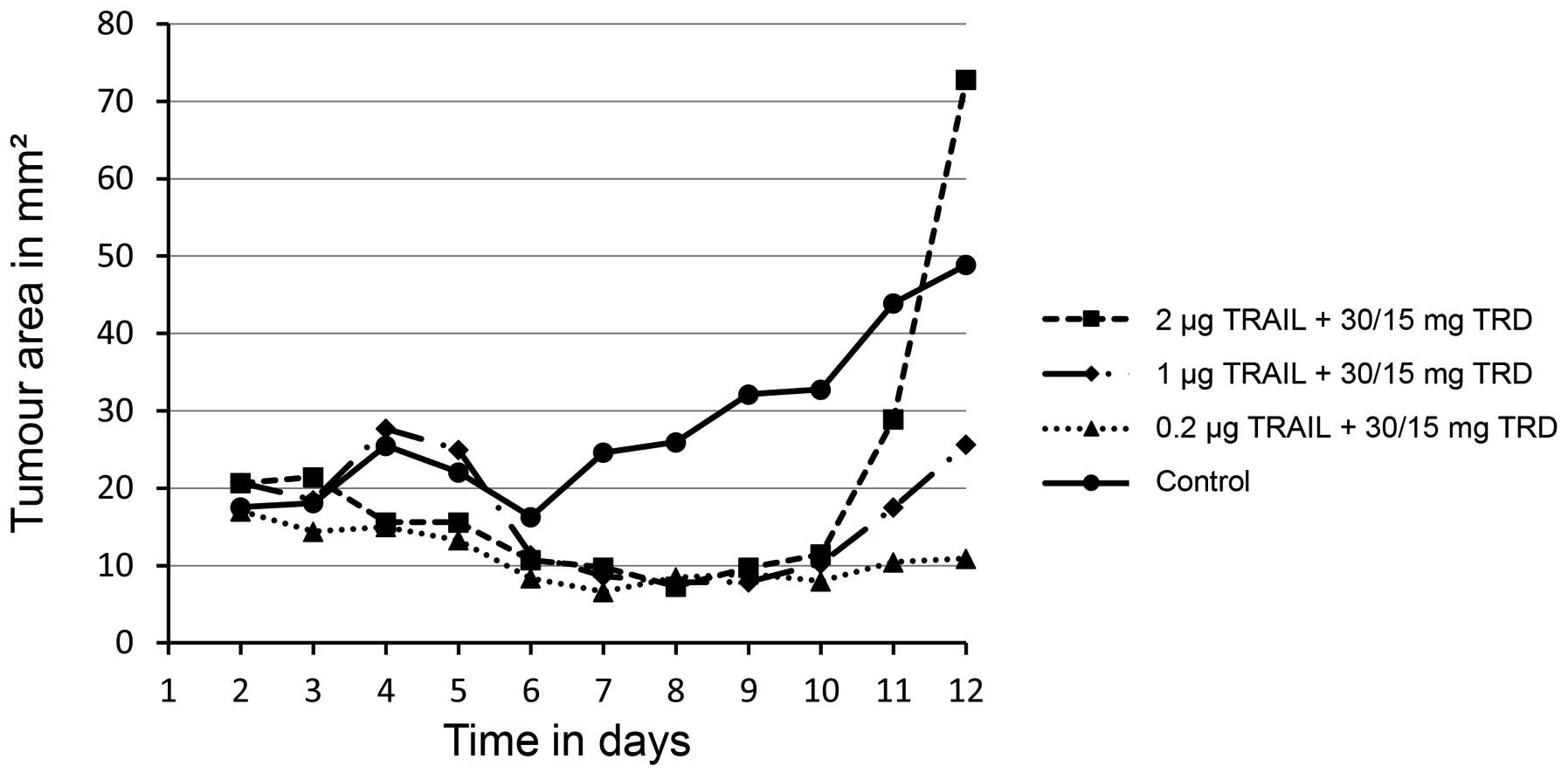
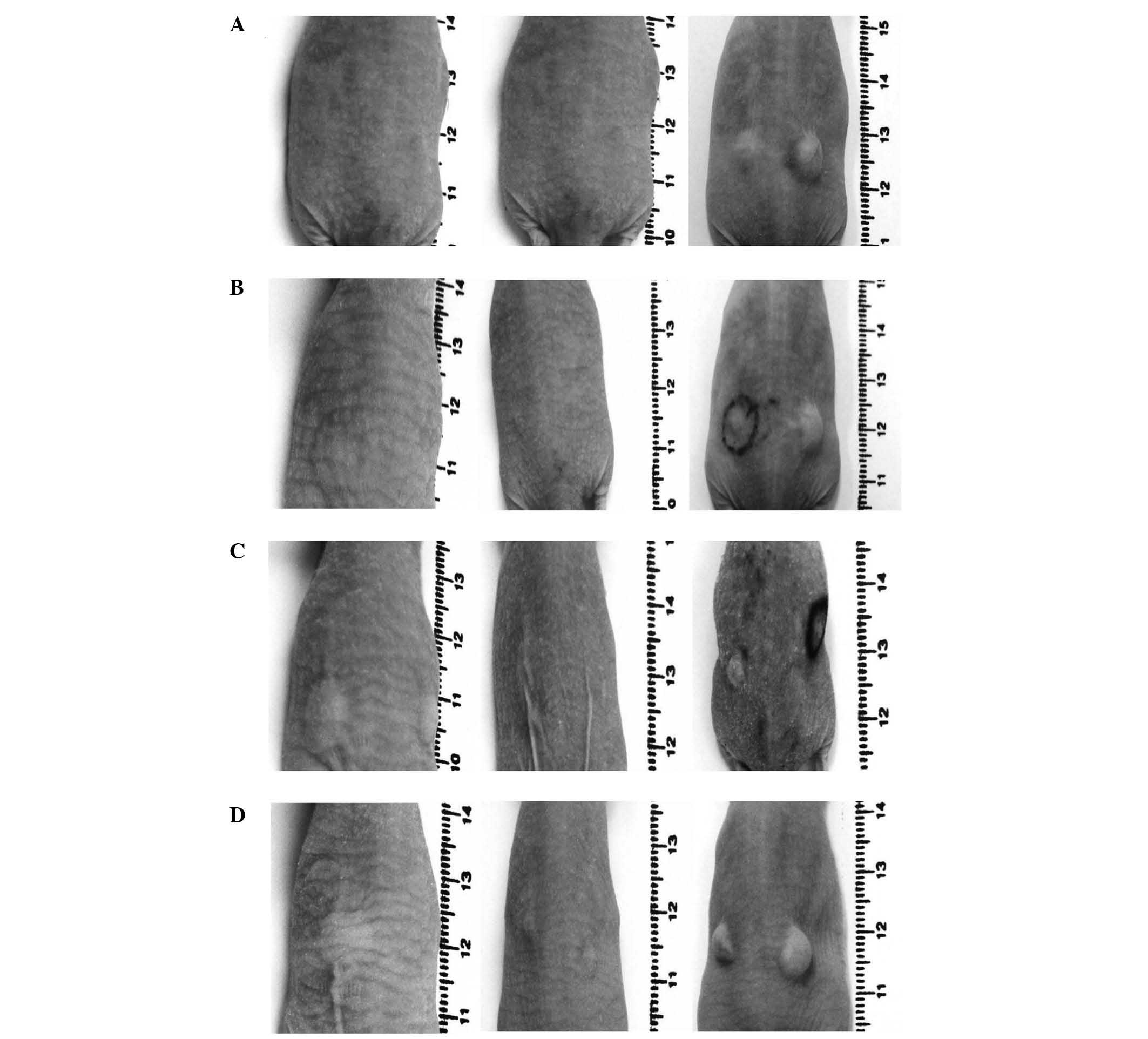
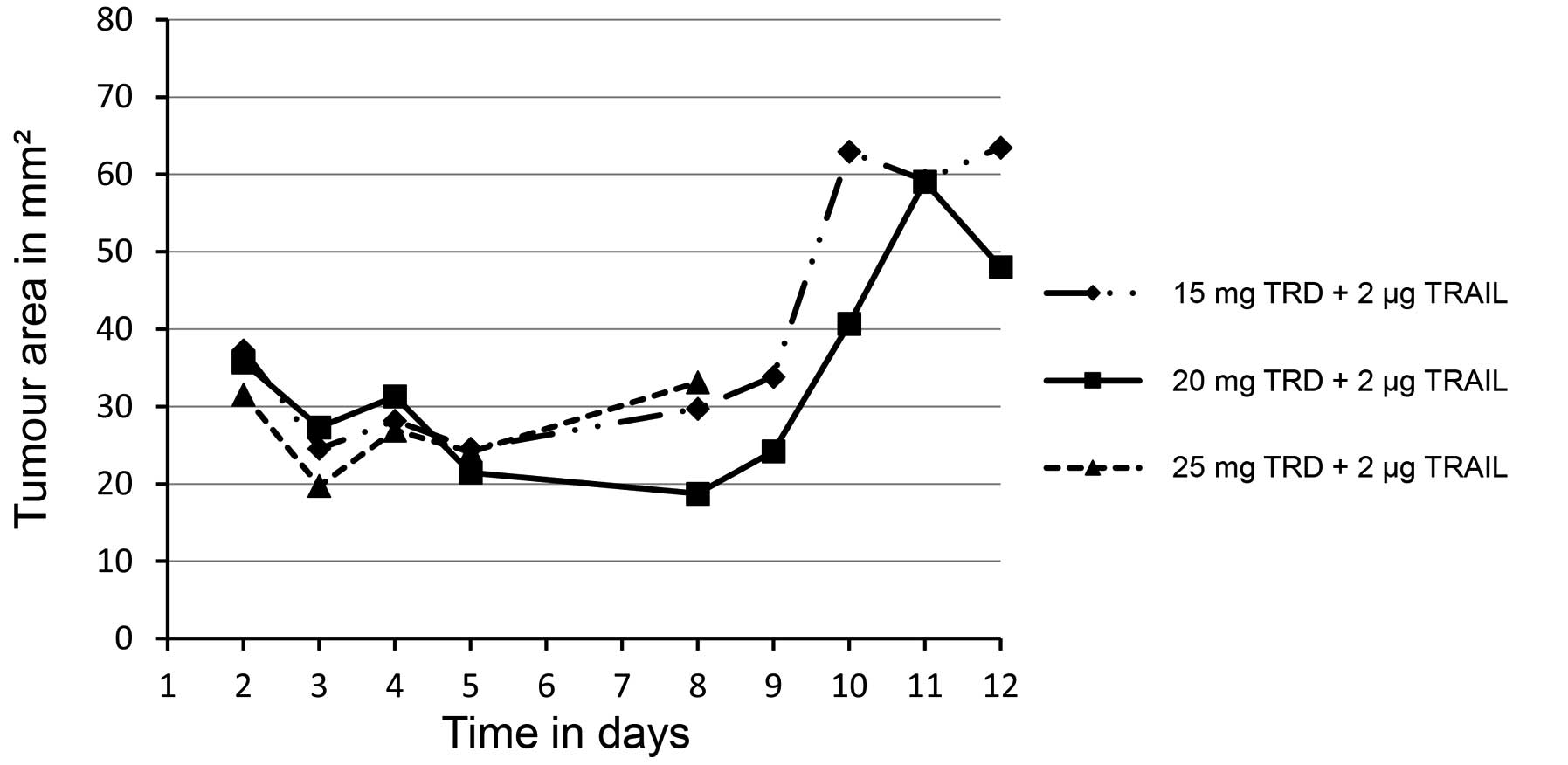
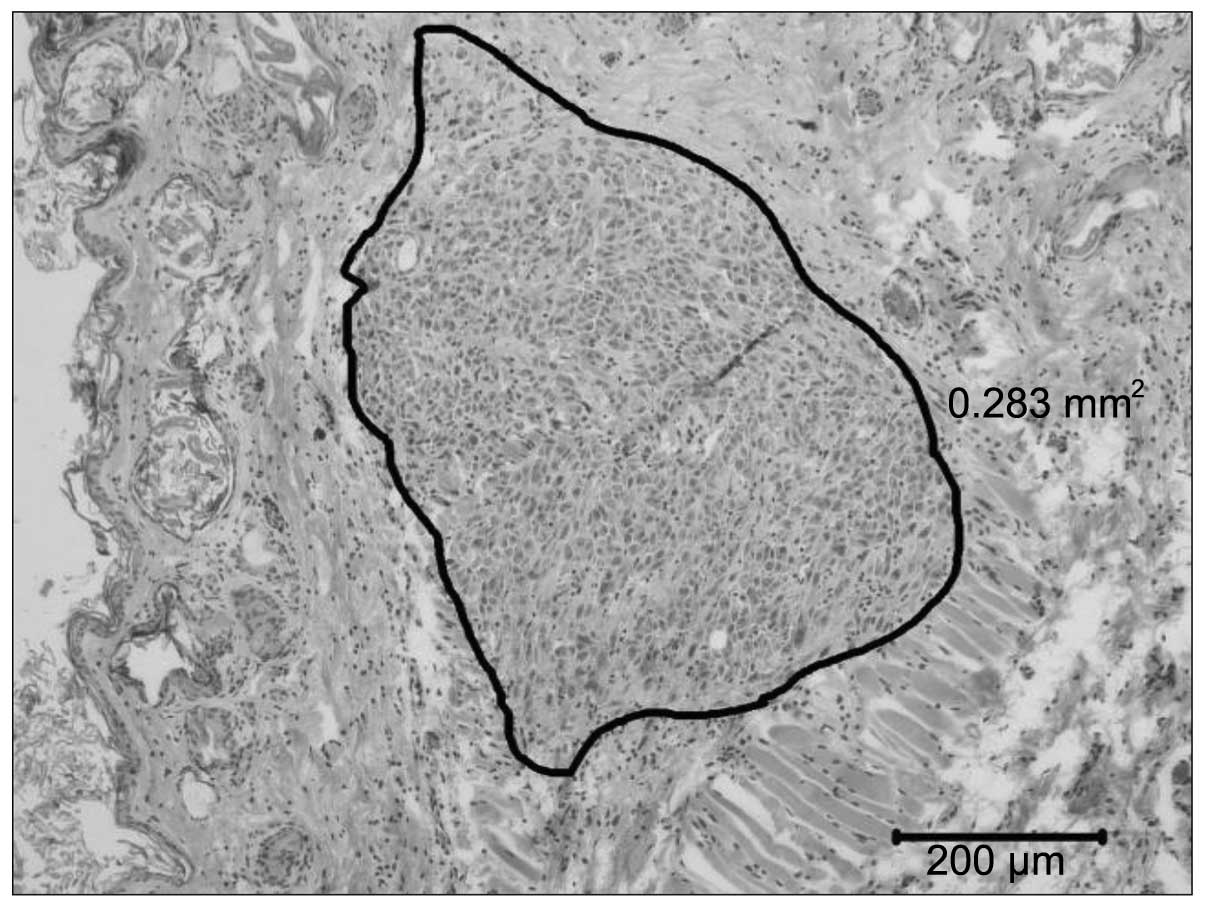
|
1
|
Nedea EA and DeLaney TF: Sarcoma and skin
radiation oncology. Hematol Oncol Clin North Am. 20:401–429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gronchi A, Casali PG, Mariani L, Miceli R,
Fiore M, Lo Vullo S, Bertulli R, Collini P, Lozza L, Olmi P and
Rosai J: Status of surgical margins and prognosis in adult soft
tissue sarcomas of the extremities: A series of patients treated at
a single institution. J Clin Oncol. 23:96–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patrikidou A, Domont J, Cioffi A and Le
