Introduction
The incidence rate of sporadic desmoid tumors is 2–4
individuals per million (1). Desmoid
tumors originate from clonal fibroblastic proliferation with
infiltrative growth and an inability to metastasize (2). The standard treatment regimen consists
of complete surgical resection, followed by anti-inflammatory
therapy, hormonal blockade and cytotoxic chemotherapy in certain
patients (3). The predominant
obstacle in the management of desmoid tumors is the high propensity
for local recurrence. Major retrospective studies report that the
rate of recurrence is between 20 and 60% at 5 years, even following
the complete ablation of the tumor (4,5). Clinical
characteristics, including the tumor site (such as the trunk)
(4), a younger age (6) and the surgical margin (7), are associated with the risk of local
recurrence of desmoid tumors.
Mutations in the adenomatous polyposis coli
(APC) or catenin (cadherin-associated protein) β1
(CTNNB1; β-catenin) genes have been identified in 89% of
sporadic desmoid tumors and also in tumors that occur in
association with familial adenomatous polyposis (2). In normal cells, the APC protein directly
binds to free β-catenin and promotes its phosphorylation by
glycogen synthase kinase-3β (GSK-3β); this targets β-catenin for
degradation via the ubiquitin-proteasome pathway (2). However, mutations in APC or β-catenin,
and particularly in the GSK-3β phosphorylation sites, inhibit the
binding of APC to β-catenin, leading to the stabilization of
β-catenin, which accumulates in the cytoplasm and nucleus of the
cell (6). Within the nucleus,
β-catenin binds directly to the T-cell factor/lymphoid enhancer
factor (TCF/LEF) group of DNA binding transcription factors and
stimulates the transcription of its target genes (2). The three most common somatic β-catenin
point mutations are located in codons 41 and 45 of exon 3. Previous
studies have suggested that the molecular profile of β-catenin may
be an important biological predictor of tumor recurrence; for
example, the S45F mutation may be associated with the risk of
desmoid tumor recurrence (6,8).
Midkine, also termed neurite growth-promoting factor
2, is a heparin-binding growth factor that is involved in various
cellular processes, including proliferation, survival and migration
(9). Numerous cell-surface receptors
have been identified to account for the multiple biological
activities of midkine, including ALK, LRP1 and integrin (9). The human midkine gene maps to chromosome
11p11.2 (9). The truncated form of
midkine is observed in several types of malignancies, including
gastric and breast cancer, pancreatic carcinoma and Wilms' tumors
(9). In addition, midkine was
demonstrated to be commonly expressed in a large cohort of human
desmoid tumor samples, and its increased expression was
significantly associated with the risk of tumor recurrence
(3). Menin, which is encoded by the
multiple endocrine neoplasia type 1 gene, is a tumor suppressor and
transcriptional regulator, and promotes the activity of the Wnt
signaling pathway (10). Menin has
been hypothesized to interact with β-catenin and promote its
translocation between the nucleus and the cytoplasm (10).
The present study performed direct sequencing of the
CTNNB1 gene and immunohistochemistry for the expression of
midkine, β-catenin, TCF-4 and menin in resected desmoid tumor
samples to assess the predictive value of these factors in the risk
of tumor recurrence.
Materials and methods
Patients and tumor samples
A total of 159 resected sporadic desmoid tumors from
initial surgeries of 159 patients were used in the present study.
They were obtained between January 1990 and December 2009 at the
Department of Pathology, Seoul National University Hospital (Seoul,
South Korea). The anatomical sites were classified as being
superficial (fascial), extra-abdominal, abdominal or
intra-abdominal (11).
Only samples with confirmed desmoid tumor histology
and an adequate amount of tissue for analysis were analyzed in the
present study. The time to recurrence was calculated as the time
between the initial surgery and the first tumor recurrence.
Postoperative adjuvant therapy, radiotherapy, chemotherapy or
combined radio- and chemotherapy were administered in 38, 12, 7 and
4 patients, respectively. The chemotherapy regimens consisted of
methotrexate, vinblastine, Glivec®, sulindac, monosodium glutamate
or tamoxifen. Detailed information, including demographics,
therapeutic regimens, histopathological findings and clinical
outcomes, were retrieved from the medical records of patients,
pathology results and the database of the Ministry of Security and
Public Administration, South Korean Government. The data retrieved
comprised the patient gender, anatomical sites of tumors, tumor
sizes, date of surgery, surgical margin, other treatments received
following surgery, date of recurrence, treatment for recurrence,
date of the last follow-up, status at the last follow-up and
patient survival information.
The present study was approved by the Institutional
Review Board of Seoul National University Hospital (approval no.
H-1209-068-427).
Mutational analysis
For the extraction of genomic DNA, the lesion region
was marked on a hematoxylin and eosin-stained slide by a
pathologist. DNA was extracted from the formalin-fixed paraffin
embedded blocks that contained ≥70% tumor content. Nested
polymerase chain reaction was performed using the following primers
for CTNNB1 exon 3 (cDNA NM_001904): Round 1 forward,
5′-ATGGAGTTGGACATGGCCAT-3′ and reverse, 5′-CCTGAGGAAGAGGATGTGGA-3′;
and round 2 forward, 5′-CTGGCAGCAACAGTCTTACC-3′ and reverse,
5′-CACTCAAGAACAAGTAG-3′ (Macrogen, Seoul, Korea). For the first
polymerase chain reaction (PCR), 200 ng of the purified DNA, 10
pmol of either forward or reverse primer and Premix Ex Taq (Takara
Bio Inc., Otsu, Japan) was made up to a final volume of 20 µl using
distilled water for the PCR, which underwent 35 cycles at 95°C for
30 sec, 58°C for 30 sec and 72°C for 1 min. A second PCR (nested
PCR) was performed with the same protocol as the first PCR using
the diluted (1:50) product of the first PCR. The amplified product
was a 172-bp fragment, which was purified using the QIAamp DNA Mini
kit (Qiagen GmbH, Hilden, Germany). Direct sequencing was performed
using the forward, 5′-ATGGAGTTGGACATGGCCAT-3′ and reverse,
5′-CACTCAAGAACAAGTAG-3′ primers (Macrogen, Seoul, Korea), and the
Applied Biosystems PRISM 3100 Genetic Analyzer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). A negative control was
performed by replacing the purified DNA with distilled water.
