Introduction
Fibrosarcomas are rare soft tissue sarcomas
originating from the intra- and intermuscular fibrous tissues,
fascia and tendons. Every year, they account for ~3% of all soft
tissue sarcomas, which themselves represent ~1% of all new cancer
diagnoses in the United States and Europe (1–3). Due to
their rarity, therapy for fibrosarcomas must be individualised and
multimodal. At present, the standard therapy comprises surgical
resection with a wide margin of healthy tissue, typically
accompanied by radiation treatment in order to reduce the chance of
local recurrence (4,5). Unfortunately, distant metastasis occurs
in ~50% of all patients, and surgical treatment is thus unavailable
(6,7).
In patients with metastatic disease, the median
survival time with or without chemotherapeutic treatment is <12
months (8,9). Few agents, such as doxorubicin,
dacarbazine and ifosfamide, have been proven to be effective in the
therapy of soft tissue sarcomas in general (4). However, these treatments have shown poor
results and are rarely associated with significant improvements in
overall survival (10). In
disseminated disease, a response rate of 20–30% has been observed
with doxorubicin, the most frequently used chemotherapeutic agent
in the treatment of soft tissue sarcomas (1,11).
Furthermore, the combined use of doxorubicin and ifosfamide
demonstrates greater efficacy, with higher response rates compared
with those of doxorubicin alone; however, this treatment is
associated with severe short- and long-term toxicities, including
cardiomyopathy and bone marrow suppression (12–14). The
recently published EORTC 62012 trial, which involved 455 patients
with locally advanced, unresectable or metastatic high-grade
soft-tissue sarcomas, concluded that an intensified therapy with
doxorubicin and ifosfamide is unsuitable for palliation of advanced
soft-tissue sarcomas due to the severe side effects, and should
only be used when the specific goal is tumour shrinkage (14). Thus, the optimal treatment,
particularly for metastatic or unresectable fibrosarcomas, remains
to be defined.
The purpose of the present study was to determine
the antitumour effects of tumour necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) and taurolidine on HT1080 human
fibrosarcoma xenografts in vivo, with the final aim of
identifying a suitable combination for the treatment of
disseminated fibrosarcomas. The apoptosis-inducing effects of
TRAIL, which is a member of the TNF-superfamily, have been observed
in a number of types of malignant cells (15–18). The
receptors for TRAIL, death receptors 4 and 5, are oligomerised upon
its binding, leading to the recruitment of FAS-associated protein
with death domain to form a functional death-inducing signaling
complex (DISC). Subsequently, DISC induces the activation of the
extrinsic apoptosis pathway via caspase 8 (19–21).
Taurolidine, an antiseptic agent derived from the
amino acid taurine, has been utilized for the treatment of
peritonitis and catheter-related infections (22). Additionally, studies have demonstrated
that taurolidine may be used effectively to treat certain malignant
diseases (23–26). In numerous malignant cell lines,
taurolidine treatment led to the inhibition of proliferation and
the induction of cell death (23–25,27–31).
Whilst its precise mechanism of action remains unclear, it appears
to involve translational inhibition and various pathways of
programmed cell death (28–30).
TRAIL and taurolidine represent a promising combined
therapy able to exert synergistic apoptotic effects on various
types of malignant cells in vitro, including soft tissue
sarcoma cell lines, such as HT1080 fibrosarcoma (32–35). In
studies conducted in vitro, the combination of TRAIL and
taurolidine was revealed to result in sustained cell death, and
this was superior to single treatment with TRAIL or taurolidine,
despite the lower concentrations of the two substances used in the
combination trials (32,33,36).
Experimental findings have also demonstrated that combined
treatment with taurolidine may reduce the potential toxic side
effects of TRAIL by reducing the required optimal dose of TRAIL,
and by modulating its effector pathways without affecting the
efficacy of its antitumour activity (33).
Inspired by these results, the present study
examined the antitumour effects of the two compounds in different
single and cumulative treatment doses on human fibrosarcoma
xenografts in athymic nude mice.
Materials and methods
Cell culture
HT1080 human fibrosarcoma cells were purchased from
the American Type Culture Collection (ATCC; Wesel, Germany; cell
line CCL-121) and maintained in minimal essential medium (Biowest,
Nuaillé, France); containing 10% foetal bovine serum (Pan-Biotech
GmbH, Aidenbach, Germany) supplemented with 1% penicillin (100
U/ml; Sigma-Aldrich, St. Louis, MO, USA) and streptomycin (100
µg/ml; Sigma-Aldrich) as well as 1% L-glutamine (Sigma-Aldrich).
The cells were grown to a sub-confluent monolayer and maintained at
37°C in a humidified atmosphere of 5% CO2.
Reagents
Taurolidine (Taurolin® 2%; Boehringer Ingelheim
Pharma GmbH & Co. KG, Ingelheim, Germany) containing 5% Povidon
was used as supplied by the manufacturer. Recombinant human TRAIL
(Bender MedSystems GmbH, Vienna, Austria) was dissolved in
distilled water according to the manufacturer's instructions.
Phosphate-buffered saline (GE Healthcare Life Sciences, Pasching,
Austria) was applied in equal volume as a control substance.
Animals
The treated animals were congenitally athymic nude
mice (Foxn1nu/nu; Harlan Winkelmann GmbH, Borchen,
Germany), 5 weeks of age and weighing 20–25 g. The animals were
housed in ventilated, pathogen-free racks under a 12 h light-dark
photoperiodicity with controlled humidity (50–60%) and temperature
(22–24°C). The animals were allowed sterile food (Ssniff
Spezialdiäten GmbH, Soest, Germany) and water ad libitum.
