Introduction
Small cell carcinoma is a frequently occurring
pulmonary neoplasm that may also be located in extrapulmonary
sites, including the salivary glands, pharynx, larynx, thymus,
esophagus, stomach, small bowel, colon, rectum, gallbladder,
kidney, uterus, skin and breasts (1).
Extrapulmonary small cell carcinoma is histologically identical to
pulmonary small cell carcinoma, with rapid local progression and
exhibiting early regional and distant metastasis (1). Patients with extrapulmonary small cell
carcinoma frequently have a poor prognosis, even if diagnosed at an
early stage (2). Small cell carcinoma
of the large bowel is extremely rare, representing ~0.2% of all
large bowel malignancies (3). To
date, there have been ~40 cases of extrapulmonary small cell
carcinoma of the rectum reported in the literature (4), the majority of which are focused on the
clinical and pathological features, treatment and prognosis of the
disease.
The pathological features of extrapulmonary small
cell carcinoma of the rectum are similar to small-cell
neuroendocrine carcinomas in other sites, exhibiting closely packed
cells with scanty cytoplasm, nuclear pleomorphism, a high mitotic
rate and necrosis (4). Neuroendocrine
differentiation can be demonstrated by immunohistochemical methods
or electron microscopy (2). The
differential diagnosis includes metastatic lung small cell
carcinoma, which can only be excluded clinically; other small cell
malignancies that may occur in this region, such as the more common
basaloid or cloacogenic carcinoma, lymphoma, embryonal
rhabdomyosarcoma and amelanotic melanoma; and other neuroendocrine
tumors, such as carcinoid (4). Long
survival (10–20 years) has been reported following resection of the
primary tumor, although a median survival of 5–11 months is most
commonly reported (4). Computed
tomography (CT) and magnetic resonance imaging (MRI), as well as
colorectal colonoscopy may be used in the diagnosis of small cell
carcinoma of the rectum (4,5).
Small cell carcinoma of the rectum is extremely
rare. The present study aimed to improve the recognition of CT and
MRI features observed in patients with small cell carcinoma of the
rectum by reporting the radiological features of 4 patients with
surgically and pathologically diagnosed small cell carcinoma of the
rectum. In particular, the present study reviews the CT and MRI
features used to diagnose the patients.
Patients and methods
Patients
The cases of 4 patients with pathologically
confirmed extrapulmonary small cell carcinoma of the rectum were
retrospectively reviewed by the present study. The patients were
treated at Sun Yat-sen University Cancer Center (Guangzhou,
Guangdong, China) between January 2001 and August 2013. The
histological diagnosis of rectal small cell carcinoma was confirmed
by endoscopic biopsy or surgery (Miles' operation, exploratory
laparotomy and sigmoidostomy). The patients consisted of 3 men and
1 woman (age range, 40–65 years; median age, 51.5 years). The
clinical records of the patients were obtained, and patient
characteristics, including age, gender, tumor stage, clinical
symptoms, digital rectal examination and colonoscopy results,
laboratory examinations [carcinoembryonic antigen, cancer antigen
19–9 and neuron-specific enolase (NSE)], treatment and follow-up
information were reviewed. Sun Yat-sen University Cancer Center
Institutional Review Board approval and written informed patient
consent were obtained prior to the commencement of the present
study.
Imaging protocol
CT was performed for 3 patients using a Brilliance
TM16 abdomen scanner (Philips Medical Systems B.V., Eindhoven, The
Netherlands) with the following parameters: 5-mm slice thickness;
120-kV voltage; 200-mA current; and a 256×256 matrix. Following
non-enhanced CT imaging, an intravenous bolus of 1.5–2.0 ml/kg of a
non-ionic iodine contrast agent (iopromide; Ultravist®; Bayer Plc.,
Newbury, UK) was administered at a rate of 2.5–3.0 ml/sec. Enhanced
CT images were obtained at 60 sec post-injection of the contrast
agent.
MRI was performed on 1 patient using a 1.5-Tesla
unit (GE Signa CVi system; GE Healthcare Life Sciences, Chalfont,
UK) with an abdomen combined coil. The following 7 sequences were
obtained: Non-enhanced T1-weighted images (T1WI) in axial plane;
non-enhanced T2-weighted images (T2WI) in axial plane; non-enhanced
fat-suppressed T2WI in axial and sagittal planes; and
contrast-enhanced fat-suppressed T1WI in axial, coronal and
sagittal planes. A 0.2-mmol/kg body weight bolus injection of
gadopentetate dimeglumine (Magnevist®; Bayer Plc.) was administered
to obtain contrast-enhanced sequences.