Immunohistochemistry
Tissue array sections of 4-µm thickness were
deparaffinized and rehydrated in graded alcohol. Antigen retrieval
was achieved by pressure-cooking the slides in 0.01 mol/l citrate
buffer for 5 min. The following primary antibodies were used:
Polyclonal mouse anti-human β-catenin (dilution, 1:800; catalog no.
610153; Transduction Laboratories™; BD Biosciences, Franklin Lakes,
NJ, USA); monoclonal mouse anti-human TCF-4 (dilution, 1:100;
clone, 6H5-3; catalog no. 05–511; Upstate Biotechnology, Inc., Lake
Placid, NY, USA); monoclonal rabbit anti-human Akt 1 (phospho S473)
(dilution, 1:100; catalog no. 2118-1; Epitomics, Burlingame, CA,
USA); polyclonal rabbit anti-human midkine (dilution, 1:200;
catalog no. 1937-1; Abcam, Cambridge, MA, USA); and monoclonal
rabbit anti-human menin (dilution, 1:200; catalog no. 2817-1;
Epitomics). Immunohistochemistry was performed and visualized using
Bond-MAX (Leica Microsystems, Wetzlar, Germany) and Bond Polymer
Refine Detection (Leica Biosystems, Newcastle, UK), respectively.
Nuclear staining was considered to indicate expression of
β-catenin, and tumor samples with >10% cells expressing
β-catenin were recorded as positive. The intensity of β-catenin
staining was recorded as mild, moderate or strong. Midkine
immunostaining was scored by intensity (0, none; 1, low; 2,
moderate/strong) and the percentage of positively-stained cells,
and samples were regarded as positive if they met the following
criteria: >10% of cells and an intensity of 2 or 3; or >50%
of cells stained and an intensity of 1. A sample was considered
TCF-4-positive if >30% of the cells were stained. Cytoplasmic
pAkt immunostaining was considered to indicate positive pAkt
expression. Negative menin expression for a sample was noted when
>90% of the cells did not exhibit staining in the nuclei.
Statistical analysis
SPSS version 20 software (IBM SPSS, Armonk, NY, USA)
was used for statistical analysis. χ2 test and Cox
regression analysis were used. All P-values were two-tailed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological profiles of
sporadic desmoid tumors
The present study evaluated the prevalence of
CTNNB1 mutations in 159 patients with sporadic desmoid
tumors. The patients consisted of 77 men (48.4%) and 82 women
(51.6%). The average age of the patients was 41.2 years (range,
7–83 years). In total, 28.3% of the desmoid tumors were superficial
(fascial; 45 patients), 44.7% were extra-abdominal (71 patients),
15.1% were abdominal (24 patients) and 11.9% were intra-abdominal
(19 patients). The association between clinicopathological
characteristics and tumor recurrence was analyzed. Tumor recurrence
was significantly associated with a female gender (P=0.016),
younger age (<30 years; P=0.002), extra-abdominal tumor site
(P<0.001) and expression of midkine (P=0.007) (Table I).
 | Table I.Association between
clinicopathological characteristics and tumor recurrence in
patients with sporadic desmoid tumors. |
Table I.
Association between
clinicopathological characteristics and tumor recurrence in
patients with sporadic desmoid tumors.