All animals were acclimated for 8 days under these conditions prior
to commencing the investigations. Injections and other
manipulations were conducted aseptically inside a laminar flow hood
(Tecniplast, Hohenpeißenberg, Germany).
The experimental procedures were conducted with
strict adherence to the rules and guidelines for the ethical use of
animals in research according to the German animal welfare law
(Tierschutzgesetz), and were approved by the State Office of
Nature, Environment and Consumer Protection of the German State of
North Rhine-Westphalia (permission number,
9.93.2.10.32.07.151).
HT1080 xenograft model
HT1080 cell suspensions were injected subcutaneously
(1×106 cells) into the right and left flanks of the
athymic nude mice to establish xenograft tumours and simulate
disseminated disease. Each day, mice were weighed and tumour area
was determined with a Vernier caliper. Tumour area was calculated
based on the following equation: Tumour area (mm2) =
length (long axis) × width (short axis).
Toxicity testing
To evaluate the potential toxic effects of TRAIL and
taurolidine, 6 athymic nude mice without xenografts received
intraperitoneal (i.p.) injections of 15 mg taurolidine and 1 µg
TRAIL on 3 days/week for 4 weeks, resulting in a cumulative
taurolidine dose of 180 mg and TRAIL dose of 12 µg per animal
within 28 days. Animals were examined daily, body weights were
recorded thrice weekly, feed and water usage once a week. A
reduction in body weight of >10% was considered significant. A
complete blood count was performed prior to and following
treatment. At 4 weeks after therapy initiation, all mice were
euthanised. Kidney, liver and skin samples were retrieved and
histologically examined.
Histological examination
Mice were euthanised under gas anaesthesia with
narcoren (Merial GmbH, Hallbergmoos, Germany). Final body weight,
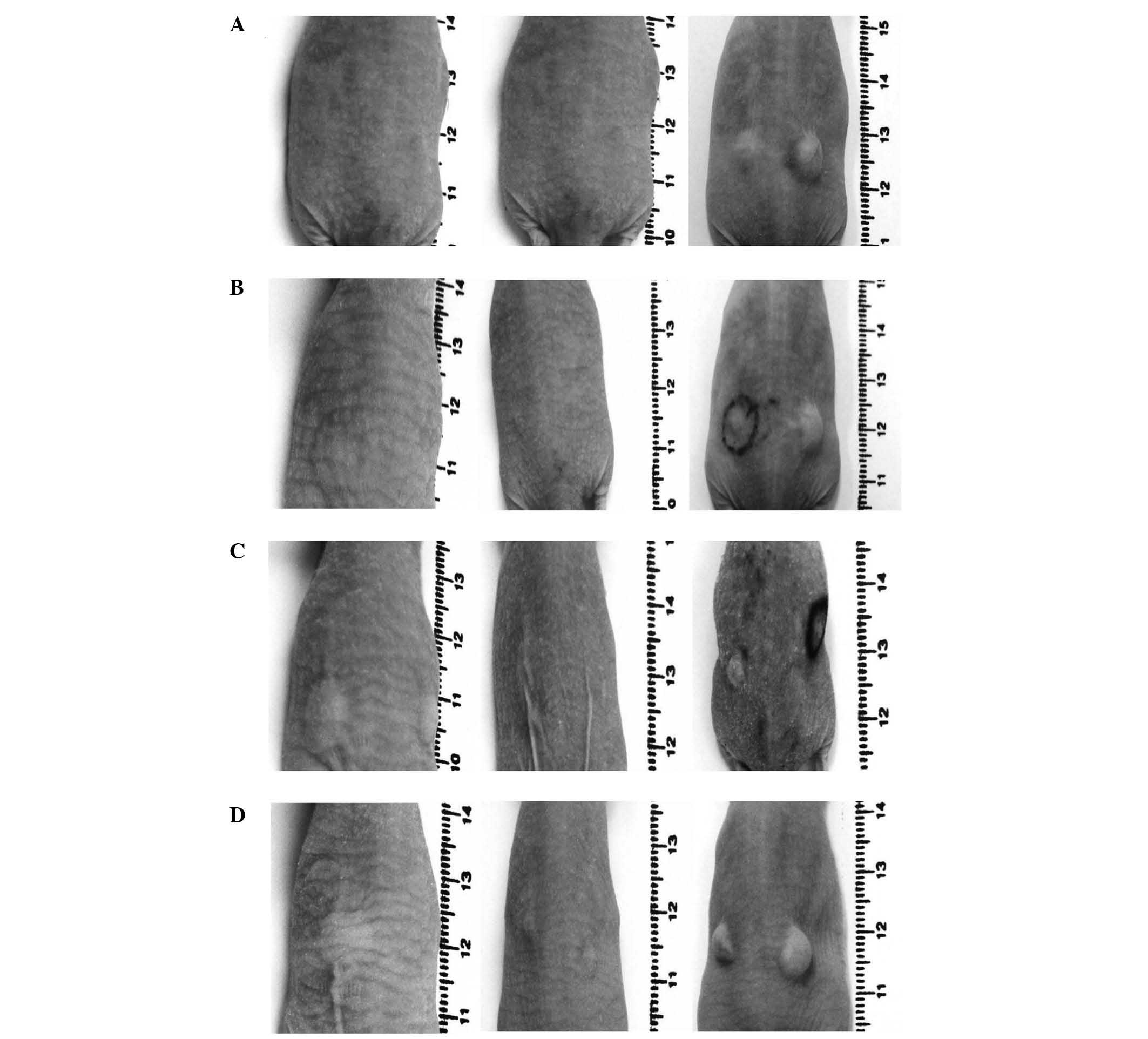
as well as the tumour size, were measured. Excised tumours and
organs (heart, lung, liver, kidney and skin samples) were fixed in
5% buffered formaldehyde. Paraffin-embedded 2–3-µm sections were
stained with haematoxylin and eosin for histological
examination.