Imaging analysis
Two experienced radiologists independently evaluated
the CT and MRI images. Any disagreements were resolved by
consensus. The CT and MRI features evaluated consisted of rectal
wall thickening, the presence of a soft-tissue tumor, local tumor
invasion, CT density, MRI signal intensity (hypointensity,
isointensity or hyperintensity in association with gluteal muscle
signals), lesion texture (homogeneous, heterogeneous or necrotic),
contrast enhancement characteristics (strong, moderate or poor),
lymphadenopathy and distant metastasis. The thick rectal wall was
measured in the axial plane and the maximum value was selected.
Strong enhancement was selected if the degree of enhancement was
stronger than that of the gluteal muscles, moderate enhancement was
selected if the degree of enhancement was similar to that of the
gluteal muscles and poor enhancement was selected if the degree of
enhancement exhibited no clear signal increase. Lymphadenopathy was
considered abnormal when the maximal short dimension was >0.5
cm, or when stronger enhancement was observed compared with that of
the adjacent gluteal muscles.
Histology
All 4 patients underwent electronic enteroscopic
biopsy. The specimens were cut to a width of 3.5 µm, and examined
by microscopic and immunohistochemical analyses in the Pathology
Department of Sun Yat-sen University Cancer Center.
Immunohistochemical examinations included synaptophysin, cluster of
differentiation 56 (CD56), chromogranin A and NSE. The specimens
were examined microscopically by hematoxylin and eosin (H&E)
staining.
Results
Clinical characteristics
The most common clinical symptoms observed in the
patients were tenesmus (n=2; patients 2 and 3), increased frequency
of defecation (n=2; patients 3 and 4), bloody stools (n=3; patients
1–3), difficulty in defecation (n=2; patients 2 and 4), anal pain
(n=1; patient 1) and dysuresia (n=1; patient 4). In total, 1 out of
the 4 patients was stage IIIC (patient 1), 1 patient was stage IVA
