Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignant tumors, with high rates of mortality and
morbidity (1,2). The worldwide incidence of HCC is
~1,000,000 cases per year and is equivalent to its mortality rate
(3). Furthermore, HCC ranks as the
third highest cause of cancer-associated mortality (4). Such statistics are largely explained by
the difficulties faced when diagnosing HCC, with diagnosis often
only confirmed once the disease is at a late stage and palliative
care is the only treatment option available. An important strategy
to improve patient outcome involves the identification of disease
risk factors and the maintenance of vigilant surveillance of
high-risk individuals to allow for recognition of the disease at a
stage that is responsive to treatment with curative intent.
Therefore, it is fundamental that high-risk groups are identified,
followed by successful implementation of a surveillance program and
recall protocol following any abnormal findings (5–8). Progress
has been made in identifying molecular markers for the initiation
and progression of HCC that will likely increase the rate of early
and potentially life-saving diagnoses (9–12). The
expanding amount of knowledge regarding the molecular foundations
of HCC makes it increasingly clear that successful therapy requires
treatment tailored to the individual patient (8).
Melanoma-associated antigens (MAGEs), members of the
cancer/testis antigen family that consists of >50 proteins, are
divided into two types, MAGE-I and MAGE-II, dependent on varying
gene structures and tissue-specific expression patterns (13,14). The
MAGE-I subgroup consists of products yielded by numerous ×
chromosome clustered genes, including MAGE-A, -B and
-C, which are typically expressed in cancer cells of various
origins, but not in adult tissues, with the exception of germ-line
cells in the placenta, ovaries and testes (15,16). By
contrast, the MAGE-II subgroup, including MAGE-D variants,
do not have defined chromosome clustering and are cancer
non-specific, with near universal expression in normal adult
tissues and germ-line cells (14,16,17). MAGE
proteins, as tumor-associated antigens, have attracted increasing
attention regarding the development of vaccine-based immunotherapy
for the treatment of cancer (18).
Our strategy for addressing this issue involves detailed analysis
of the literature to identify potential markers and targets for
therapy of HCC (15). For example, a
previous meta-analysis indicated that glypican-3,
des-γ-carboxyprothrombin, α-L-fucosidase and vascular endothelial
growth factor may serve as suitable serological markers for HCC
(19). Meta-analyses are incisive and
may potentially be more informative compared with omic surveys that
are expensive, technology-intensive and arguably more
time-consuming. This lead the current study to focus on the 86-kDa
NRAGE protein, also known as MAGE-D1, which is encoded by the
NRAGE gene located on the × chromosome (20–22). Cells
of diverse embryonic and adult tissues, particularly those of the
nervous system, express NRAGE, which subsequently interacts with
proteins that regulate cell adhesion and migration, the cell cycle,
cell differentiation, apoptosis and gene transcription (22–24).
However, little is understood regarding the physiological relevance
of these interactions.
NRAGE serves a role in the process of
apoptosis through interactions with the p75 neurotrophin receptor
(p75NTR) (25) and
apoptosis-antagonizing transcription factor (AATF) (26,27). As
NRAGE interacts with proteins with diverse functions, it is not
unexpected that its effects are cell-type specific. For example,
the downregulation of NRAGE transcription serves an
important function in apoptosis, and when expressed ectopically,
NRAGE inhibits the proliferation of breast cancer cells (27). Furthermore, downregulation of NRAGE in
colorectal cancer is associated with a negative clinical course
(24,28,29). By
contrast, NRAGE functions as an oncogene in esophageal and
lung cancers (22,30). Yang et al (22) demonstrated that the overexpression of
NRAGE exerts tumor-promoting effects by interacting with the
DNA polymerase III domain of proliferating cell nuclear antigen
(PCNA) and consequently inhibits K48-polyubiquitin
chain-mediated proteasome degradation of PCNA in esophageal cancer.
However, to the best of our knowledge, there are currently no
studies concerning NRAGE expression in HCC or its role in
the pathogenesis of this disease. Therefore, the aim of the present
study was to assess the clinical significance of NRAGE
expression in HCC, as well as its relevance as a novel biomarker
for tumor progression.
Materials and methods
Sample collection
The HCC cell lines (Hep3B, HepG2, HLE, HLF, HuH1,
HuH2, HuH7, PLC/PRF/5 and SK-Hep1) were obtained from the American
Type Culture Collection (Manassas, VA, USA). All cell lines were
cultured in Dulbecco's modified Eagle's Medium (Sigma-Aldrich, St.
Louis, MO, USA), supplemented with 10% fetal bovine serum at 37°C
in an atmosphere containing 5% CO2 (6). The primary HCC tissues and the
corresponding non-cancerous tissues were collected from 151
patients with HCC who had undergone liver resection at the Nagoya
University Hospital (Nagoya, Japan) between January 1998 and July
2012. The specimens were classified histologically according to the
criteria published in the Classification of Malignant Tumours,
Union for International Cancer Control (UICC) (31). Clinicopathological data were collected
from medical records, and written informed consent for the use of
clinical samples and data was obtained from all patients, as
required by the Institutional Review Board of Nagoya University,
Japan (32).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Expression levels of mRNA in all samples were
analyzed using RT-qPCR, which was performed using an Applied
Biosystems StepOne Plus (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) in triplicate. Total RNA (10 µg) isolated from the HCC
cell lines, and the 151 primary HCC specimens and corresponding
adjacent non-cancerous tissues, was used as the template for the
synthesis of complementary DNA. RT-qPCR was performed using the
SYBR® Green PCR Core Reagents kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and specific primers for NRAGE (Hokkaido
System Science Co., Ltd., Tokyo, Japan) as follows: One cycle at
95°C for 10 min, 40 cycles at 95°C for 5 sec and 60°C for 30 sec.