Cesne A: Treating soft tissue sarcomas with adjuvant chemotherapy.
Current Treat Options Oncol. 12:21–31. 2011. View Article : Google Scholar
|
|
5
|
Kaushal A and Citrin D: The role of
radiation therapy in the management of sarcomas. Surg Clin North
Am. 88:629–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Brien GC, Cahill RA, Bouchier-Hayes DJ
and Redmond HP: Co-immunotherapy with interleukin-2 and taurolidine
for progressive metastatic melanoma. Ir J Med Sci. 175:10–14. 2006.
View Article : Google Scholar
|
|
7
|
Solomon LR, Cheesbrough JS, Bhargava R,
Mitsides N, Heap M, Green G and Diggle P: Observational study of
need for thrombolytic therapy and incidence of bacteremia using
taurolidine-citrate-heparin, taurolidine-citrate and heparin
catheter locks in patients treated with hemodialysis. Semin Dial.
25:233–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karavasilis V, Seddon BM, Ashley S,
Al-Muderis O, Fisher C and Judson I: Significant clinical benefit
of first-line palliative chemotherapy in advanced soft-tissue
sarcoma: Retrospective analysis and identification of prognostic
factors in 488 patients. Cancer. 112:1585–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Billingsley KG, Lewis JJ, Leung DH, Casper
ES, Woodruff JM and Brennan MF: Multifactorial analysis of the
survival of patients with distant metastasis arising from primary
extremity sarcoma. Cancer. 85:389–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pezzi CM, Pollock RE, Evans HL, Lorigan
JG, Pezzi TA, Benjamin RS and Romsdahl MM: Preoperative
chemotherapy for soft-tissue sarcomas of the extremities. Ann Surg.
211:476–481. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Donato Paola E and Nielsen OS: EORTC
Soft Tissue and Bone Sarcoma Group: The EORTC soft tissue and bone
sarcoma group: The EORTC soft tissue and bone sarcoma group.