Treatment
The antitumour activity of different doses of
combined TRAIL and taurolidine was analysed in the HT1080
xenografts. In the first series, varying doses of TRAIL (0.2, 1 or
2 µg/injection) were applied i.p. with a consistent dose of
taurolidine (30 mg/injection in the first 4-day cycle and 15
mg/injection in the second 4-day cycle) within a time range of 12
days in 40 mice bearing n=80 xenografts. From day 1 after tumour
inoculation, TRAIL was administered daily at 0.2, 1 or 2
µg/injection in combination with 30 mg/injection taurolidine for 4
days. Following a break of 2 days, treatment was continued with
administration of TRAIL at 0.2, 1 or 2 µg/injection and taurolidine
at 15 mg/injection for another 4 days. Accordingly, the first
series consisted of one control group and three treatment groups
(each n=20) with different cumulative dosages of TRAIL (1.6, 8 or
16 µg) but the same cumulative dosage of taurolidine (180 mg).
Similarly, in the second series, varying doses of taurolidine (15,
20 or 25 mg/injection) were applied with a consistent dose of TRAIL
(2 µg/injection) within a time range of 12 days in 9 mice bearing a
total of 18 xenografts. With the exception of dosage, all
procedures were performed the same way as in the first series. The
second series consisted of three treatment groups (each n=6) with
different cumulative dosage of taurolidine (120 mg, 160 mg or 200
mg) but the same cumulative dosage of TRAIL (16 µg). Tumour area
and body weight were recorded daily, feed and water usage weekly.
At 12 days after the beginning of treatment, all surviving animals
were euthanised and tumour, liver, heart, lung, kidney and skin
samples were removed and histologically examined.
Statistical analysis
SPSS version 18 (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. Comparisons between experimental
groups and trend lines were performed using one-way analysis of
variance and Tukey's post hoc test. P≤0.05 was considered to
indicate a statistically significant difference.
Results
I.p. injections of TRAIL and
taurolidine are well-tolerated in athymic nude mice without
fibrosarcoma xenografts
In the preliminary toxicity tests, combined