For standardization, glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) mRNA (TaqMan GAPDH Control Reagents; Applied Biosystems;
Thermo Fisher Scientific, Inc.) was quantified in each sample.
Expression levels are presented as the value of NRAGE mRNA divided
by the value of GAPDH mRNA (33,34).
Expression levels of mRNAs were normalized by the serially diluted
standards (35). AATF, p75NTR and
PCNA encode proteins that may interact with NRAGE, and the specific
primers (Hokkaido System Science Co., Ltd.) used for each of these
genes are listed in Table I.
 | Table I.Primers and annealing
temperatures. |
Table I.
Primers and annealing
temperatures.
| Gene | Oligo sequence
(5′–3′) | Product size,
bp | Annealing
temperature, °C |
|---|
| NRAGE |
|
|
|
|
Forward |
GATTCCCTCAGACCTTTGC | 170 | 60 |
|
Reverse |
GAAGGAATCTGAGGCTTCAG |
|
|
| AATF |
|
|
|
|
Forward |
ACAAAGGTGGCCCAGAATTT | 103 | 62 |
|
Reverse |
TGGAAAAGCAACTCTTCCTGA |
|
|
| p75NTR |
|
|
|
|
Forward |
CTGCTGCTGTTGCTGCTTCT | 98 | 60 |
|
Reverse |
CAGGCTTTGCAGCACTCAC |
|
|
| PCNA |
|
|
|
|
Forward |
TGCAAGTGGAGAACTTGGAA | 128 | 58 |
|
Reverse |
TCAGGTACCTCAGTGCAAAAG |
|
|
| GAPDH |
|
|
|
|
Forward |
GAAGGTGAAGGTCGGAGTC | 226 | 60 |
|
Probe |
CAAGCTTCCCGTTCTCAGCC |
|
|
|
Reverse |
GAAGATGGTGATGGGATTTC |
|
|
Immunohistochemistry (IHC)
IHC was performed to determine the expression and
localization of NRAGE in 30 representative well-preserved HCC
samples. Formalin-fixed, paraffin-embedded tissue samples were
dewaxed twice in xylene for 5 min, rehydrated sequentially using a
graded series of alcohol concentrations (100, 90 and 70%) for 2 min
each and treated with 3% H2O2 to inhibit
endogenous peroxidases. Antigen retrieval was performed by
incubating the sections 5 times in citrate buffer (10 mM) at 95°C
for 5 min. The samples were then washed with phosphate-buffered
saline, followed by a 10-min incubation with biotinylated goat
anti-rabbit IgG secondary antibody (Histofine SAB PO(R) kit; Code
424032; Nichirei Corporation, Tokyo, Japan) for 5 min to limit
non-specific reactivity, and incubated for 1 h with a rabbit
polyclonal anti-human NRAGE antibody (catalog no., LS-C100414;
LifeSpan BioSciences, Inc., Seattle, WA, USA) in a 1:200 dilution
with ChemMateT antibody diluent (Dako Japan Co., Ltd., Tokyo,
Japan). The samples were then washed with phosphate-buffered
saline, followed by a 10-min incubation with biotinylated goat
anti-rabbit IgG secondary antibody (Histofine SAB-PO(R) kit;
Nichirei Corporation) in a 1:1,000 dilution with ChemMateT antibody
dilutent. Subsequently, the sections were incubated for 1 min with
liquid 3,3′-diaminobenzidine (Nichirei Corporation) to detect
antigen-antibody complexes. Staining of NRAGE was evaluated using
vessels as internal controls. To avoid bias when interpreting data,
specimens were randomized and coded prior to analysis by two
independent observers who were uninformed of the status of the
samples. Each observer evaluated all specimens at least twice
within a given time interval to minimize intraobserver variation
(36,37).
Statistical analysis
The significance of the association between the
levels of NRAGE mRNA and the clinicopathological features
was evaluated using the χ2 test, and differences between
groups were evaluated using the Mann-Whitney U test. Correlations
between the level of NRAGE mRNA and encoding AATF,
p75NTR and PCNA, as well as those of pre-operative
serum tumor markers, were analyzed using Spearman's rank
correlation coefficient. Disease-specific survival rates were
calculated using the Kaplan-Meier method, and the differences in
survival curves were evaluated using the generalized Wilcoxon
rank-sum test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using JMP® software, version 10 (SAS Institute Inc., Cary, NC,
USA).