| Variable | Total, n (%) | Recurrence, n
(%) | No recurrence, n
(%) | P-value |
|---|
| Total patients | 157
(100.0) | 67 (42.7) | 90 (57.3) |
|
| Gender |
|
|
|
0.016 |
|
Female | 81
(51.6) | 42 (51.9) | 39 (48.1) |
|
| Male | 76
(48.4) | 25 (32.9) | 51 (67.1) |
|
| Age, years |
|
|
|
0.002 |
|
<30 | 47
(29.9) | 29 (61.7) | 18 (38.3) |
|
| ≥30 | 110 (70.1) | 38 (34.5) | 72 (65.5) |
|
| Tumor size, cm |
|
|
|
0.983 |
|
<6.0 | 95
(61.3) | 41 (43.2) | 54 (56.8) |
|
| ≥6.0 | 60
(38.7) | 26 (43.3) | 34 (56.7) |
|
| Site |
|
|
| <0.001 |
|
Superficial (fascial) | 45
(28.7) | 12 (26.7) | 33 (73.3) |
|
|
Extra-abdominal | 69
(43.9) | 45 (65.2) | 24 (34.8) |
|
|
Abdominal | 24
(15.3) | 5
(20.8) | 19 (79.2) |
|
|
Intra-abdominal | 19
(12.1) | 5
(26.3) | 14 (73.7) |
|
| Growth |
|
|
|
0.361 |
|
Nodular | 39
(26.9) | 14 (35.9) | 25 (64.1) |
|
|
Infiltrative | 106 (73.1) | 47 (44.3) | 59 (55.7) |
|
| Resection margin |
|
|
|
0.594 |
| Not
involved | 65
(53.7) | 31 (47.7) | 34 (52.3) |
|
|
Involved | 56
(46.3) | 24 (42.9) | 32 (57.1) |
|
| CTNNB1 |
|
|
|
0.116 |
|
Wild-type | 48
(30.6) | 16 (33.3) | 32 (66.7) |
|
|
Mutation | 109 (69.4) | 51 (46.8) | 58 (53.2) |
|
| CTNNB1 |
|
|
|
0.113 |
|
Wild-type | 48
(30.6) | 16 (33.3) | 32 (66.7) |
|
| T41
mutation | 88
(56.1) | 44 (50.0) | 44 (50.0) |
|
| S45
mutation | 18
(11.5) | 7
(38.9) | 11 (61.1) |
|
| Other
mutation | 3
(1.9) | 0 (0.0) | 3
(100.0) |
|
| β-catenin
intensity |
|
|
|
0.876 |
|
Weak | 60
(40.8) | 26 (43.3) | 34 (56.7) |
|
|
Moderate | 64
(43.5) | 29 (45.3) | 35 (54.7) |
|
|
Strong | 23
(15.6) | 9
(39.1) | 14 (60.9) |
|
| TCF-4 |
|
|
|
0.105 |
|
Negative | 63
(42.6) | 22 (34.9) | 41 (65.1) |
|
|
Positive | 85
(57.4) | 41 (48.2) | 44 (51.8) |
|
| Menin |
|
|
|
0.696 |
|
Negative | 11 (7.6) | 4
(36.4) | 7
(63.6) |
|
|
Positive | 132 (91.7) | 56 (42.4) | 76 (57.6) |
|
| pAkt |
|
|
|
0.857 |
|
Negative | 121 (84.6) | 52 (43.0) | 69 (57.0) |
|
|
Positive | 22
(15.4) | 9
(40.9) | 13 (59.1) |
|
| Midkine |
|
|
|
0.007 |
|
Negative | 111 (76.0) | 41 (36.9) | 70 (63.1) |
|
|
Positive | 35
(24.0) | 22 (62.9) | 13 (37.1) |
|
CTNNB1 genotype in sporadic desmoid
tumors
Mutations in CTNNB1 were detected in 69.8%
(111/159) of the tumors. Point mutations were identified in 4
codons, corresponding to T41, S45, T42 and G48 amino acids
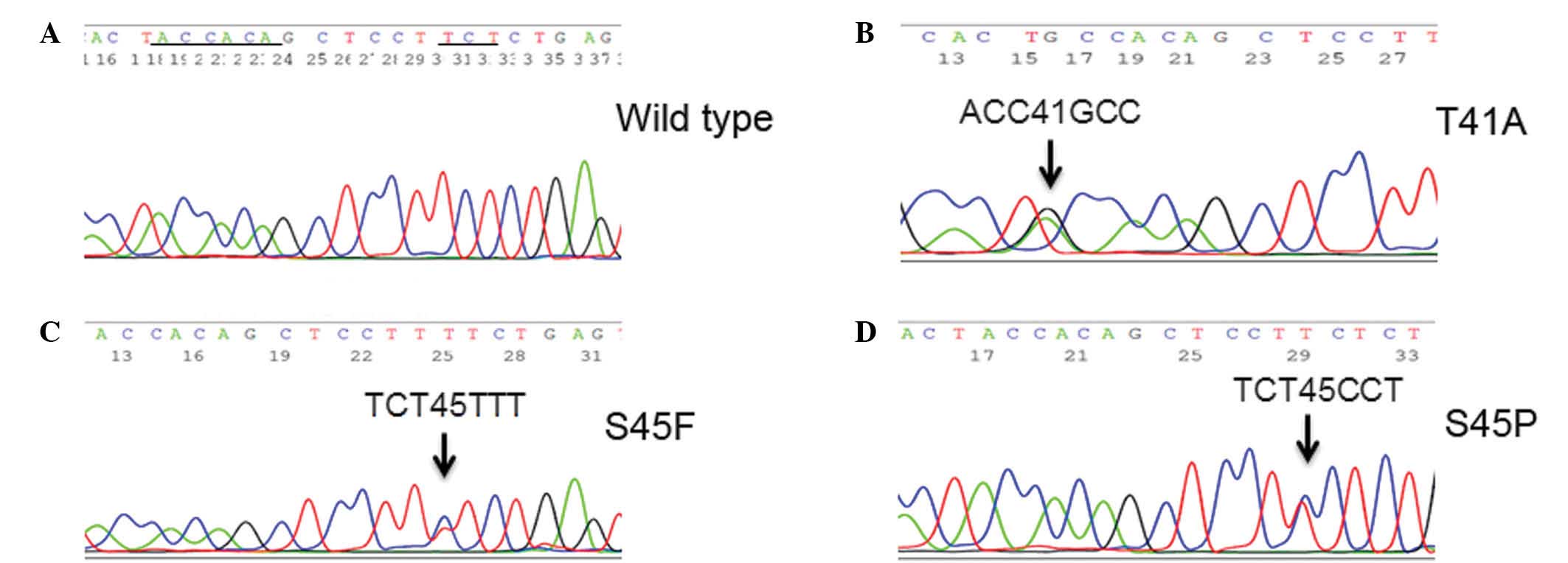
(Fig. 1). The replacement of
threonine by alanine at codon 41 (p.T41A) was observed in 56% of
the tumors (n=89). At codon 45, replacement of serine by
phenylalanine (p.S45F), serine by proline (p.S45P) or serine by
asparagine (p.S45N) was identified in 8.2% (13/159), 1.9% (3/159)
and 1.9% (3/159) of the tumors, respectively. A point mutation in
T42, resulting in the replacement of threonine by alanine (p.T42A)
was observed in 1 tumor (0.6%). A silent mutation in G48,
consisting of a codon alteration from GGT to GGC, was observed in 1
tumor (0.6%). The intensity of β-catenin expression was not
associated with the presence of the CTNNB1 mutation or
genotype (Table II).
 | Table II.Association between
clinicopathological characteristics and CTNNB1 mutations in
patients with sporadic desmoid tumors. |
Table II.