administration of 15 mg taurolidine and 1 µg TRAIL i.p. was not
associated with any acute toxic events. Furthermore, the cumulative
doses of 12 µg TRAIL and 180 mg taurolidine applied within 28 days
were also well-tolerated by the 6 tested immunosuppressed mice. No
significant weight losses or changes in feed and water usage were
observed. Only 1 animal died on day 22 of treatment during
anaesthesia. Otherwise, there was no evidence for significantly
increased mortality. Skin samples, liver and kidneys exhibited no
relevant histological changes, and a complete blood count revealed
no pathological alterations (data not shown).
Combined TRAIL and taurolidine
treatment leads to marked tumour growth inhibition in a TRAIL
dose-dependent manner, but is associated with significantly
increased mortality
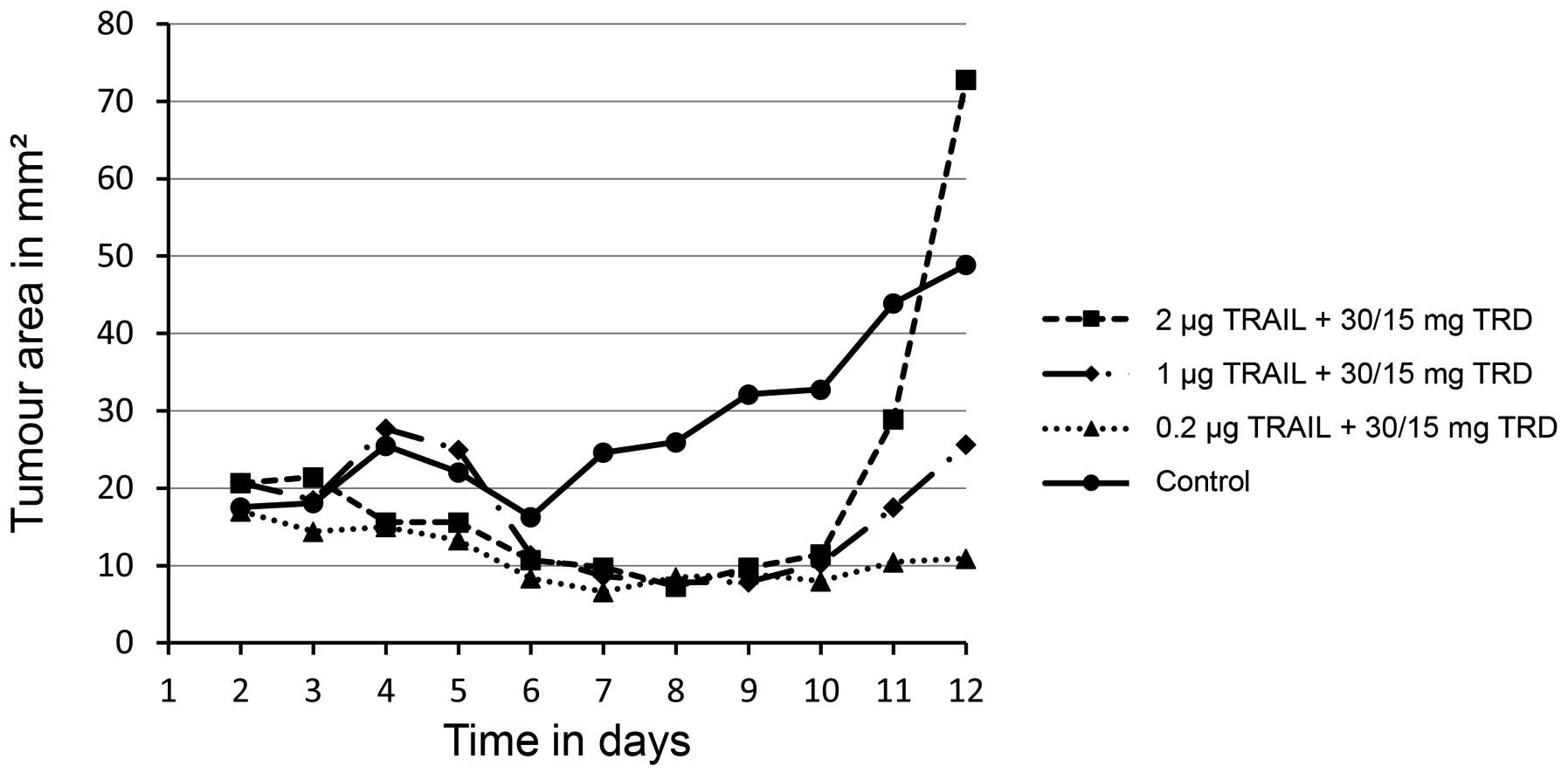
Treatment with varying doses of TRAIL (0.2 µg, 1 µg
or 2 µg/injection) and a consistent dose of taurolidine (30 mg and
15 mg/injection) significantly decreased the size of the inoculated
xenografts when compared with the untreated control group (0.2 µg,
P=0.0010; 1 µg, P=0.0080; 2 µg, P=0.0100) (Figs. 1 and 2).
Of the 58 successfully inoculated xenografts, 34 exhibited a
complete remission when examined histologically following the
treatment. The growth of a further 14 tumours was inhibited
significantly, whereas only 10 tumours increased steadily. In the
control group, 17 of 20 implanted xenografts exhibited a sustained
growth. The antitumoural effect was observed in a TRAIL
dose-dependent manner; however, the differences between the
treatment groups with various TRAIL dosages were not statistically
significant. Treatment with 2 µg TRAIL and 30 mg/15 mg taurolidine
reduced the mean size of implanted xenografts to 10.9
mm2, compared to 48.9 mm2 in the control
group (P=0.0100) (Fig. 1).
Notably, 24 of the 30 animals died during the
combination treatment with TRAIL (0.2 µg, 1 µg or 2 µg/injection)
and taurolidine (30 mg and 15 mg/injection). The mean survival
times were 7.5 days (0.2 µg TRAIL, 30/15 mg taurolidine), 8.3 days
(1 µg TRAIL, 30/15 mg taurolidine) and 7.9 days (0.2 µg TRAIL,
30/15 mg taurolidine) in the three treatment groups. Despite the