Results
Analysis of NRAGE, AATF, p75NTR and
PCNA mRNA expression in HCC cell lines
The relative levels of NRAGE mRNA and those
of its putative interacting partners, AATF, p75NTR
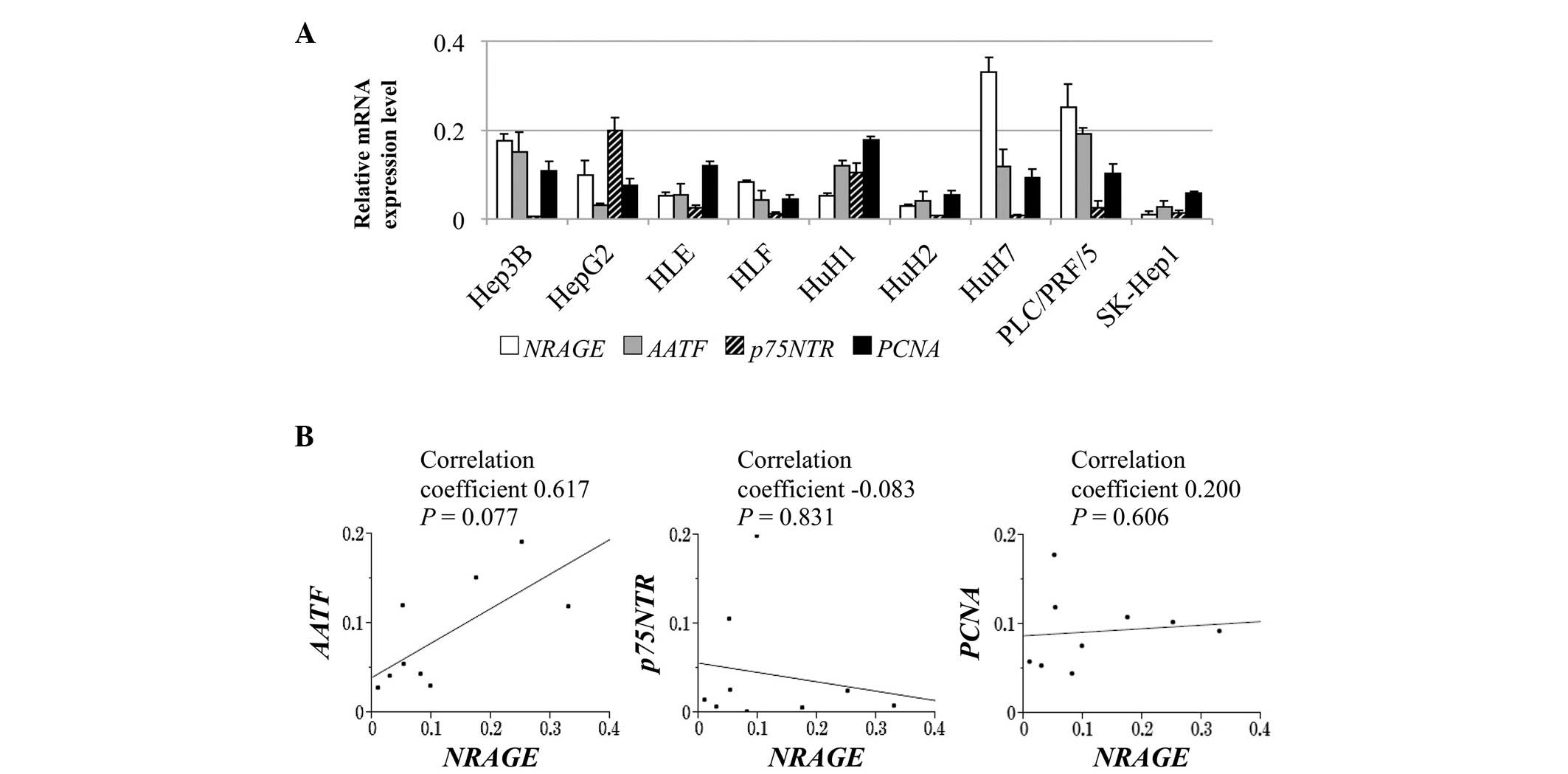
and PNCA, in the HCC cell lines are presented in Fig. 1A. Heterogeneity was observed in the
NRAGE mRNA levels between 9 HCC cell lines, which correlated
significantly with those of AATF (correlation coefficient,
0.617), whereas no significant correlation was observed between the
expression of NRAGE and p75NTR or PCNA
(Fig. 1B).
Patient characteristics
The ages of the 151 patients ranged from 34–84 years
(mean ± standard deviation, 64.7±9.8 years), and the male to female
ratio was 5:1. A total of 37 and 84 patients were infected with
hepatitis B and C virus, respectively. The numbers of patients with
a normal liver, chronic hepatitis or cirrhosis were 10, 87 and 54,
respectively. When classified according to the UICC's
tumor-node-metastasis classification, 94, 39 and 18 patients were
in stages I, II and III, respectively.