Association between
clinicopathological characteristics and CTNNB1 mutations in
patients with sporadic desmoid tumors.
|
|
| CTNNB1 | CTNNB1
mutation |
|---|
|
|
|
|
|
|---|
| Variable | Total, n (%) | Wild-type, n
(%) | Mutation, n
(%) |
P-valuea | T41, n (%) | S45, n (%) | Other, n (%) |
P-valueb |
|---|
| Total | 159
(100.0) | 48 (30.2) | 111 (69.8) |
| 89 (56.0) | 19
(11.9) | 3 (1.9) |
|
| Gender |
|
|
| 0.100 |
|
|
| 0.211 |
|
Female | 82
(51.6) | 20 (24.4) | 62
(75.6) |
| 52 (63.4) | 8
(9.8) | 2 (2.4) |
|
|
Male | 77
(48.4) | 28 (36.4) | 49
(63.6) |
| 37 (48.1) | 11
(14.3) | 1 (1.3) |
|
| Age, years |
|
|
| 0.227 |
|
|
| 0.158 |
|
<30 | 47
(29.6) | 11 (23.4) | 36
(76.6) |
| 27 (57.4) | 9
(19.1) | 0 (0.0) |
|
|
≥30 | 112 (70.4) | 37 (33.0) | 75
(67.0) |
| 62 (55.4) | 10
(8.9) | 3 (2.7) |
|
| Tumor size, cm |
|
|
| 0.060 |
|
|
| 0.407 |
|
<6.0 | 96
(61.1) | 34 (35.4) | 62
(64.6) |
| 52 (54.2) | 8
(8.3) | 2 (2.1) |
|
|
≥6.0 | 61
(38.9) | 13 (21.3) | 48
(78.7) |
| 36 (59.0) | 11
(18.0) | 1 (1.6) |
|
| Site |
|
|
| 0.014 |
|
|
| 0.005 |
|
Superficial (fascial) | 45
(28.3) | 22 (48.9) | 23
(51.1) |
| 21 (46.7) | 1
(2.2) |
1(2.2) |
|
|
Extra-abdominal | 71
(44.7) | 16 (22.5) | 55
(77.5) |
| 43 (60.6) | 12
(16.9) | 0 (0.0) |
|
|
Abdominal | 24
(15.1) | 5
(20.8) | 19
(79.2) |
| 12 (50.0) | 5
(20.8) | 2 (8.3) |
|
|
Intra-abdominal | 19
(11.9) | 5
(26.3) | 14
(73.7) |
| 13 (68.4) | 1
(5.3) | 0 (0.0) |
|
| Growth |
|
|
| 0.563 |
|
|
| 0.619 |
|
Nodular | 39
(26.5) | 10 (25.6) | 29
(74.4) |
| 21 (53.8) | 7
(17.9) | 1 (2.6) |
|
|
Infiltrative | 108 (73.5) | 33 (30.6) | 75
(69.4) |
| 62 (57.4) | 11
(10.2) | 2 (1.9) |
|
| Resection
margin |
|
|
| 0.358 |
|
|
| 0.68 |
| Not
involved | 65
(53.7) | 16 (24.6) | 49
(75.4) |
| 39 (60.0) | 9
(13.8) | 1 (1.5) |
|
|
Involved | 56
(46.3) | 18 (32.1) | 38
(67.9) |
| 29 (51.8) | 7
(12.5) | 2 (3.6) |
|
| β-catenin
intensity |
|
|
| 0.063 |
|
|
| 0.184 |
|
Weak | 60
(40.3) | 25 (41.7) | 35
(58.3) |
| 29 (48.3) | 6
(10.0) | 0 (0.0) |
|
|
Moderate | 66
(44.3) | 18 (27.3) | 48
(72.7) |
| 35 (53.0) | 11
(16.7) | 2 (3.0) |
|
|
Strong | 23
(15.4) | 4
(17.4) | 19
(82.6) |
| 16 (69.6) | 2
(8.7) | 1 (4.3) |
|
| TCF-4 |
|
|
| 0.245 |
|
|
| 0.589 |
|
Negative | 63
(42.0) | 23 (36.5) | 40
(63.5) |
| 33 (52.4) | 6
(9.5) | 1 (1.6) |
|
|
Positive | 87
(58.0) | 24 (27.6) | 63
(72.4) |
| 48 (55.2) | 13
(14.9) | 2 (2.3) |
|
| Menin |
|
|
| 0.731 |
|
|
| 0.924 |
|
Negative | 11 (7.6) | 4
(36.4) | 7
(63.6) |
| 6 (54.5) | 1
(9.1) | 0 (0.0) |
|
|
Positive | 134 (92.4) | 42 (31.3) | 92
(68.7) |
| 71 (53.0) | 18
(13.4) | 3 (2.2) |
|
| pAkt |
|
|
| 0.360 |
|
|
| 0.663 |
|
Negative | 123 (84.8) | 40 (32.5) | 83
(67.5) |
| 64 (52.0) | 16
(13.0) | 3 (2.4) |
|
|
Positive | 22
(15.2) | 5
(22.7) | 17
(77.3) |
| 14 (63.6) | 3
(13.6) | 0 (0.0) |
|
| Midkine |
|
|
| 0.024 |
|
|
| 0.131 |
|
Negative | 111 (75.0) | 40 (36.0) | 71
(64.0) |
| 57 (51.4) | 12
(10.8) | 2 (1.8) |
|
|
Positive | 37
(25.0) | 6
(16.2) | 31
(83.8) |
| 23 (62.2) | 7
(18.9) | 1 (2.7) |
|
Immunohistochemical staining for
midkine and Wnt pathway proteins
All successful tumor samples expressed β-catenin
[weak intensity, 40.3% (60/149); moderate to strong intensity,
59.7% (89/149)]. In certain cases, immunostaining failed and these
results were excluded. Therefore, 58.0% (87/150) of the tumors
expressed TCF-4, 92.4% (134/145) expressed menin, 15.2% (22/145)
expressed pAkt and 25.0% (37/148) expressed midkine (Fig. 2). Positive expression of midkine was
significantly associated with tumor recurrence (P=0.007). However,
there was no significant association between tumor recurrence and
CTNNB1 mutations and TCF-4, menin and pAkt expression.
