manifestation of cancer of
the thyroid gland, representing 70–80% of all cancers of the
thyroid gland (11). Examination by
B-ultrasound has become the first choice for auxiliary examination
of thyroid nodular disease, whilst pathological examination remains
the ‘gold standard’ for the diagnosis of cancer (12). However, differentiating PTC from
benign papillary hyperplasia of the thyroid gland based on its
morphology (particularly if it exhibits PTC-like nuclear
alterations) is challenging. Hence, identifying sensitive and
specific IHC markers to differentiate between benign thyroid
nodular disease and PTC is urgently required. CK-19, HBME-1 and
galectin-3 have been demonstrated to show higher expression in PTC
than in benign follicular lesions of the thyroid gland (13); however, results among studies have
varied (14,15). The present study assessed ICAM-1
expression and evaluated the diagnostic importance of PTC, as well
as the potential value of measuring ICAM-1 expression in HT with
PTC-like features.
In healthy individuals, ICAM-1 is expressed at low
levels on various cell types, including endothelial cells,
fibroblasts, and certain types of leukocyte (2). Recently, it has also been reported to
play an important role in promoting progression in various types of
cancer. Hayes et al (2)
observed ICAM-1 expression in 300 tissue cores from multiple arrays
of normal, malignant and metastatic tissues by IHC analyses. They
observed ICAM-1 expression to be associated with various cancer
types, and it appeared to play a part in cancer metastasis
(2). Several studies have
demonstrated upregulation of ICAM-1 expression in PTC (16,17).
Buitrago et al (7) identified
expression of the ICAM-1 gene to be higher in PTC and LNM when
compared with benign tumors. In accordance with those results, 113
of 132 PTC samples exhibited overexpression of ICAM-1 in the
present study, whereas no cases of follicular cancer, follicular
adenoma, nodular goiter or normal thyroid tissues were
immunoreactive. A constant diagnostic challenge occurs when
differentiating the follicular variant of PTC from follicular
lesions (adenoma and cancer). In the present study, 10 of 16
follicular-variant PTC cases exhibited moderate to high expression
of ICAM-1, supporting the notion of ICAM-1 as a specific marker in
differentiating between follicular-type lesions in thyroid
tissues.
Furthermore, ICAM-1 expression was demonstrated to
be associated with certain clinicopathological characteristics of
patients. The growth pattern of the majority of PTC cases
expressing ICAM-1 tended to have extra-thyroidal invasion and LNM,
suggesting aggressive behavior. ICAM-1 has been demonstrated to
facilitate the spread of metastatic cancer cells via the
recruitment of inflammatory cells, by stimulating their
proliferation, angiogenesis and invasion (18). However, the underlying mechanism of
this action remains unclear.
The association between HT and PTC is controversial.
The prevalence of cancer in HT has been reported to range from
<1 to 32% (19–21). Jankovic et al (19) undertook a systematic review of
original studies that investigated the correlation between HT and
PTC. Notably, studies based on fine-needle aspiration biopsy
reported no link between HT and PTC, whereas many of the studies
using thyroidectomy specimens revealed a positive association.
Several authors have postulated that the inflammatory response may
cause DNA mutations that eventually lead to the development of PTC
(22,23). Certain studies have observed a higher
risk of PTC in patients with HT, particularly those who harbor
focal PTC-like nuclear alterations in thyroid epithelial cells
(e.g., nuclear overlapping, enlargement, chromatin clearing,
intranuclear grooves and inclusions), which may be observed in
almost one-third of HT cases on routine microscopic examination
(24–26). A number of studies have reported that
focal PTC-like changes suggest the possibility of focal, early
premalignant transformation in some cases of HT, which eventually
lead specifically to PTC (27,28). It is
likely that there is a morphological continuum between PTC-like
thyrocytes, follicular hyperplasia and metaplasia of Hürthle cells,
and the reliability of valuable IHC markers is uncertain (29).
In this work, 21 of 72 HT cases had features of
PTC-like nuclear changes, most of which exhibited clusters and
micronodules that were histologically similar to PTC. Galectin-3,
CK-19 and ICAM-1 exhibited high (71.4, 66.7 and 57.1%,
respectively) and diffuse expression. Comparatively, HBME-1
expression was lower (38.1%), and was focused in thyrocytes with
PTC-like nuclear changes. Prasad et al (24) noted focal expression of galectin-3
(87%), CK-19 (65%) and HBME-1 (26%) primarily in nodules in HT
cases, thereby demonstrating unique morphological features that
overlap with PTC, in accordance with the current results. The
utility of CK-19 expression has been studied extensively and it has
been hypothesized to be the most sensitive marker for the diagnosis
of PTC, and galectin-3 is also believed to be a valuable marker for
distinguishing PTC from benign conditions (13). However, CK-19 was observed in ~14% of
cases of follicular adenoma, and galectin-3 could accumulate in
inflammatory cells to react with the normal epithelium (13). Thus, CK-19 and galectin-3 may be less
specific markers for the discrimination of benign changes from
pre-malignant changes. How to best use these antibodies in routine
practice (particularly if dealing with questionable PTC features)
must be addressed fully.