(patient 2) and the remaining 2 patients were stage IVB (patients 3
and 4), according to the National Comprehensive Cancer Network
guidelines (6). Blood tests revealed
that 1 out of 4 patients (patient 1) exhibited an abnormal
carcinoembryonic antigen level of 8.19 ng/ml (normal range, 0–5
ng/ml). The 2 patients who were tested for cancer antigen 19–9
possessed normal levels (patient 1, 8.6 U/ml; patient 2, 6.9 U/ml;
normal range, 0–35 U/ml). Blood NSE was evaluated in 1 patient
(patient 3), at an abnormal level of 114.8 ng/ml (normal range,
0.0–15.2 ng/ml). In total, 1 patient underwent Miles' operation
(patient 1), 1 patient underwent concurrent chemotherapy and
radiotherapy (patient 2), 1 patient underwent an exploratory
laparotomy, sigmoidostomy and post-operative chemotherapy (patient
3), and 1 patient did not receive any treatment (patient 4).
Imaging findings
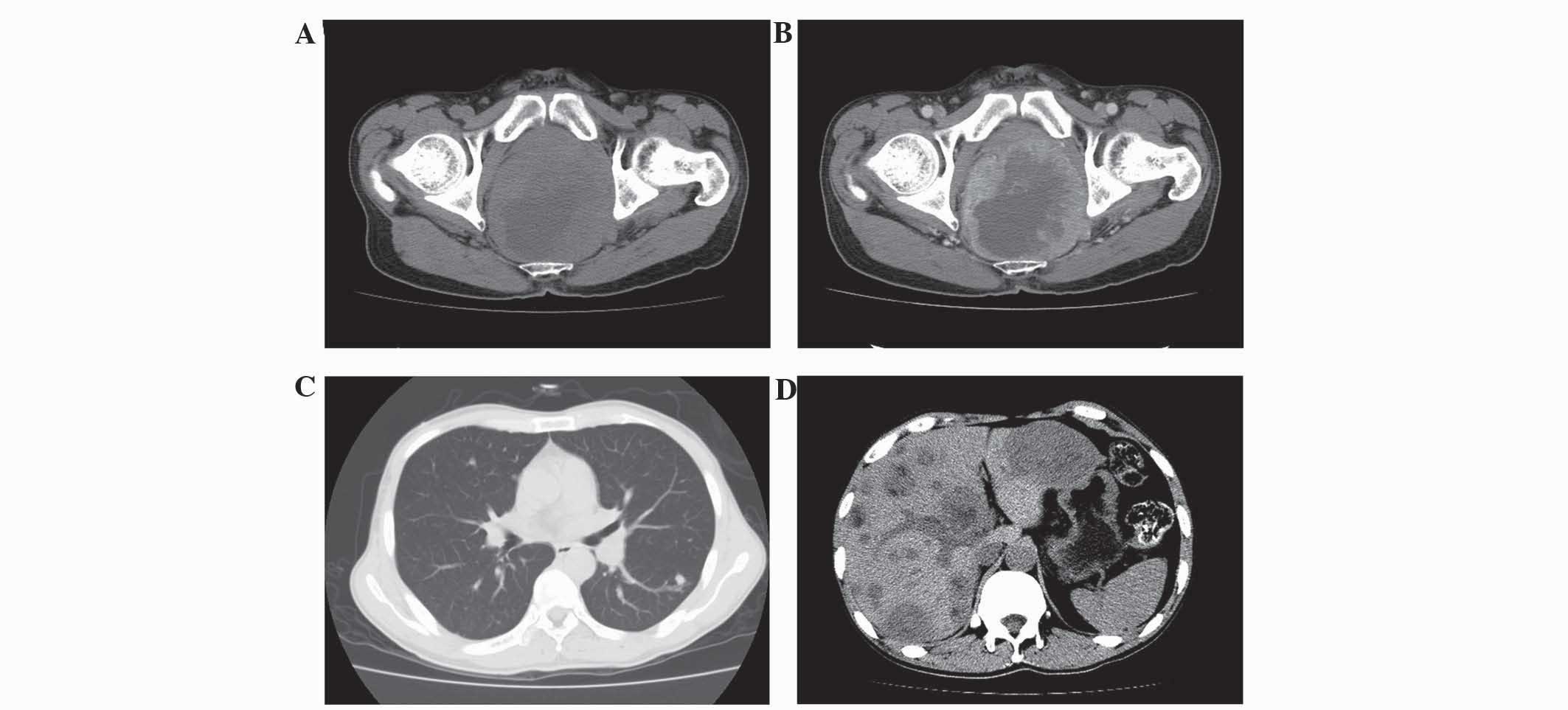
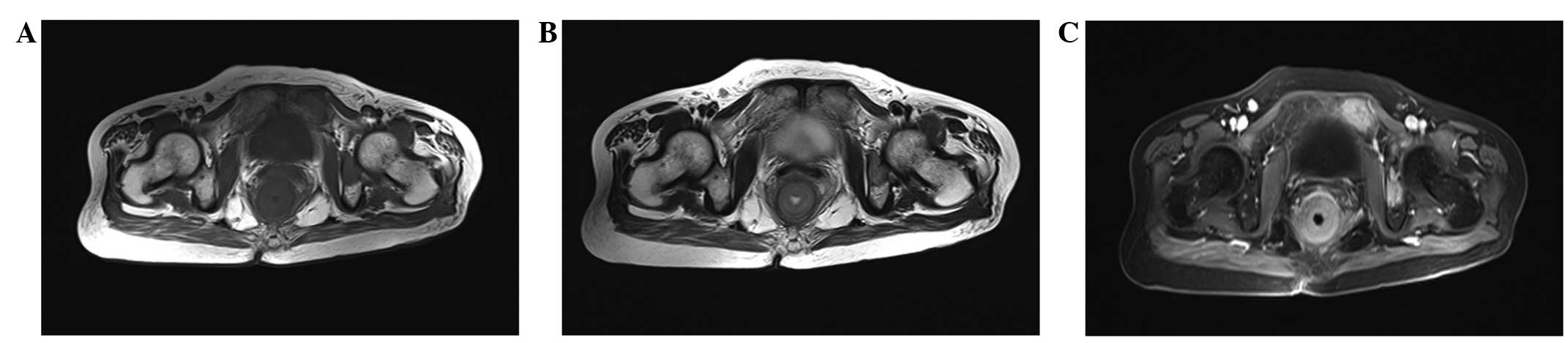
The CT and MRI results are summarized in Table I. All 4 patients exhibited ring-like
rectal wall thickening, hypodensity compared with the gluteus
muscles on non-enhanced CT images, isointensity on T1WI and
hyperintensity on T2WI. Thickening of the left wall of the rectum
with patchy low attenuation and the presence of an exophytic mass
was observed in 1 patient (patient 4; Fig. 1). Local tumor invasion of the
perirectal fat spaces was observed in 4 patients. Tumor invasion of
the seminal vesicle was exhibited by 1 patient (patient 1), and
tumor invasion of the seminal vesicle and prostate gland was
exhibited by 1 patient (patient 4). Heterogeneous attenuation was
found on non-enhanced CT images in 3 lesions (patients 1, 2 and 4),
and 1 lesion exhibited heterogeneous intensity on non-enhanced MRI
(patient 3). All the lesions demonstrated strong enhancement
following contrast imaging. Lymphadenopathy was observed in 4
patients, pulmonary metastasis in 2 patients (patients 3 and 4),
liver metastasis in 3 patients (patient 2–4) and multiple bone
metastases in 1 patient (patient 3; Fig.