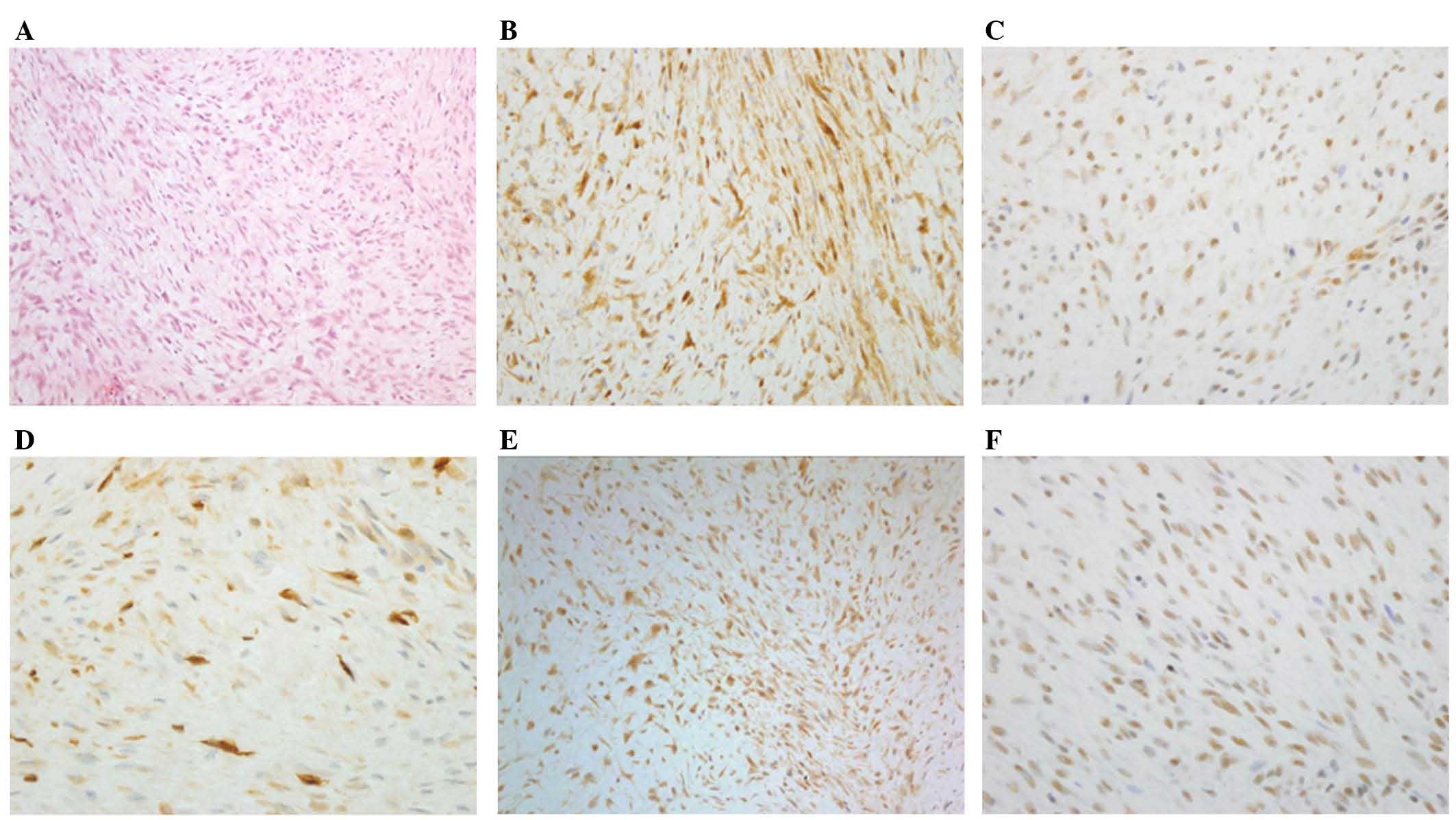 | Figure 2.Immunohistochemical staining of
β-catenin, TCF-4, midkine, pAkt, and menin in sporadic desmoid
tumors. (A) Hematoxylin and eosin-stained section of a sporadic
desmoid tumor, (B) β-catenin staining, (C) TCF-4 staining, (D)
midkine staining, (E) pAkt staining and (F) menin staining.