The present study demonstrated ICAM-1 to be a
sensitive IHC marker of PTC. Furthermore, positive staining with
ICAM-1, if strong and diffuse, may be useful for distinguishing
PTC-like alterations in HT from histological mimics. All 5 HT cases
diagnosed with contralateral thyroid cancer following surgery were
strongly positive for ICAM-1 expression, suggesting that ICAM-1 is
a potential marker for predicting the possibility of progression to
PTC in HT with PTC-like features. It was previously reported that
expression of RET/PTC-1 and RET/PTC-3 oncogenes in patients with HT
may identify HT as a pre-neoplastic lesion (25,26).
Reports have also stated that p63 protein (30,31), and
the loss of heterozygosity of 8-oxoguanine DNA glycosylase may be
involved in neoplastic transformation from HT to PTC (32). To date, however, no affirmative
genetic linkage has been confirmed. We suggest that ICAM-1 be used
as part of a panel that may include CK-19 and galectin-3, depending
on the differential diagnosis that is considered.
ICAM-1 appears to be a sensitive and diagnostically
useful marker of PTC. Thus, it is proposed that ICAM-1 be used as
part of a panel (and not in isolation) for the detection of
PTC-like changes in thyrocytes in patients with HT. A statistically
significant positive relationship between HT and PTC was not
observed; however, careful observation and follow-up of HT patients
is recommended (particularly for those with PTC-like
alterations).
Acknowledgements
This research was supported by the Scientific
Research Fund for the Health Development of Shandong Province,
China (no. 2011HW053) and the National Nature Science Foundation
for Young Scientists of China (no. 81202092).
References
|
1
|
Cheema Y, Repplinger D, Elson D and Chen
H: Is tumor size the best predictor of outcome for papillary
thyroid cancer? Ann Surg Oncol. 13:1524–1528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayes SH and Seigel GM: Immunoreactivity
of ICAM-1 in human tumors, metastases and normal tissues. Int J
Clin Exp Pathol. 2:553–560. 2009.PubMed/NCBI
|
|
3
|
Chen H, Hernandez W, Shriver MD, Ahaghotu
CA and Kittles RA: ICAM gene cluster SNPs and prostate cancer risk
in African Americans. Hum Genet. 120:69–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosette C, Roth RB, Oeth P, Braun A,
Kammerer S, Ekblom J and Denissenko MF: Role of ICAM1 in invasion
of human breast cancer cells. Carcinogenesis. 26:943–950. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Usami Y, Ishida K, Sato S, Kishino M,
Kiryu M, Ogawa Y, Okura M, Fukuda Y and Toyosawa S: Intercellular
adhesion molecule-1 (ICAM-1) expression correlates with oral cancer
progression and induces macrophage/cancer cell adhesion. Int J
Cancer. 133:568–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakashima M, Eguchi K, Ishikawa N,
Yamashita I, Sakai M, Ida H, Kawabe Y, Ito K and Nagataki S:
Expression of adhesion molecule ICAM-1 (CD54) in thyroid papillary
adenocarcinoma. J Endocrinol Invest. 17:843–848. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buitrago D, Keutgen XM, Crowley M,
Filicori F, Aldailami H, Hoda R, Liu YF, Hoda RS, Scognamiglio T,
Jin M, et al: Intercellular adhesion molecule-1 (ICAM-1) is
upregulated in aggressive papillary thyroid carcinoma. Ann Surg
Oncol. 19:973–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DeLellis RA and Williams ED: Tumours of
the thyroid and parathyroid. World Health Organization
Classification of Tumours. Pathology and Genetics of Endocrine
Organs (Lyon). IARC Press. 58–70. 2004.
|
|
9
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cvejic D, Savin S, Petrovic I, Paunovic I,
Tatic S, Krgovic K and Havelka M: Galectin-3 expression in
papillary microcarcinoma of the thyroid. Histopathology.
47:209–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheema Y, Olson S, Elson D and Chen H:
What is the biology and optimal treatment for papillary
microcarcinoma of the thyroid? J Surg Res. 134:160–162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kabaker AS, Tublin ME, Nikiforov YE,
Armstrong MJ, Hodak SP, Stang MT, McCoy KL, Carty SE and Yip L:
Suspicious ultrasound characteristics predict BRAF V600E-positive
papillary thyroid carcinoma. Thyroid. 22:585–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scognamiglio T, Hyjek E, Kao J and Chen
YT: Diagnostic usefulness of HBME1, galectin-3, CK19 and CITED1 and
evaluation of their expression in encapsulated lesions with
questionable features of papillary thyroid carcinoma. Am J Clin
Pathol. 126:700–708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El Demellawy D, Nasr A and Alowami S:
Application of CD56, P63 and CK19 immunohistochemistry in the
diagnosis of papillary carcinoma of the thyroid. Diagn Pathol.