steadily growing tumours, all 10 control animals were alive at day
12 and were euthanised for further investigations. The mortality
rate was significantly higher in the treatment groups compared with
the control group (0.2 µg, P<0.0001; 1 µg, P<0.0020; 2 µg,
P<0.0010). Furthermore, the treated animals exhibited a
significant loss in body weight compared with untreated animals
(P<0.0001) (data not shown). This was accompanied by
significantly decreased feed (0.2 µg, P=0.0020; 1 µg, P=0.0050; 2
µg, P=0.0040) and water usage (0.2 µg, P=0.0070; 1 µg, P=0.0020; 2
µg, P<0.0010) by treated animals (data not shown).
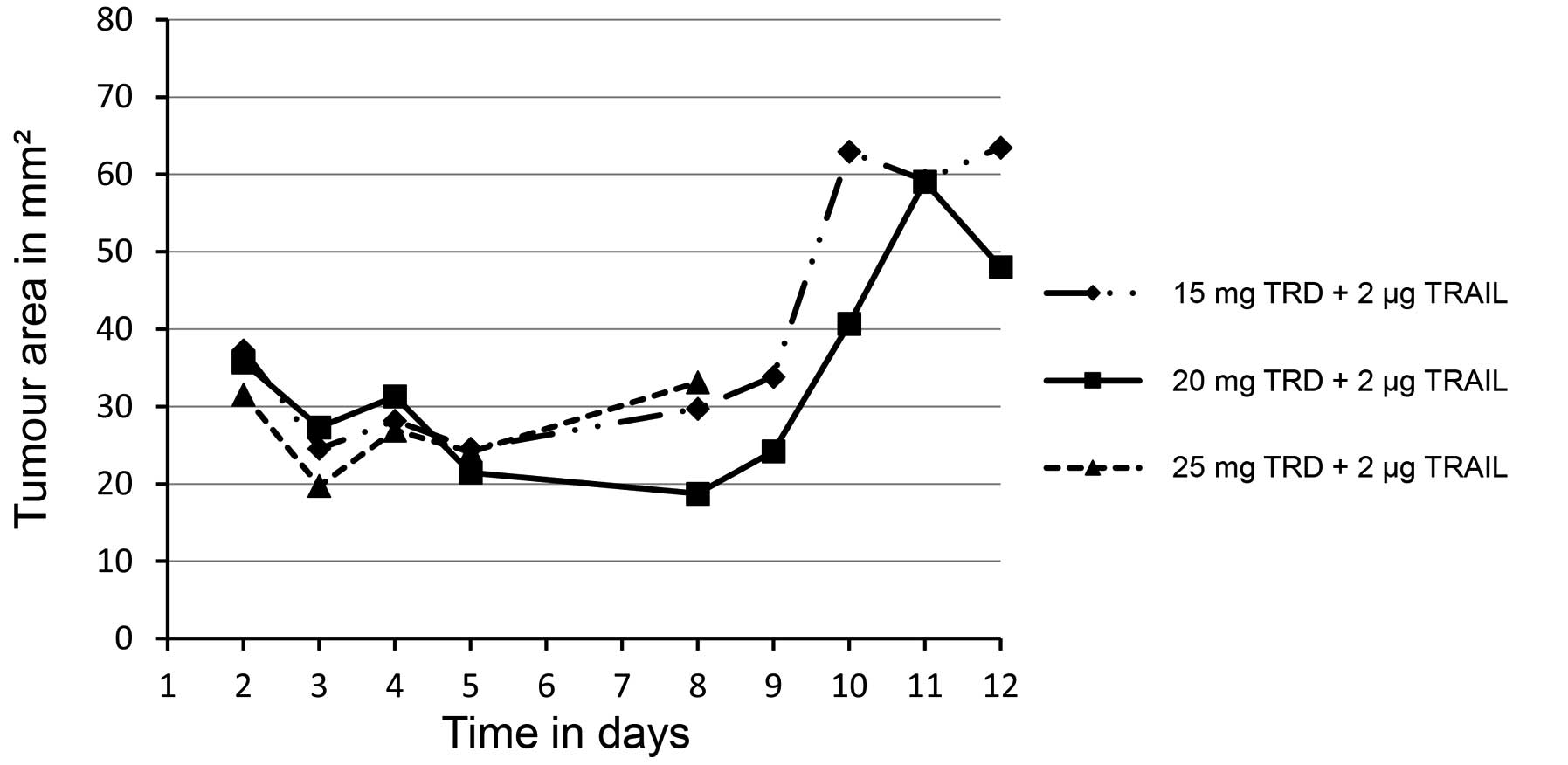
Increase of taurolidine dosage exerts
no additive effects on tumour growth inhibition and is associated
with diminished survival
Tumour growth was unaffected by altering taurolidine
dosage (Fig. 3). All 3 animals in the
treatment group with the highest taurolidine dosage (25
mg/injection in combination with 2 µg TRAIL) died within 8 days and
exhibited a mean body-weight loss of 15.6%. In addition, the feed
and water usage of this group was significantly lower when compared
with that of the other groups (P<0.0010) (data not shown).
Animals exposed to lower taurolidine dosage (15 mg or 20
mg/injection) survived through the 12 days of treatment. However,
in these treatment groups, xenografted tumours grew
continuously.
Histological analyses of organs reveal
no clear evidence for systemic toxic damages
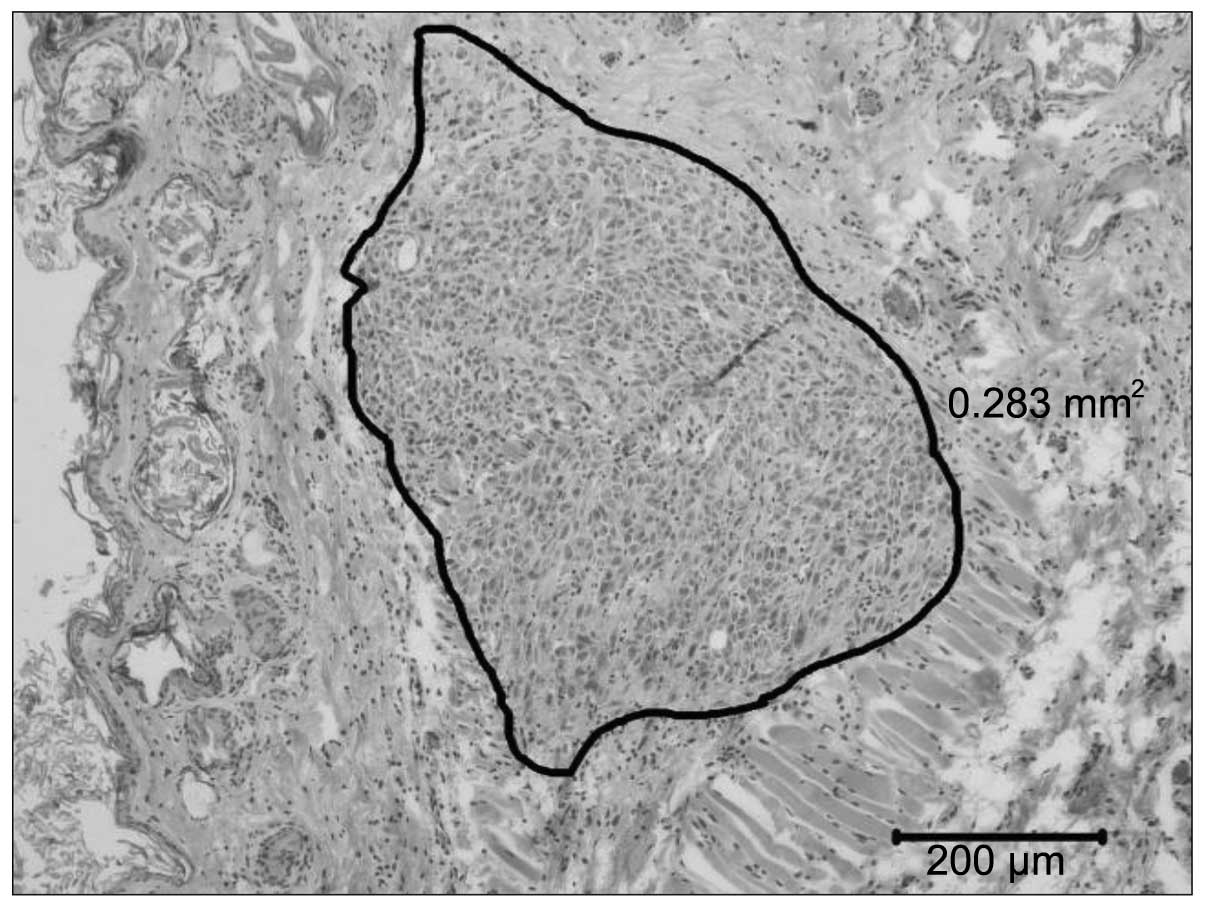
The histological examinations classified all
successfully inoculated tumours as human fibrosarcomas (Fig. 4). The skin samples, hearts and livers
of all animals exhibited no histological abnormalities. Notably, in
the majority of the treated animals, oedema was observed in the
tubulus system of the kidneys in the absence of any evidence for
necrosis. By contrast, none of the control animals had an altered
tubulus system. Small liver necrosis under the capsule was detected
only in a few treated mice. However, the majority of the treated
mice did not display any toxic organ damages. Overall, no relevant
toxic organ alterations that may be causative of the mortality of
the treated mice were observed.