Analysis of NRAGE and AATF mRNA levels
in surgically resected liver tissues
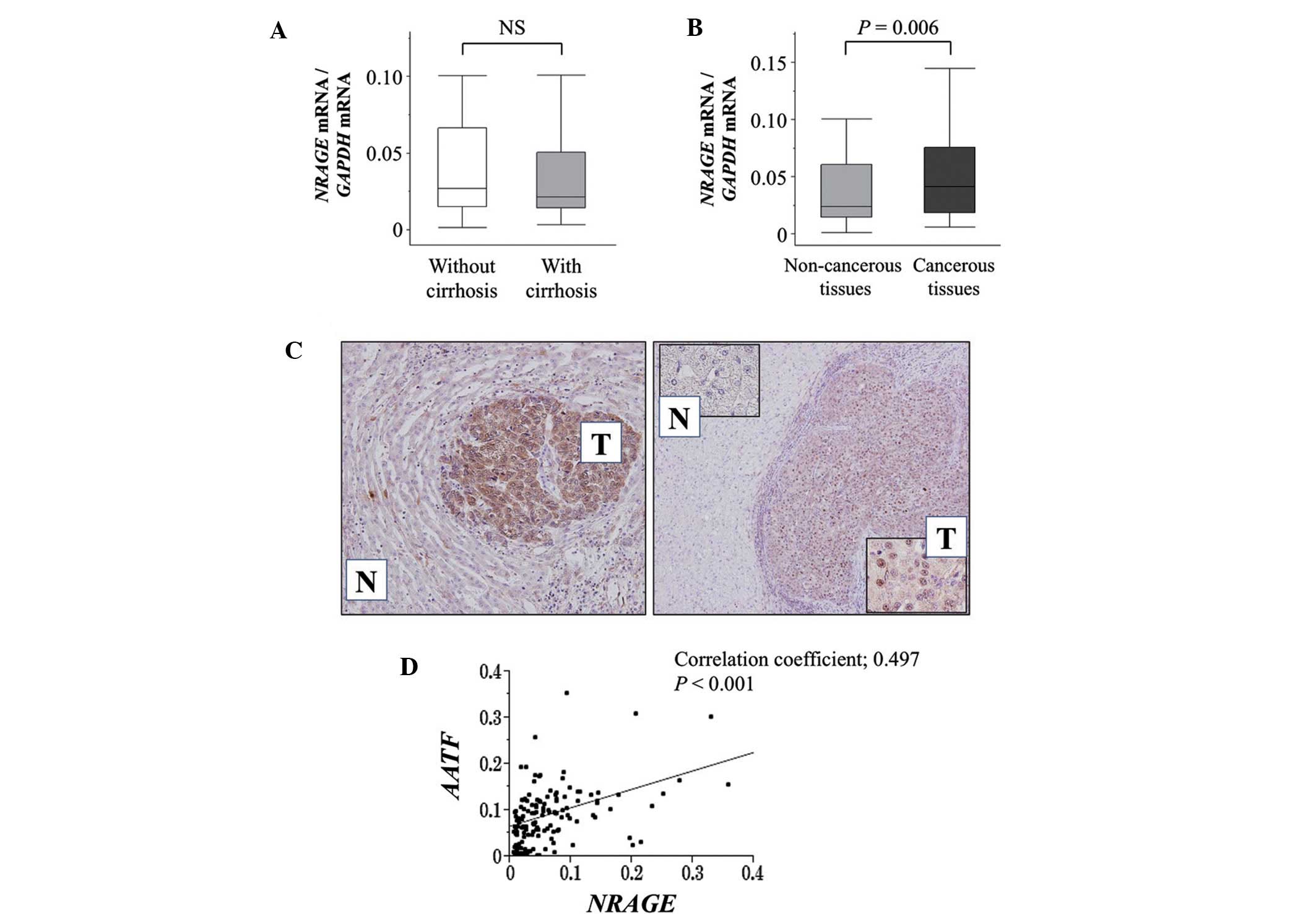
There were no significant differences observed in
the NRAGE mRNA levels in the non-cancerous tissue samples
between patients with or without cirrhosis (Fig. 2A). However, the mean level of
NRAGE mRNA in the HCC tissues was significantly higher
compared with that in the corresponding normal tissues (P=0.006;
Fig. 2B).
IHC analysis was conducted to determine whether
NRAGE expression correlated with NRAGE mRNA levels in the
tumor and non-cancerous tissues. Representative images showing
strong staining intensities of NRAGE in cancer tissues are
presented in Fig. 2C. The expression
patterns of NRAGE protein and mRNA in the HCC and
non-cancerous tissues were consistent among 30 representative pairs
of specimens. It was observed that the level of NRAGE and
AATF mRNA was directly correlated, consistent with results
of the analysis of the HCC cell lines (Fig. 2C).
Clinical significance of NRAGE mRNA
expression
Tissue sections were assigned to two groups (high
and low expression) according to their level of NRAGE mRNA.