Magnification, ×200. TCF-4; T cell-factor 4; pAkt, phosphorylated
protein kinase B. |
Analysis of recurrence-free survival
(RFS) time
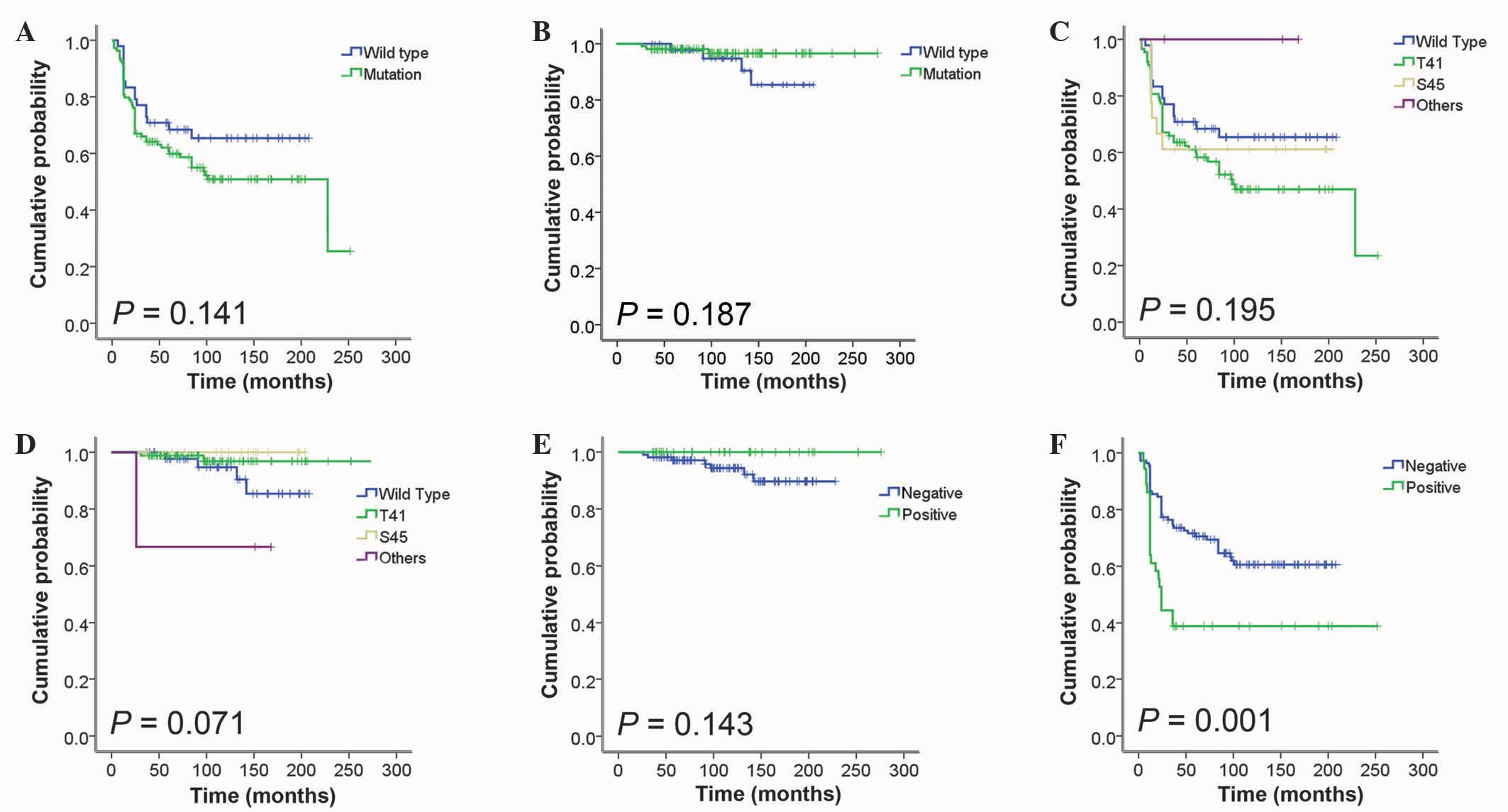
Kaplan-Meier analysis for RFS time demonstrated that
midkine expression was significantly associated with a shorter RFS
time (P=0.001; Fig. 3). In the
multivariate Cox regression analysis, four clinical characteristics
(age, ≥30 vs. <30 years; tumor site, extra-abdominal vs. others;
CTNNB1 genotype, mutation vs. wild-type; midkine expression,
positive vs. negative) were included as covariates. This revealed
that an extra-abdominal tumor site [hazard ratio (HR), 2.625;
P=0.001] and positive midkine expression (HR, 2.077; P=0.009) were
independent predictors of RFS time (Table III).
 | Table III.Multivariate Cox analysis of
recurrence-free survival and overall survival time in patients with
sporadic desmoid tumors. |
Table III.
Multivariate Cox analysis of
recurrence-free survival and overall survival time in patients with
sporadic desmoid tumors.
| Variable | n | P-value | HR | 95% CI |
|---|
| Total | 145 |
|
|
|
| Age, years |
| 0.064 | 1.644 | 0.972–2.781 |
|
≥30 | 100 |
|
|
|
|
<30 | 45 |
|
|
|
| Site |
| 0.001 | 2.625 | 1.491–4.623 |
|
Extra-abdominal | 66 |
|
|
|
|
Other | 79 |
|
|
|
| CTNNB1 |
| 0.517 | 1.229 | 0.659–2.289 |
|
Mutation | 99 |
|
|
|
|
Wild-type | 46 |
|
|
|
| Midkine
expression |
| 0.009 | 2.077 | 1.203–3.587 |
|
Positive | 35 |
|
|
|
|
Negative | 110 |
|
|
|
Discussion
The present study confirmed that positive midkine
expression in sporadic desmoid tumors is significantly associated
with tumor recurrence. In a previous target gene screening study
for sporadic desmoid tumors, 98 genes were identified to be
overexpressed. Among the genes containing a TCF/LEF
consensus-binding site, midkine was demonstrated to have a
significant association with tumor recurrence (3). Midkine binds with high affinity to the
chondroitin sulfate portion of protein tyrosine phosphatase (PTP)ζ,
and to the cell-surface nucleolin receptor with low affinity.