European organisation for research and treatment of cancer. Eur J
Cancer. 38(Suppl 4): S138–S141. 2002.PubMed/NCBI
|
|
12
|
Brodowicz T, Schwameis E, Widder J, Amann
G, Wiltschke C, Dominkus M, Windhager R, Ritschl P, Pötter R, Kotz
R and Zielinski CC: Intensified adjuvant IFADIC chemotherapy for
adult soft tissue sarcoma: A prospective randomized feasibility
trial. Sarcoma. 4:151–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frustaci S, Gherlinzoni F, De Paoli A,
Bonetti M, Azzarelli A, Comandone A, Olmi P, Buonadonna A, Pignatti
G, Barbieri E, et al: Adjuvant chemotherapy for adult soft tissue
sarcomas of the extremities and girdles: Results of the Italian
randomized cooperative trial. J Clin Oncol. 19:1238–1247.
2001.PubMed/NCBI
|
|
14
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: Doxorubicin alone versus intensified
doxorubicin plus ifosfamide for first-line treatment of advanced or
metastatic soft-tissue sarcoma: A randomised controlled phase 3
trial. Lancet Oncol. 15:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yagita H, Takeda K, Hayakawa Y, Smyth MJ
and Okumura K: TRAIL and its receptors as targets for cancer
therapy. Cancer Sci. 95:777–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouralexis S, Findlay DM and Evdokiou A:
Death to the bad guys: Targeting cancer via Apo2L/TRAIL. Apoptosis.
10:35–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rowinsky EK: Targeted induction of
apoptosis in cancer management: The emerging role of tumor necrosis
factor-related apoptosis-inducing ligand receptor activating
agents. J Clin Oncol. 23:9394–9407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daniel P: Molecular principles of
apoptosis. Principles of Molecular Medicine. Ganten D and Ruckpaul
K: (3rd). (Berlin Heidelberg). Springer. 159–203. 2008.
|
|
20
|
Newsom-Davis T, Prieske S and Walczak H:
Is TRAIL the holy grail of cancer therapy? Apoptosis. 14:607–623.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chromik AM, Daigeler A, Bulut D, Flier A,
May C, Harati K, Roschinsky J, Sülberg D, Ritter PR, Mittelkötter
U, et al: Comparative analysis of cell death induction by
Taurolidine in different malignant human cancer cell lines. J Exp
Clin Cancer Res. 29:212010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacobi CA, Menenakos C and Braumann C:
Taurolidine-a new drug with anti-tumor and anti-angiogenic effects.
Anticancer Drugs. 16:917–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCourt M, Wang JH, Sookhai S and Redmond
HP: Taurolidine inhibits tumor cell growth in vitro and
in vivo. Ann Surg Oncol. 7:685–691. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petrovic L, Schlegel KA, Ries J, Park J,
Diebel E, Schultze-Mosgau S and Wiltfang J: In vitro effect of
taurolidine on squamous cell carcinoma in the oral cavity. Mund
Kiefer Gesichtschir. 7:102–107. 2003.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gallagher KA, Liu ZJ, Xiao M, Chen H,
Goldstein LJ, Buerk DG, Nedeau A, Thom SR and Velazquez OC:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1 alpha.
J Clin Invest. 117:1249–1259. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calabresi P, Goulette FA and Darnowski JW:
Taurolidine: Cytotoxic and mechanistic evaluation of a novel
antineoplastic agent. Cancer Res. 61:6816–6821. 2001.PubMed/NCBI
|
|
28
|
Braumann C, Henke W, Jacobi CA and Dubiel
W: The tumor-suppressive reagent taurolidine is an inhibitor of
protein biosynthesis. Int J Cancer. 112:225–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnowski JW, Goulette FA, Cousens LP,
Chatterjee D and Calabresi P: Mechanistic and antineoplastic
evaluation of taurolidine in the DU145 model of human prostate
cancer. Cancer Chemother Pharmacol. 54:249–258. 2004.PubMed/NCBI
|
|
30
|
Stendel R, Biefer HR, Dékány GM, Kubota H,
Münz C, Wang S, Mohler H, Yonekawa Y and Frei K: The antibacterial
substance taurolidine exhibits anti-neoplastic action based on a
mixed type of programmed cell death. Autophagy. 5:194–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stendel R, Scheurer L,
Stoltenburg-Didinger G, Brock M and Möhler H: Enhancement of
fas-ligand-mediated programmed cell death by taurolidine.