3:52008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rossi ED, Straccia P, Palumbo M, Stigliano
E, Revelli L, Lombardi CP, Santeusanio G, Pontecorvi A and Fadda G:
Diagnostic and prognostic role of HBME-1, galectin-3, and β-catenin
in poorly differentiated and anaplastic thyroid carcinomas. Appl
Immunohistochem Mol Morphol. 21:237–241. 2013.PubMed/NCBI
|
|
16
|
Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J,
Wang J, Zhang D, Cheng S and Liu S: Expression of intercellular
adhesion molecule 1 by hepatocellular carcinoma stem cells and
circulating tumor cells. Gastroenterology. 144:1031–1041. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jenkinson C, Elliott V, Menon U,
Apostolidou S, Fourkala OE, Gentry-Maharaj A, Pereira SP, Jacobs I,
Cox TF, Greenhalf W, et al: Evaluation in pre-diagnosis samples
discounts ICAM-1 and TIMP-1 as biomarkers for earlier diagnosis of
pancreatic cancer. J Proteomics. 113:400–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin YC, Shun CT, Wu MS and Chen CC: A
novel anticancer effect of thalidomide: Inhibition of intercellular
adhesion molecule-1-mediated cell invasion and metastasis through
suppression of nuclear factor-kappaB. Clin Cancer Res.
12:7165–7173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jankovic B, Le KT and Hershman JM:
Clinical review: Hashimoto's thyroiditis and papillary thyroid
carcinoma: Is there a correlation? J Clin Endocrinol Metab.
98:474–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn D, Heo SJ, Park JH, Kim JH, Sohn JH,
Park JY, Park SK and Park J: Clinical relationship between
Hashimoto's thyroiditis and papillary thyroid cancer. Acta Oncol.
50:1228–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng V, Brainard J and Nasr C:
Co-occurrence of papillary thyroid carcinoma and primary lymphoma
of the thyroid in a patient with long-standing Hashimoto's
thyroiditis. Thyroid. 22:647–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tafani M, De Santis E, Coppola L, Perrone
GA, Carnevale I, Russo A, Pucci B, Carpi A, Bizzarri M and Russo
MA: Bridging hypoxia, inflammation and estrogen receptors in
thyroid cancer progression. Biomed Pharmacother. 68:1–5. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muzza M, Degl'Innocenti D, Colombo C,
Perrino M, Ravasi E, Rossi S, Cirello V, Beck-Peccoz P, Borrello MG
and Fugazzola L: The tight relationship between papillary thyroid
cancer, autoimmunity and inflammation: Clinical and molecular
studies. Clin Endocrinol (Oxf). 72:702–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prasad ML, Huang Y, Pellegata NS, de la
Chapelle A and Kloos RT: Hashimoto's thyroiditis with papillary
thyroid carcinoma (PTC)-like nuclear alterations express molecular
markers of PTC. Histopathology. 45:39–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sadow PM, Heinrich MC, Corless CL,
Fletcher JA and Nose V: Absence of BRAF, NRAS, KRAS, HRAS mutations
and RET/PTC gene rearrangements distinguishes dominant nodules in
Hashimoto thyroiditis from papillary thyroid carcinomas. Endocr
Pathol. 21:73–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rhoden KJ, Unger K, Salvatore G, Yilmaz Y,
Vovk V, Chiappetta G, Qumsiyeh MB, Rothstein JL, Fusco A, Santoro
M, et al: RET/papillary thyroid cancer rearrangement in
nonneoplastic thyrocytes: Follicular cells of Hashimoto's
thyroiditis share low-level recombination events with a subset of
papillary carcinoma. J Clin Endocrinol Metab. 91:2414–2423. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z,
Wang F, Duan Z, Xin S and Zhang J: Hashimoto's thyroiditis as a
risk factor of papillary thyroid cancer may improve cancer
prognosis. Otolaryngol Head Neck Surg. 148:396–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konturek A, Barczyński M, Wierzchowski W,
Stopa M and Nowak W: Coexistence of papillary thyroid cancer with
Hashimoto thyroiditis. Langenbecks Arch Surg. 398:389–394. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Pasquale M, Rothstein JL and Palazzo
JP: Pathologic features of Hashimoto's-associated papillary thyroid
carcinomas. Hum Pathol. 32:24–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Unger P, Ewart M, Wang BY, Gan L, Kohtz DS
and Burstein DE: Expression of p63 in papillary thyroid carcinoma
and in Hashimoto's thyroiditis: A pathobiologic link? Hum Pathol.
34:764–769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burstein DE, Nagi C, Wang BY and Unger P:
Immunohistochemical detection of p53 homolog p63 in solid cell
nests, papillary thyroid carcinoma and hashimoto's thyroiditis: A
stem cell hypothesis of papillary carcinoma oncogenesis. Hum
Pathol. 35:465–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Royer MC, Zhang H, Fan CY and Kokoska MS:
Genetic alterations in papillary thyroid carcinoma and hashimoto
thyroiditis: An analysis of hOGG1 loss of heterozygosity. Arch
Otolaryngol Head Neck Surg. 136:240–242. 2010. View Article : Google Scholar : PubMed/NCBI
|