2).
 | Table I.CT and MRI results reviewed in 4
patients with small cell carcinoma of the rectum. |
Table I.
CT and MRI results reviewed in 4
patients with small cell carcinoma of the rectum.
|
|
|
|
|
| MRI |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Rectal wall
thickening, mm | Tumor size, mm | Local invasion | CT density | T1WI | T2WI | Lesion texture | CEC | Lymphadenopathy | Distant
metastasis |
|---|
| 1 | 18 | NA | Perirectal fat
spaces, left seminal vesicle | Slight hypo | NA | NA | Hetero | Strong | Perirectal space | NA |
| 2 | 22 | NA | Perirectal fat
spaces | Slight hypo | NA | NA | Hetero | Strong | Perirectal space,
presacral space | Liver |
| 3 | 17 | NA | Perirectal fat
spaces | NA | Iso | Hyper | Hetero | Strong | Mediastinum, hilus
pulmonis, hepatic hilar region, retroperitoneal, presacral space,
perirectal space, iliac blood vessel region | Pulmonary, liver,
bone |
| 4 | 21 | 104×110 | Perirectal spaces,
prostate fat glands, seminal vesicle | Hypo | NA | NA | Hetero, necrosis | Strong | Perirectal space,
presacral space, hilus pulmonis | Pulmonary, liver |
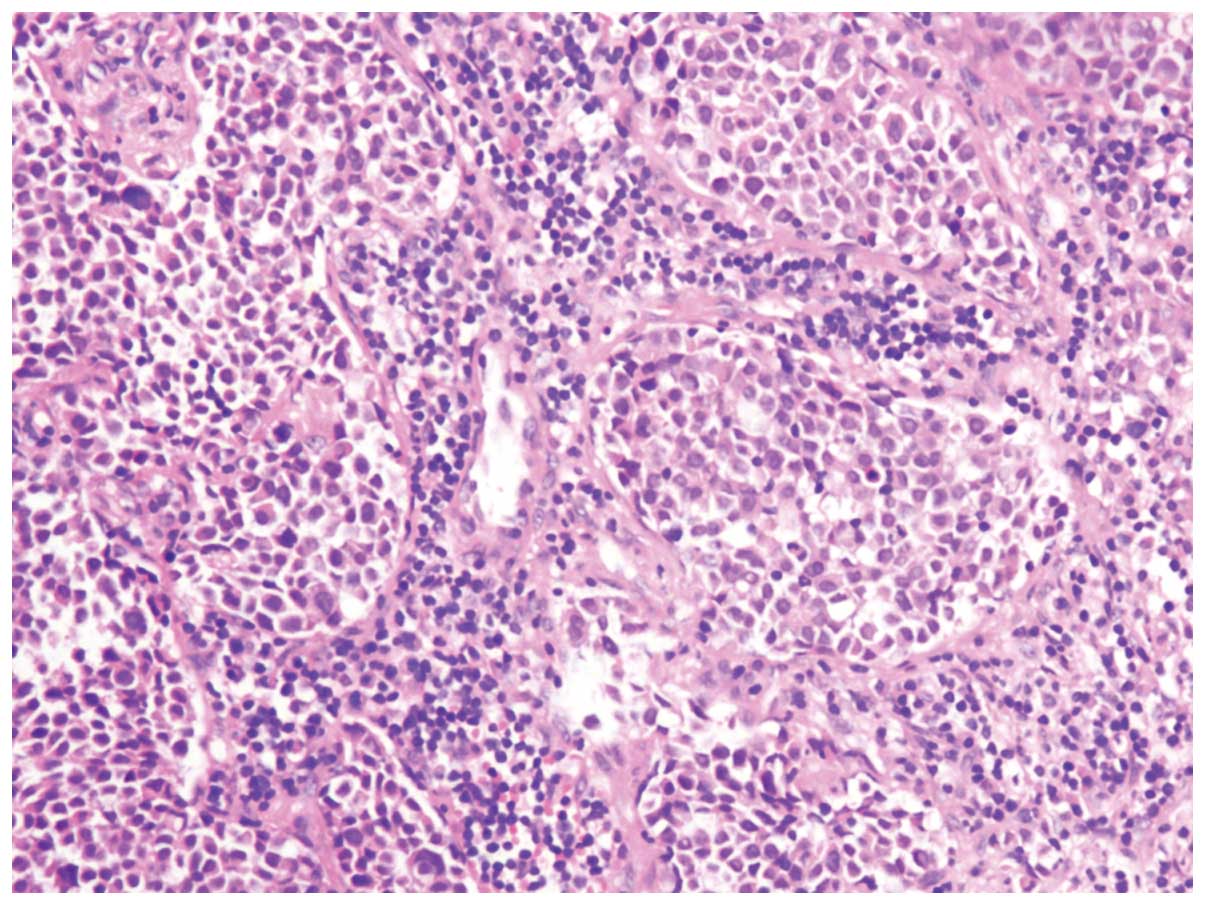
Pathological findings
All 4 patients were diagnosed with extrapulmonary
small cell carcinoma of the rectum using H&E staining (patient
2; Fig. 3) and immunohistochemistry.
Immunohistochemical analysis was performed on 3 post-operative
specimens (patients 2–4). Of the total patients, 1 was diagnosed
with a mixed cell type, consisting of small cell carcinoma and
moderately-differentiated adenocarcinoma (patient 1), observed
using H&E staining. Immunohistochemical examinations revealed
that the tumor cells expressed synaptophysin and CD56 in 3 patients
(patients 2–4), and chromogranin A and NSE in 2 patients (patients
2 and 3). None of the patients were negative for the antibodies
relevant to small carcinoma of the rectum.
Discussion
The etiology of extrapulmonary small cell carcinoma
remains unknown. Certain patients with extrapulmonary small cell
carcinoma may have an ectopic production of hormones, including
corticotropin, calcitonin, somatostatin and gastrin, which is
similar to small cell carcinoma of the lung, although clinical
manifestations resulting from hormone production are rare (7). The largest series of cases of
extrapulmonary small cell carcinoma were retrospectively reviewed