The high and low expression groups included 93 and 58 patients,
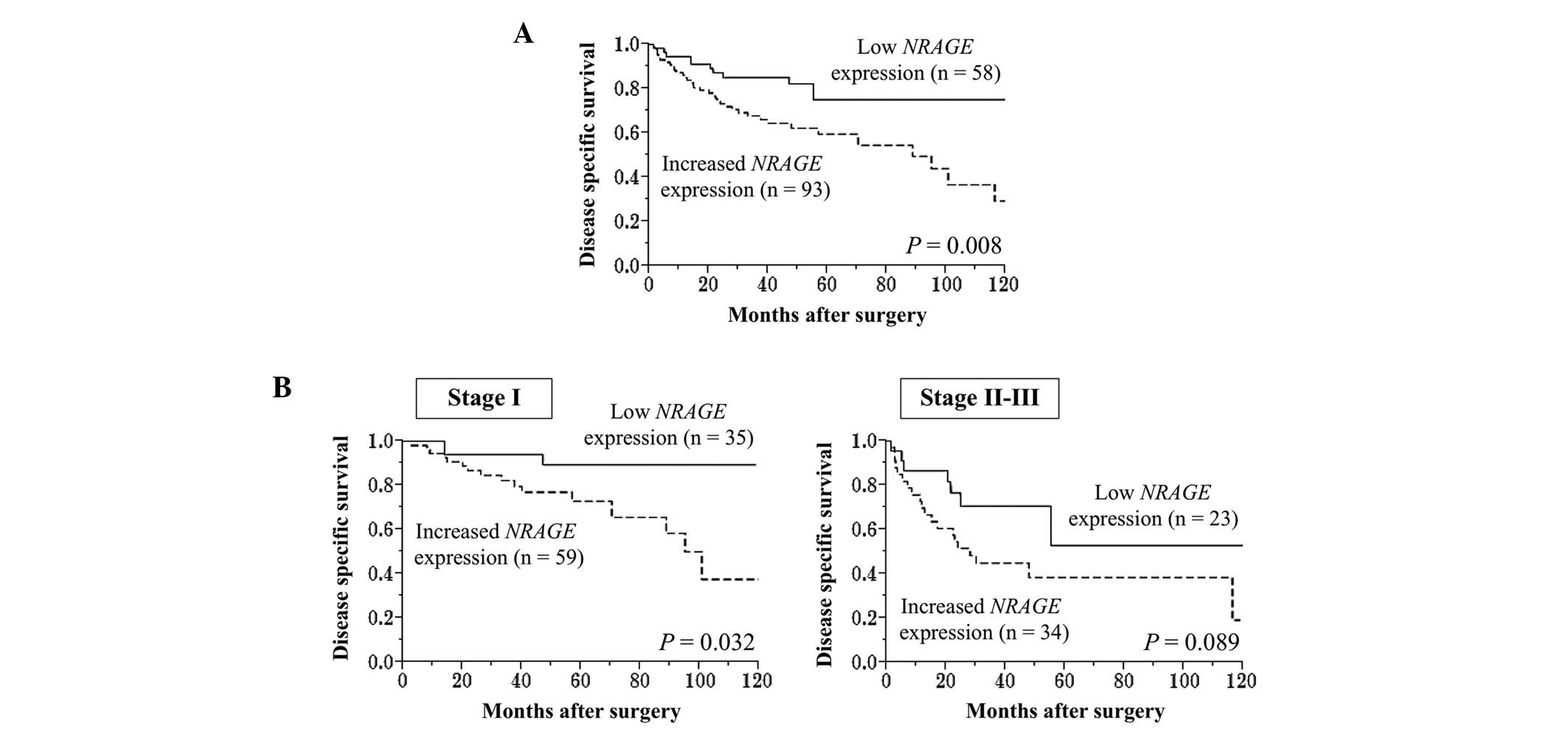
respectively. The high expression group was associated with
increased age and an α-fetoprotein level of >20 ng/ml (P=0.014
and P=0.020, respectively; Table
II). The disease-specific survival time of the patients was
significantly shorter in the high expression group compared with
the low expression group (5-year survival rate, 60 vs. 75%;
P=0.008; Fig. 3A). Subgroup analysis
according to UICC staging indicated that the disease-specific
survival curves showed a similar tendency of differences between
high and low NRAGE expression groups in each disease stage
(Fig. 3B). Multivariate analysis
identified vascular invasion and increased NRAGE expression
as independent prognostic factors (hazard ratios, 2.24 and 2.23,
respectively; Table III).
 | Table II.Association between NRAGE mRNA
levels and clinicopathological parameters of 151 hepatocellular
carcinoma patients. |
Table II.
Association between NRAGE mRNA
levels and clinicopathological parameters of 151 hepatocellular
carcinoma patients.