Following binding to its ligand, PTPζ undergoes dimerization, which
inactivates the phosphatase domain and leads to an increase in
intracellular tyrosine phosphate. β-catenin has been identified as
the substrate of PTPζ (12).
The high expression of midkine in Wilms' tumors
suggests that midkine is a target gene of Wilms' tumor 1 (WT1)
(13). The wild-type WT1 gene
is markedly overexpressed in β-catenin-mutant desmoid tumors. The
Wnt signaling pathway interacts with WT1 in normal kidney
development and commonly plays a role in the genesis of Wilms'
tumors. WT1 overexpression is hypothesized to be involved in the
association between the β-catenin mutation and midkine
overexpression in sporadic desmoid tumors (13,14).
The majority of sporadic desmoid tumors (~85%) have
mutations in the β-catenin gene CTNNB1, which is located on
the short arm of chromosome 3. In addition, frequent point
mutations at codons 41 and 45 (T41A, S45F and S45P) have been
identified in sporadic desmoid tumors (6,7). A
previous large series of sporadic desmoid tumors suggested that
T41A is the most common mutation, and patients harboring S45F
mutations are at an increased risk of tumor recurrence (6). This finding was also confirmed by a
multicenter validation study (8). The
CTNNB1 gene, which encodes a component of the Wnt signaling
pathway, is hypothesized to cause dysregulation of proliferation
and invasiveness in fibroblasts (7,15,16).
In the present study, a mutation frequency of 70%
was observed; these mutations occurred predominantly in T41 (T41A,
56% of all patients) or S45 (12%), whilst the remaining 2% harbored
other mutations. Notably, compared with previous studies, the
mutation rate in the present study was lower (70 vs. 83–87%), the
frequency of the T41 mutation was higher (56 vs. 35–50%) and the
frequency of the S45 mutation was lower (12 vs. 35–52%) (6,17,18).
Colombo et al (8) observed a specific association between
the type of CTNNB1 mutation and the site of the origin of
desmoid tumors; a predominance of the S45F mutation was observed in
extremity desmoid tumors, indicating that the S45F mutation is
associated with a poor outcome of patients. By contrast, the
association between the type of CTNNB1 mutation and the site
of origin of the desmoid tumor differed in the present study; a
higher frequency of the S45 mutation was identified in the
extra-abdominal and abdominal sites compared with the superficial
or intra-abdominal sites. The T41 mutation was more common in the
intra-abdominal and head and neck fibromatoses. These results are
supported by a previous study that demonstrated that the T41
mutation is more frequent in mesenteric fibromatoses (80.4%)
compared with the abdominal wall and extra-abdominal fibromatoses
(46.4%) (19). In addition, the
current study observed that there was no significant association
between the CTNNB1 mutation and clinicopathological
characteristics, which is supported by the results from a previous
study (18).
It is possible that ethnic differences between
Western and Asian countries affects the frequency of the mutations.
Notably, two previous Japanese studies reported that the
frequencies of CTNNB1 mutations in patients with sporadic
desmoid tumors were 38.9% (7/18 patients) (20) and 52.4% (22/42 patients) (21). Independent multicenter studies with
large cohorts of patients are required to further investigate these
ethnic variations.
Occasionally, desmoid tumors are focally positive
for nuclear β-catenin, and protein expression levels may vary
within the tumor (22,23). Therefore, CTNNB1 genotyping may
be beneficial in the differential diagnosis of desmoid tumors.
However, we suggest that extensive statistical data and analysis
are required for the clinical application of this genotyping
(18).
In conclusion, the present study demonstrated that
midkine-positivity in sporadic desmoid tumors is significantly
associated with the risk of tumor recurrence. Therefore, midkine
expression may be considered as a predictive marker for the
recurrence of sporadic desmoid tumors, and may be useful for
determining treatment regimens for patients.
Acknowledgements
The present study was supported by the Seoul
National University Hospital Research Fund (grant no.
03-2012-0190).
References
|
1
|
Reitamo JJ, Häyry P, Nykyri E and Saxén E:
The desmoid tumor. I. Incidence, sex-, age- and anatomical
distribution in the Finnish population. Am J Clin Pathol.
77:665–673. 1982.PubMed/NCBI
|
|
2
|
Alman BA, Li C, Pajerski ME, Diaz-Cano S
and Wolfe HJ: Increased beta-catenin protein and somatic APC
mutations in sporadic aggressive fibromatoses (desmoid tumors). Am
J Pathol. 151:329–334. 1997.PubMed/NCBI
|
|
3
|
Colombo C, Creighton CJ, Ghadimi MP,
Bolshakov S, Warneke CL, Zhang Y, Lusby K, Zhu S, Lazar AJ, West
RB, et al: Increased midkine expression correlates with desmoid
tumour recurrence: A potential biomarker and therapeutic target. J
Pathol. 225:574–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fiore M, Rimareix F, Mariani L, Domont J,
Collini P, Le Péchoux C, Casali PG, Le Cesne A, Gronchi A and
Bonvalot S: Desmoid-type fibromatosis: A front-line conservative
approach to select patients for surgical treatment. Ann Surg Oncol.