Discussion
Fibrosarcomas are rare tumours and respond poorly to
chemotherapy and radiation. Despite excellent rates of local
disease control, treatment options in distant metastatic disease,
particularly in pulmonary locations, are extremely limited and have
an associated median survival of <12 months (8,9). Due to
the rarity of fibrosarcomas, the development of new therapeutics
has been challenging, and the lack of novel chemotherapy protocols
remains a major problem.
In a previous experimental study, our group
demonstrated that TRAIL and taurolidine synergistically induced
apoptosis and inhibited cell proliferation of HT1080 fibrosarcoma
cells in vitro (36). Inspired
by these results, the present study treated human fibrosarcoma
xenografts in athymic nude mice with TRAIL and taurolidine in order
to evaluate the safety and antitumour efficacy of these two
compounds. The first step of the current study analysed the
potential systemic toxic effects exerted by the combination of
these two substances. Tested in athymic nude mice without
xenografts, a cumulative dosage of 180 mg taurolidine and 12 µg
TRAIL per animal applied i.p. within 28 days was well-tolerated. In
the second step, the combination of the two compounds administered
in the same and slightly different cumulative dosages was examined
in athymic nude mice bearing HT1080 fibrosarcoma xenografts. Due to
the rapid growth of the fibrosarcoma xenografts, the cumulative
dosages were applied within 12 days instead of the previously
tested, and initially intended, 28 days, resulting in shorter
dosing intervals and higher cumulative dose intensities (CDIs).
Here, treatment with TRAIL and taurolidine reduced tumour growth
significantly in a TRAIL dose-dependent manner when comparing the
growth rates. However, treatment with TRAIL and taurolidine was
associated with a severely increased mortality rate, raising
concern regarding the used CDI and the safety of the two compounds.
However, reconsidering the current findings and the results of
previous in vivo studies, increased mortality was associated
primarily with high dosages of taurolidine. In two previous
studies, the antineoplastic effects of taurolidine were analysed in
athymic nude mice weighing 20–25 g. Cumulative dosages of 180 mg
(applied via nine 20-mg i.p. injections within 21 days) and 105 mg
(applied via seven 15-mg i.p. injections within 14 days) were
well-tolerated with no significant mortality (37,38).
Single doses were applied i.p. and ranged from 15 to 20 mg.
Notably, 20 mg taurolidine per injection and day was identified by
Calabresi et al (27) to be
the maximally tolerated dose in athymic nude mice, with a mortality
of 13% following 3 days of treatment (CDI, 60 mg/3 days). By
contrast, 30 mg taurolidine per injection and day resulted in a
mortality rate of 100% following 3 days of treatment (CDI, 90 mg/3
days). In the current study, effective antineoplastic treatment
required applications of 30 mg taurolidine per injection and day
for 4 subsequent days (CDI, 120 mg/4 days); however animals treated
with this dosage of taurolidine exhibited a markedly increased
mortality rate. These findings correlate with the results of
Calabresi et al (27) and
indicate that the high mortality rate was attributable to the high
CDI of taurolidine. Nevertheless, in the present study,
drug-related mortality was not accompanied by histologically
detectable damage to the examined organs (heart, lungs, liver,
kidneys and skin). Notably, lower taurolidine dosage did not
increase mortality, but also failed to inhibit tumour growth.
Although taurolidine has been assumed to be
well-tolerated, severe side effects have been observed in
preclinical studies (39–41). In a recent study evaluating the
antineoplastic effects of taurolidine in immunocompetent mice with
osteosarcoma, taurolidine was associated with severe liver
toxicity, with a notable increase of liver enzymes, and led to
pathological alterations of hepatocytes as detected by electron
microscopy (39). Further studies
assessing the safety of taurolidine in dogs and canines with
osteosarcoma demonstrated that taurolidine enhanced the systemic
toxicity of chemotherapeutics like doxorubicin and carboplatin
(40,41). Although initial clinical tests in
humans reported low toxicity (42–45), the
present findings suggest that cautious use is advisable, and raise
concern with regard to the therapeutic index of taurolidine, which
must be analysed by forthcoming studies with detailed toxicity
tests.