| Clinicopathological
parameters | Increased
expression of NRAGE mRNA, n | Others, n | P-value |
|---|
| Age, years |
|
| 0.014a |
|
<65 | 34 | 33 |
|
|
≥65 | 59 | 25 |
|
| Gender |
|
| 0.096 |
|
Male | 74 | 52 |
|
|
Female | 19 | 6 |
|
| Background of
liver |
|
| 0.628 |
|
Normal | 6 | 4 |
|
| Chronic
hepatitis | 51 | 36 |
|
|
Cirrhosis | 36 | 18 |
|
| Child-Pugh
classification |
|
| 0.621 |
| A | 87 | 53 |
|
| B | 6 | 5 |
|
| Hepatitis
virus |
|
| 0.728 |
|
Absent | 17 | 13 |
|
|
HBV | 22 | 15 |
|
|
HCV | 54 | 30 |
|
| AFP, ng/ml |
|
| 0.020a |
|
≤20 | 43 | 38 |
|
|
>20 | 50 | 20 |
|
| PIVKA II,
mAU/ml |
|
| 0.350 |
|
≤40 | 33 | 25 |
|
|
>40 | 60 | 33 |
|
| Tumor
multiplicity |
|
| 0.707 |
|
Solitary | 73 | 44 |
|
|
Multiple | 20 | 14 |
|
| Tumor size, cm |
|
| 0.733 |
|
<3.0 | 28 | 19 |
|
|
≥3.0 | 65 | 39 |
|
|
Differentiation |
|
| 0.314 |
|
Well | 19 | 16 |
|
|
Moderate to poor | 74 | 42 |
|
| Growth type |
|
| 0.208 |
|
Expansive | 81 | 46 |
|
|
Invasive | 12 | 12 |
|
| Serosal
infiltration |
|
| 0.389 |
|
Absent | 68 | 46 |
|
|
Present | 25 | 12 |
|
| Formation of
capsule |
|
| 0.289 |
|
Absent | 26 | 21 |
|
|
Present | 67 | 37 |
|
| Infiltration to
capsule |
|
| 0.101 |
|
Absent | 37 | 31 |
|
|
Present | 56 | 27 |
|
| Septum
formation |
|
| 0.407 |
|
Absent | 35 | 18 |
|
|
Present | 58 | 40 |
|
| Vascular
invasion |
|
| 0.386 |
|
Absent | 68 | 46 |
|
|
Present | 25 | 12 |
|
| UICC pathological
stage |
|
| 0.919 |
| I | 59 | 35 |
|
| II | 23 | 16 |
|
|
III | 11 | 7 |
|
 | Table III.Prognostic factors of 151 patients
with hepatocellular carcinoma. |
Table III.
Prognostic factors of 151 patients
with hepatocellular carcinoma.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | n | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age ≥65 years | 84 | 1.92 | 1.07–3.57 |
0.030a | 1.39 | 0.75–2.65 | 0.301 |
| Male gender | 126 | 1.27 | 0.60–3.13 |
0.553 |
|
|
|
| Cirrhosis of
liver |
4 | 1.58 | 0.88–2.81 |
0.123 |
|
|
|
| Child-Pugh
classification B | 11 | 0.93 | 0.28–2.32 |
0.889 |
|
|
|
| AFP >20
ng/ml | 70 | 1.90 | 1.07–3.42 |
0.029a | 1.30 | 0.70–2.44 | 0.790 |
| PIVKA II >40
mAU/ml | 93 | 2.10 | 1.14–4.07 |
0.016a | 1.12 | 0.55–2.40 | 0.770 |
| Tumor
multiplicity | 34 | 2.09 | 1.11–3.76 |
0.023a | 1.32 | 0.67–2.53 | 0.416 |
| Tumor size ≥3.0
cm | 104 | 2.20 | 1.13–4.71 |
0.020a | 1.87 | 0.82–4.64 | 0.140 |
| Well-differentiated
tumor | 35 | 0.55 | 0.25–1.10 |
0.095 |
|
|
|
| Invasive
growth | 24 | 1.44 | 0.69–2.76 |
0.318 |
|
|
|
| Serosal
infiltration | 37 | 2.51 | 1.32–4.61 |
0.006a | 1.53 | 0.76–2.95 | 0.225 |
| Formation of
capsule | 104 | 1.05 | 0.57–2.02 |
0.884 |
|
|
|
| Infiltration to
capsule | 83 | 1.20 | 0.67–2.18 |
0.537 |
|
|
|
| Septum
formation | 98 | 0.87 | 0.49–1.60 |
0.651 |
|
|
|
| Vascular
invasion | 37 | 3.40 | 1.87–6.07 |
<0.001a | 2.24 | 1.12–4.41 | 0.022a |
| Increased
NRAGE expression | 93 | 2.42 | 1.27–4.99 | 0.006a | 2.23 | 1.06–3.83 | 0.020a |
Discussion
Originally identified by Salehi et al
(25) in a two-hybrid screen
utilizing p75NTR as bait, NRAGE is a signaling
cascade component that mediates apoptosis by interacting with
p75NTR to antagonize its association with the nerve growth
factor receptor tropomyosin receptor kinase A. Studies have
indicated that NRAGE promotes apoptosis through the
ubiquitination of AATF (26,27),
therefore suggesting that NRAGE functions as a tumor
suppressor by inducing tumor cell apoptosis. However,
overexpression of NRAGE accelerates the proliferation and
migration of esophageal cancer cells via interactions with
PCNA. A genome-wide association study demonstrated that the
initiation of NRAGE signals through the JNK pathway
is associated with non-small cell lung cancer (22,30). Xue
et al (38) identified an
association between elevated NRAGE expression and the
increased radioresistance of esophageal carcinoma cells. Such
studies indicate that NRAGE functions to inhibit or promote
oncogenesis depending on cell type, leading to the rationale behind
the present study.
The current study demonstrated that NRAGE
mRNA levels positively correlated with those of AATF, but
not those of p75NTR or PCNA. The presence of
cirrhosis had a minimal affect on NRAGE expression, and
NRAGE mRNA levels were significantly higher in the HCC
tissues compared with the corresponding non-cancerous tissues; this
suggests that NRAGE is involved in hepatocarcinogenesis or
the subsequent progression of HCC.