16:2587–2593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ballo MT, Zagars GK, Pollack A, Pisters PW
and Pollack RA: Desmoid tumor: Prognostic factors and outcome after
surgery, radiation therapy, or combined surgery and radiation
therapy. J Clin Oncol. 17:158–167. 1999.PubMed/NCBI
|
|
6
|
Lazar AJ, Tuvin D, Hajibashi S, Habeeb S,
Bolshakov S, Mayordomo-Aranda E, Warneke CL, Lopez-Terrada D,
Pollock RE and Lev D: Specific mutations in the beta-catenin gene
(CTNNB1) correlate with local recurrence in sporadic desmoid
tumors. Am J Pathol. 173:1518–1527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lazar AJ, Hajibashi S and Lev D: Desmoid
tumor: From surgical extirpation to molecular dissection. Curr Opin
Oncol. 21:352–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colombo C, Miceli R, Lazar AJ, Perrone F,
Pollock RE, Le Cesne A, Hartgrink HH, Cleton-Jansen AM, Domont J,
Bovée JV, et al: CTNNB1 45F mutation is a molecular
prognosticator of increased postoperative primary desmoid tumor
recurrence: An independent, multicenter validation study. Cancer.
119:3696–3702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakamoto K and Kadomatsu K: Midkine in the
pathology of cancer, neural disease, and inflammation. Pathol Int.
62:445–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y, Liu R, Jiang X, Lu J, Jiang J,
Zhang C, Li X and Ning G: Nuclear-cytoplasmic shuttling of menin
regulates nuclear translocation of {beta}-catenin. Mol Cell Biol.
29:5477–5487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiss SW and Goldblum JR: Fibromatoses.
Enzinger and Weiss's Soft Tissue Tumors. 1:(5th). (Philadelphia,
PA). Elsevier, Inc. 2282008.
|
|
12
|
Meng K, Rodriguez-Peña A, Dimitrov T, Chen
W, Yamin M, Noda M and Deuel TF: Pleiotrophin signals increased
tyrosine phosphorylation of beta beta-catenin through inactivation
of the intrinsic catalytic activity of the receptor-type protein
tyrosine phosphatase beta/zeta. Proc Natl Acad Sci USA.
97:2603–2608. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nik Amini S, Hohenstein P, Jadidizadeh A,
Van Dam K, Bastidas A, Berry RL, Patek CE, Van der Schueren B,
Cassiman JJ and Tejpar S: Upregulation of Wilms' tumor gene 1 (WT1)
in desmoid tumors. Int J Cancer. 114:202–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adachi Y, Matsubara S, Pedraza C, Ozawa M,
Tsutsui J, Takamatsu H, Noguchi H, Akiyama T and Muramatsu T:
Midkine as a novel target gene for the Wilms' tumor suppressor gene
(WT1). Oncogene. 13:2197–2203. 1996.PubMed/NCBI
|
|
15
|
Mignemi NA, Itani DM, Fasig JH, Keedy VL,
Hande KR, Whited BW, Homlar KC, Correa H, Coffin CM, Black JO, et
al: Signal transduction pathway analysis in desmoid-type
fibromatosis: Transforming growth factor-β, COX2 and sex steroid
receptors. Cancer Sci. 103:2173–2180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheon S, Poon R, Yu C, Khoury M, Shenker
R, Fish J and Alman BA: Prolonged beta-catenin stabilization and
tcf-dependent transcriptional activation in hyperplastic cutaneous
wounds. Lab Invest. 85:416–425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amary MF, Pauwels P, Meulemans E, Roemen
GM, Islam L, Idowu B, Bousdras K, Diss TC, O'Donnell P and Flanagan
AM: Detection of beta-catenin mutations in paraffin-embedded
sporadic desmoid-type fibromatosis by mutation-specific restriction
enzyme digestion (MSRED): An ancillary diagnostic tool. Am J Surg
Pathol. 31:1299–1309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dômont J, Salas S, Lacroix L, Brouste V,
Saulnier P, Terrier P, Ranchère D, Neuville A, Leroux A, Guillou L,
et al: High frequency of β-catenin heterozygous mutations in
extra-abdominal fibromatosis: A potential molecular tool for
disease management. Br J Cancer. 102:1032–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huss S, Nehles J, Binot E, Wardelmann E,
Mittler J, Kleine MA, Künstlinger H, Hartmann W, Hohenberger P,
Merkelbach-Bruse S, et al: β-catenin (CTNNB1) mutations and
clinicopathological features of mesenteric desmoid-type
fibromatosis. Histopathology. 62:294–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito T, Oda Y, Tanaka K, Matsuda S,
Tamiya S, Iwamoto Y and Tsuneyoshi M: Beta-catenin nuclear
expression correlates with cyclin D1 overexpression in sporadic
desmoid tumours. J Pathol. 195:222–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tejpar S, Nollet F, Li C, Wunder JS,
Michils G, dal Cin P, Van Cutsem E, Bapat B, van Roy F, Cassiman JJ
and Alman BA: Predominance of beta-catenin mutations and
beta-catenin dysregulation in sporadic aggressive fibromatosis
(desmoid tumor). Oncogene. 18:6615–6620. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coffin CM, Hornick JL, Zhou H and Fletcher
CD: Gardner fibroma: A clinicopathologic and immunohistochemical
analysis of 45 patients with 57 fibromas. Am J Surg Pathol.
31:410–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Montgomery E and Folpe AL: The diagnostic
value of beta-catenin immunohistochemistry. Adv Anat Pathol.
12:350–356. 2005. View Article : Google Scholar : PubMed/NCBI
|