References
|
1
|
Nedea EA and DeLaney TF: Sarcoma and skin
radiation oncology. Hematol Oncol Clin North Am. 20:401–429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gronchi A, Casali PG, Mariani L, Miceli R,
Fiore M, Lo Vullo S, Bertulli R, Collini P, Lozza L, Olmi P and
Rosai J: Status of surgical margins and prognosis in adult soft
tissue sarcomas of the extremities: A series of patients treated at
a single institution. J Clin Oncol. 23:96–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patrikidou A, Domont J, Cioffi A and Le
Cesne A: Treating soft tissue sarcomas with adjuvant chemotherapy.
Current Treat Options Oncol. 12:21–31. 2011. View Article : Google Scholar
|
|
5
|
Kaushal A and Citrin D: The role of
radiation therapy in the management of sarcomas. Surg Clin North
Am. 88:629–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Brien GC, Cahill RA, Bouchier-Hayes DJ
and Redmond HP: Co-immunotherapy with interleukin-2 and taurolidine
for progressive metastatic melanoma. Ir J Med Sci. 175:10–14. 2006.
View Article : Google Scholar
|
|
7
|
Solomon LR, Cheesbrough JS, Bhargava R,
Mitsides N, Heap M, Green G and Diggle P: Observational study of
need for thrombolytic therapy and incidence of bacteremia using
taurolidine-citrate-heparin, taurolidine-citrate and heparin
catheter locks in patients treated with hemodialysis. Semin Dial.
25:233–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karavasilis V, Seddon BM, Ashley S,
Al-Muderis O, Fisher C and Judson I: Significant clinical benefit
of first-line palliative chemotherapy in advanced soft-tissue
sarcoma: Retrospective analysis and identification of prognostic
factors in 488 patients. Cancer. 112:1585–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Billingsley KG, Lewis JJ, Leung DH, Casper
ES, Woodruff JM and Brennan MF: Multifactorial analysis of the
survival of patients with distant metastasis arising from primary
extremity sarcoma. Cancer. 85:389–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pezzi CM, Pollock RE, Evans HL, Lorigan
JG, Pezzi TA, Benjamin RS and Romsdahl MM: Preoperative
chemotherapy for soft-tissue sarcomas of the extremities. Ann Surg.
211:476–481. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Donato Paola E and Nielsen OS: EORTC
Soft Tissue and Bone Sarcoma Group: The EORTC soft tissue and bone
sarcoma group: The EORTC soft tissue and bone sarcoma group.
European organisation for research and treatment of cancer. Eur J
Cancer. 38(Suppl 4): S138–S141. 2002.PubMed/NCBI
|
|
12
|
Brodowicz T, Schwameis E, Widder J, Amann
G, Wiltschke C, Dominkus M, Windhager R, Ritschl P, Pötter R, Kotz
R and Zielinski CC: Intensified adjuvant IFADIC chemotherapy for
adult soft tissue sarcoma: A prospective randomized feasibility
trial. Sarcoma. 4:151–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frustaci S, Gherlinzoni F, De Paoli A,
Bonetti M, Azzarelli A, Comandone A, Olmi P, Buonadonna A, Pignatti
G, Barbieri E, et al: Adjuvant chemotherapy for adult soft tissue
sarcomas of the extremities and girdles: Results of the Italian
randomized cooperative trial. J Clin Oncol. 19:1238–1247.
2001.PubMed/NCBI
|
|
14
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: Doxorubicin alone versus intensified
doxorubicin plus ifosfamide for first-line treatment of advanced or
metastatic soft-tissue sarcoma: A randomised controlled phase 3
trial. Lancet Oncol. 15:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yagita H, Takeda K, Hayakawa Y, Smyth MJ
and Okumura K: TRAIL and its receptors as targets for cancer
therapy. Cancer Sci. 95:777–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouralexis S, Findlay DM and Evdokiou A:
Death to the bad guys: Targeting cancer via Apo2L/TRAIL. Apoptosis.
10:35–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rowinsky EK: Targeted induction of
apoptosis in cancer management: The emerging role of tumor necrosis
factor-related apoptosis-inducing ligand receptor activating
agents. J Clin Oncol. 23:9394–9407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daniel P: Molecular principles of
apoptosis. Principles of Molecular Medicine. Ganten D and Ruckpaul
K: (3rd). (Berlin Heidelberg). Springer. 159–203. 2008.
|
|
20
|
Newsom-Davis T, Prieske S and Walczak H:
Is TRAIL the holy grail of cancer therapy? Apoptosis. 14:607–623.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chromik AM, Daigeler A, Bulut D, Flier A,
May C, Harati K, Roschinsky J, Sülberg D, Ritter PR, Mittelkötter
U, et al: Comparative analysis of cell death induction by
Taurolidine in different malignant human cancer cell lines. J Exp
Clin Cancer Res. 29:212010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacobi CA, Menenakos C and Braumann C:
Taurolidine-a new drug with anti-tumor and anti-angiogenic effects.
Anticancer Drugs. 16:917–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCourt M, Wang JH, Sookhai S and Redmond
HP: Taurolidine inhibits tumor cell growth in vitro and
in vivo. Ann Surg Oncol. 7:685–691. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petrovic L, Schlegel KA, Ries J, Park J,
Diebel E, Schultze-Mosgau S and Wiltfang J: In vitro effect of
taurolidine on squamous cell carcinoma in the oral cavity. Mund
Kiefer Gesichtschir. 7:102–107. 2003.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gallagher KA, Liu ZJ, Xiao M, Chen H,
Goldstein LJ, Buerk DG, Nedeau A, Thom SR and Velazquez OC:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1 alpha.