Multivariate analysis of disease-specific survival
time following curative hepatectomy and subgroup analysis,
according to UICC staging, identified that increased NRAGE
mRNA expression in the HCC tissues functioned as an independent
prognostic factor. These results indicate that NRAGE serves
as a tumor promoting factor, and that the levels of NRAGE
mRNA in resected primary lesions may function as a biomarker for
the progression of HCC. Furthermore, the results of IHC analysis
demonstrated that NRAGE expression correlated with mRNA
level, subsequently indicating the physiological significance of
the latter. Therefore, future investigation into the function of
NRAGE in HCC may be facilitated using RT-qPCR analysis of
NRAGE mRNA levels.
In conclusion, NRAGE mediates the progression of HCC
and may serve as a novel biomarker for the malignant phenotype of
the disease. The present study indicated that the analysis of
NRAGE expression may enhance the clinical management of HCC.
For example, the levels of NRAGE mRNA in liver biopsies or
surgically resected tissues may facilitate risk stratification of
patients with HCC, and may also serve as a criterion for
determining the most appropriate therapy tailored to individual
patients. Furthermore, the findings of the current study
demonstrate promise for the development of novel therapies for HCC
that employ small molecules or antibodies that target NRAGE
and its interacting partners, including AATF. Research to
further investigate the signaling pathways that are regulated by or
function through NRAGE may expose alternative targets for
the treatment of HCC.
References
|
1
|
Shiraha H, Yamamoto K and Namba M: Human
hepatocyte carcinogenesis (review). Int J Oncol. 42:1133–1138.
2013.PubMed/NCBI
|
|
2
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GLOBOCAN Estimated Cancer Incidence,
Mortality and Prevalence Worldwide in 2012. Stomach Cancer.
2012.
|
|
5
|
Zhao YJ, Ju Q and Li GC: Tumor markers for
hepatocellular carcinoma. Mol Clin Oncol. 1:593–598.
2013.PubMed/NCBI
|
|
6
|
Kanda M, Sugimoto H, Nomoto S, Oya H,
Hibino S, Shimizu D, Takami H, Hashimoto R, Okamura Y, Yamada S, et
al: B-cell translocation gene 1 serves as a novel prognostic
indicator of hepatocellular carcinoma. Int J Oncol. 46:641–648.
2015.PubMed/NCBI
|
|
7
|
Flores A and Marrero JA: Emerging trends
in hepatocellular carcinoma: Focus on diagnosis and therapeutics.
Clin Med Insights Oncol. 8:71–76. 2014.PubMed/NCBI
|
|
8
|
Giannelli G, Rani B, Dituri F, Cao Y and
Palasciano G: Moving towards personalised therapy in patients with
hepatocellular carcinoma: The role of the microenvironment. Gut.
63:1668–1676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanda M, Nomoto S, Okamura Y, et al:
Detection of metallothionein 1G as a methylated tumor suppressor
gene in human hepatocellular carcinoma using a novel method of
double combination array analysis. Int J Oncol. 35:477–483.
2009.PubMed/NCBI
|
|
10
|
Miki D, Ochi H, Hayes CN, Aikata H and
Chayama K: Hepatocellular carcinoma: Towards personalized medicine.
Cancer Sci. 103:846–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khare S, Zhang Q and Ibdah JA: Epigenetics
of hepatocellular carcinoma: Role of microRNA. World J
Gastroenterol. 19:5439–5445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanda M, Nomoto S, Oya H, Takami H, Hibino
S, Hishida M, Suenaga M, Yamada S, Inokawa Y, Nishikawa Y, et al:
Downregulation of DENND2D by promoter hypermethylation is
associated with early recurrence of hepatocellular carcinoma. Int J
Oncol. 44:44–52. 2014.PubMed/NCBI
|
|
13
|
Chomez P, De Backer O, Bertrand M, De
Plaen E, Boon T and Lucas S: An overview of the MAGE gene family
with the identification of all human members of the family. Cancer
Res. 61:5544–5551. 2001.PubMed/NCBI
|
|
14
|
Tseng HY, Chen LH, Ye Y, Tay KH, Jiang CC,
Guo ST, Jin L, Hersey P and Zhang XD: The melanoma-associated
antigen MAGE-D2 suppresses TRAIL receptor 2 and protects against
TRAIL-induced apoptosis in human melanoma cells. Carcinogenesis.
33:1871–1881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takami H, Kanda M, Oya H, Hibino S,
Sugimoto H, Suenaga M, Yamada S, Nishikawa Y, Asai M, Fujii T, et
al: Evaluation of MAGE-D4 expression in hepatocellular carcinoma in
Japanese patients. J Surg Oncol. 108:557–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sang M, Wang L, Ding C, Zhou X, Wang B,
Wang L, Lian Y and Shan B: Melanoma-associated antigen genes - an
update. Cancer Lett. 302:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oya H, Kanda M, Takami H, Hibino S,
Shimizu D, Niwa Y, Koike M, Nomoto S, Yamada S, Nishikawa Y, et al:
Overexpression of melanoma-associated antigen D4 is an independent
prognostic factor in squamous cell carcinoma of the esophagus. Dis
Esophagus. 28:188–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang CC, Campoli M, Luo W, Zhao W,
Zaenker KS and Ferrone S: Immunotherapy of melanoma targeting human
high molecular weight melanoma-associated antigen: Potential role
of nonimmunological mechanisms. Ann NY Acad Sci. 1028:340–350.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hussein TD: Serological tumor markers of
hepatocellular carcinoma: A meta-analysis. Int J Biol Markers.