Anticancer Res. 23:2309–2314. 2003.PubMed/NCBI
|
|
32
|
Chromik AM, Daigeler A, Hilgert C, Bulut
D, Geisler A, Liu V, Otte JM, Uhl W and Mittelkötter U: Synergistic
effects in apoptosis induction by taurolidine and TRAIL in HCT-15
colon carcinoma cells. J Invest Surg. 20:339–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daigeler A, Chromik AM, Geisler A, Bulut
D, Hilgert C, Krieg A, Klein-Hitpass L, Lehnhardt M, Uhl W and
Mittelkötter U: Synergistic apoptotic effects of taurolidine and
TRAIL on squamous carcinoma cells of the esophagus. Int J Oncol.
32:1205–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daigeler A, Chromik AM, Haendschke K,
Emmelmann S, Siepmann M, Hensel K, Schmitz G, Klein-Hitpass L,
Steinau HU, Lehnhardt M and Hauser J: Synergistic effects of
sonoporation and taurolidin/TRAIL on apoptosis in human
fibrosarcoma. Ultrasound Med Biol. 36:1893–1906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karlisch C, Harati K, Chromik AM, Bulut D,
Klein-Hitpass L, Goertz O, Hirsch T, Lehnhardt M, Uhl W and
Daigeler A: Effects of TRAIL and taurolidine on apoptosis and
proliferation in human rhabdomyosarcoma, leiomyosarcoma and
epithelioid cell sarcoma. Int J Oncol. 42:945–956. 2013.PubMed/NCBI
|
|
36
|
Daigeler A, Brenzel C, Bulut D, Geisler A,
Hilgert C, Lehnhardt M, Steinau HU, Flier A, Steinstraesser L,
Klein-Hitpass L, et al: TRAIL and taurolidine induce apoptosis and
decrease proliferation in human fibrosarcoma. J Exp Clin Cancer
Res. 27:822008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun BS, Wang JH, Liu LL, Gong SL and
Redmond HP: Taurolidine induces apoptosis of murine melanoma cells
in vitro and in vivo by modulation of the Bcl-2
family proteins. J Surg Oncol. 96:241–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nici L, Monfils B and Calabresi P: The
effects of taurolidine, a novel antineoplastic agent, on human
malignant mesothelioma. Clin Cancer Res. 10:7655–7661. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arlt MJ, Walters DK, Banke IJ, Steinmann
P, Puskas GJ, Bertz J, Rentsch KM, Ehrensperger F, Born W and Fuchs
B: The antineoplastic antibiotic taurolidine promotes lung and
liver metastasis in two syngeneic osteosarcoma mouse models and
exhibits severe liver toxicity. Int J Cancer. 131:E804–E812. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marley K, Helfand SC, Simpson J, Mata JE,
Tracewell WG, Brownlee L, Bracha S and Séguin B: Pharmacokinetic
study and evaluation of the safety of taurolidine for dogs with
osteosarcoma. J Exp Clin Cancer Res. 32:742013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marley K, Helfand SC, Edris WA, Mata JE,
Gitelman AI, Medlock J and Séguin B: The effects of taurolidine
alone and in combination with doxorubicin or carboplatin in canine
osteosarcoma in vitro. BMC Vet Res. 9:152013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Braumann C, Winkler G, Rogalla P,
Menenakos C and Jacobi CA: Prevention of disease progression in a
patient with a gastric cancer-re-recurrence. Outcome after
intravenous treatment with the novel antineoplastic agent
taurolidine. Report of a case. World J Surg Oncol. 4:342006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Möhler H, Pfirrmann RW and Frei K:
Redox-directed cancer therapeutics: Taurolidine and piperlongumine
as broadly effective antineoplastic agents (review). Int J Oncol.
45:1329–1336. 2014.PubMed/NCBI
|
|
44
|
Imhof L, Goldinger SM, Baumann K, Schad K,
French LE, Röthlisberger P and Dummer R: The antibacterial
substance, taurolidine in the second/third-line treatment of very
advanced stage IV melanoma including brain metastases: Results of a
phase 2, open-label study. Melanoma Res. 21:80–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stendel R, Picht T, Schilling A,
Heidenreich J, Loddenkemper C, Jänisch W and Brock M: Treatment of
glioblastoma with intravenous taurolidine. First clinical
experience. Anticancer Res. 24:1143–1147. 2004.PubMed/NCBI
|