J Clin Invest. 117:1249–1259. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calabresi P, Goulette FA and Darnowski JW:
Taurolidine: Cytotoxic and mechanistic evaluation of a novel
antineoplastic agent. Cancer Res. 61:6816–6821. 2001.PubMed/NCBI
|
|
28
|
Braumann C, Henke W, Jacobi CA and Dubiel
W: The tumor-suppressive reagent taurolidine is an inhibitor of
protein biosynthesis. Int J Cancer. 112:225–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnowski JW, Goulette FA, Cousens LP,
Chatterjee D and Calabresi P: Mechanistic and antineoplastic
evaluation of taurolidine in the DU145 model of human prostate
cancer. Cancer Chemother Pharmacol. 54:249–258. 2004.PubMed/NCBI
|
|
30
|
Stendel R, Biefer HR, Dékány GM, Kubota H,
Münz C, Wang S, Mohler H, Yonekawa Y and Frei K: The antibacterial
substance taurolidine exhibits anti-neoplastic action based on a
mixed type of programmed cell death. Autophagy. 5:194–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stendel R, Scheurer L,
Stoltenburg-Didinger G, Brock M and Möhler H: Enhancement of
fas-ligand-mediated programmed cell death by taurolidine.
Anticancer Res. 23:2309–2314. 2003.PubMed/NCBI
|
|
32
|
Chromik AM, Daigeler A, Hilgert C, Bulut
D, Geisler A, Liu V, Otte JM, Uhl W and Mittelkötter U: Synergistic
effects in apoptosis induction by taurolidine and TRAIL in HCT-15
colon carcinoma cells. J Invest Surg. 20:339–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daigeler A, Chromik AM, Geisler A, Bulut
D, Hilgert C, Krieg A, Klein-Hitpass L, Lehnhardt M, Uhl W and
Mittelkötter U: Synergistic apoptotic effects of taurolidine and
TRAIL on squamous carcinoma cells of the esophagus. Int J Oncol.
32:1205–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daigeler A, Chromik AM, Haendschke K,
Emmelmann S, Siepmann M, Hensel K, Schmitz G, Klein-Hitpass L,
Steinau HU, Lehnhardt M and Hauser J: Synergistic effects of
sonoporation and taurolidin/TRAIL on apoptosis in human
fibrosarcoma. Ultrasound Med Biol. 36:1893–1906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karlisch C, Harati K, Chromik AM, Bulut D,
Klein-Hitpass L, Goertz O, Hirsch T, Lehnhardt M, Uhl W and
Daigeler A: Effects of TRAIL and taurolidine on apoptosis and
proliferation in human rhabdomyosarcoma, leiomyosarcoma and
epithelioid cell sarcoma. Int J Oncol. 42:945–956. 2013.PubMed/NCBI
|
|
36
|
Daigeler A, Brenzel C, Bulut D, Geisler A,
Hilgert C, Lehnhardt M, Steinau HU, Flier A, Steinstraesser L,
Klein-Hitpass L, et al: TRAIL and taurolidine induce apoptosis and
decrease proliferation in human fibrosarcoma. J Exp Clin Cancer
Res. 27:822008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun BS, Wang JH, Liu LL, Gong SL and
Redmond HP: Taurolidine induces apoptosis of murine melanoma cells
in vitro and in vivo by modulation of the Bcl-2
family proteins. J Surg Oncol. 96:241–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nici L, Monfils B and Calabresi P: The
effects of taurolidine, a novel antineoplastic agent, on human
malignant mesothelioma. Clin Cancer Res. 10:7655–7661. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arlt MJ, Walters DK, Banke IJ, Steinmann
P, Puskas GJ, Bertz J, Rentsch KM, Ehrensperger F, Born W and Fuchs
B: The antineoplastic antibiotic taurolidine promotes lung and
liver metastasis in two syngeneic osteosarcoma mouse models and
exhibits severe liver toxicity. Int J Cancer. 131:E804–E812. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marley K, Helfand SC, Simpson J, Mata JE,
Tracewell WG, Brownlee L, Bracha S and Séguin B: Pharmacokinetic
study and evaluation of the safety of taurolidine for dogs with
osteosarcoma. J Exp Clin Cancer Res. 32:742013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marley K, Helfand SC, Edris WA, Mata JE,
Gitelman AI, Medlock J and Séguin B: The effects of taurolidine
alone and in combination with doxorubicin or carboplatin in canine
osteosarcoma in vitro. BMC Vet Res. 9:152013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Braumann C, Winkler G, Rogalla P,
Menenakos C and Jacobi CA: Prevention of disease progression in a
patient with a gastric cancer-re-recurrence. Outcome after
intravenous treatment with the novel antineoplastic agent
taurolidine. Report of a case. World J Surg Oncol. 4:342006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Möhler H, Pfirrmann RW and Frei K:
Redox-directed cancer therapeutics: Taurolidine and piperlongumine
as broadly effective antineoplastic agents (review). Int J Oncol.
45:1329–1336. 2014.PubMed/NCBI
|
|
44
|
Imhof L, Goldinger SM, Baumann K, Schad K,
French LE, Röthlisberger P and Dummer R: The antibacterial
substance, taurolidine in the second/third-line treatment of very
advanced stage IV melanoma including brain metastases: Results of a
phase 2, open-label study. Melanoma Res. 21:80–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stendel R, Picht T, Schilling A,
Heidenreich J, Loddenkemper C, Jänisch W and Brock M: Treatment of
glioblastoma with intravenous taurolidine. First clinical
experience. Anticancer Res. 24:1143–1147. 2004.PubMed/NCBI
|