30:e32–e42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jordan BW, Dinev D, LeMellay V, Troppmair
J, Gotz R, Wixler L, Sendtner M, Ludwig S and Rapp UR: Neurotrophin
receptor-interacting mage homologue is an inducible inhibitor of
apoptosis protein-interacting protein that augments cell death. J
Biol Chem. 276:39985–39989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Gao X and Xu Y: MAGED1: Molecular
insights and clinical implications. Ann Med. 43:347–355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Q, Ou C, Liu M, Xiao W, Wen C and Sun
F: NRAGE promotes cell proliferation by stabilizing PCNA in a
ubiquitin-proteasome pathway in esophageal carcinomas.
Carcinogenesis. 35:1643–1651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barker PA and Salehi A: The MAGE proteins:
Emerging roles in cell cycle progression, apoptosis, and
neurogenetic disease. J Neurosci Res. 67:705–712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du Q, Zhang Y, Tian XX, Li Y and Fang WG:
MAGE-D1 inhibits proliferation, migration and invasion of human
breast cancer cells. Oncol Rep. 22:659–665. 2009.PubMed/NCBI
|
|
25
|
Salehi AH, Roux PP, Kubu CJ, Zeindler C,
Bhakar A, Tannis LL, Verdi JM and Barker PA: NRAGE, a novel MAGE
protein, interacts with the p75 neurotrophin receptor and
facilitates nerve growth factor-dependent apoptosis. Neuron.
27:279–288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Certo MG, Corbi N, Bruno T, Iezzi S, De
Nicola F, Desantis A, Ciotti MT, Mattei E, Floridi A, Fanciulli M
and Passananti C: NRAGE associates with the anti-apoptotic factor
Che-1 and regulates its degradation to induce cell death. J Cell
Sci. 120:1852–1858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Passananti C and Fanciulli M: The
anti-apoptotic factor Che-1/AATF links transcriptional regulation,
cell cycle control, and DNA damage response. Cell Div. 2:212007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krämer BF, Schoor O, Krüger T, Reichle C,
Müller M, Weinschenk T, Hennenlotter J, Stenzl A, Rammensee HG and
Stevanovic S: MAGED4-expression in renal cell carcinoma and
identification of an HLA-A*25-restricted MHC class I ligand from
solid tumor tissue. Cancer Biol Ther. 4:943–948. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng ZL, Wu WJ, Yang J, Tang ZJ, Chen DL,
Qiu MZ, Luo HY, Wang ZQ, Jin Y, Wang DS and Xu RH: Prognostic
relevance of melanoma antigen D1 expression in colorectal
carcinoma. J Transl Med. 10:1812012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee D, Lee GK, Yoon KA and Lee JS:
Pathway-based analysis using genome-wide association data from a
Korean non-small cell lung cancer study. PLoS One. 8:e653962013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer. TNM Classification of
Malignant Tumors (7th). (New York). Wiley-Blackwell. 2009.
|
|
32
|
Kanda M, Shimizu D, Nomoto S, Hibino S,
Oya H, Takami H, Kobayashi D, Yamada S, Inokawa Y, Tanaka C, et al:
Clinical significance of expression and epigenetic profiling of
TUSC1 in gastric cancer. J Surg Oncol. 110:136–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanda M, Nomoto S, Oya H, Shimizu D,
Takami H, Hibino S, Hashimoto R, Kobayashi D, Tanaka C, Yamada S,
et al: Dihydropyrimidinase-like 3 facilitates malignant behavior of
gastric cancer. J Exp Clin Cancer Res. 33:662014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanda M, Shimizu D, Nomoto S, Takami H,
Hibino S, Oya H, Hashimoto R, Suenaga M, Inokawa Y, Kobayashi D, et
al: Prognostic impact of expression and methylation status of
DENN/MADD domain-containing protein 2D in gastric cancer. Gastric
Cancer. 18:288–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kubista M, Andrade JM, Bengtsson M,
Forootan A, Jonák J, Lind K, Sindelka R, Sjöback R, Sjögreen B,
Strömbom L, et al: The real-time polymerase chain reaction. Mol
Aspects Med. 27:95–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kanda M, Nomoto S, Oya H, Hashimoto R,
Takami H, Shimizu D, Sonohara F, Kobayashi D, Tanaka C, Yamada S,
et al: Decreased expression of prenyl diphosphate synthase subunit
2 correlates with reduced survival of patients with gastric cancer.
J Exp Clin Cancer Res. 33:882014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kanda M, Nomoto S, Okamura Y, Hayashi M,
Hishida M, Fujii T, Nishikawa Y, Sugimoto H, Takeda S and Nakao A:
Promoter hypermethylation of fibulin 1 gene is associated with
tumor progression in hepatocellular carcinoma. Mol Carcinog.
50:571–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue XY, Liu ZH, Jing FM, Li YG, Liu HZ and
Gao XS: Relationship between NRAGE and the radioresistance of
esophageal carcinoma cell line TE13R120. Chin J Cancer. 29:900–906.
2010. View Article : Google Scholar : PubMed/NCBI
|