from a cancer database in Southeast England over 34 years (8). The study identified 1,600 patients with
extrapulmonary small cell carcinoma, of which 33% were
gastrointestinal in origin, followed by genitourinary (20%), head
and neck (11%) and breast (10%) origins. The majority of
gastrointestinal small cell carcinoma occurs in the esophagus
(53–71%) and the colon (13%) (1,9).
Colorectal small cell carcinoma is an extremely rare tumor, with an
incidence of <0.2% in all types of colorectal cancers (2). The present study retrospectively
reviewed the cases of patients with small cell carcinoma of the
rectum, who presented at Sun Yat-sen University Cancer Center
between January 2001 and August 2013. A total of 4 patients were
identified from the picture archiving and communication system of
the center.
In a retrospective study of 64 cases in the USA, the
most common locations identified for extrapulmonary small cell
carcinoma were the colon and rectum (10). The median age at presentation was 55
years and a slight male predominance was observed. The median
survival time ranged between 6 and 12 months for treated patients,
down to only weeks for untreated patients. A median survival time
of 5 months has also been reported for extrapulmonary small cell
carcinoma in an additional study (7).
In the present study, the median age at presentation was 51.5 years
and the patients consisted of 3 men and 1 woman. In total, 2
patients were lost to follow-up and 2 patients succumbed to the
carcinoma ~1 and 2 months after discharge from hospital,
respectively.
Histologically, extrapulmonary small cell carcinoma
is identical to pulmonary small cell carcinoma (11). In the current study, all the patients
met the histopathological criteria for the diagnosis of
extrapulmonary small cell carcinoma, namely the presence of small
round or spindle-shaped cells with intensely hyperchromatic nuclei,
scant cytoplasm and frequent mitoses (1,5).
Immunohistochemically, extrapulmonary small cell carcinoma often
expresses synaptophysin and chromogranin A (11). In the present study, the tumors of 3
patients expressed synaptophysin and the tumors of 2 patients
expressed chromogranin A and NSE.
Small cell carcinomas are hypothesized to be derived
from a pluripotent neuroendocrine stem cell (4). Ihtiyar et al (2) reported a case of tubulovillous adenoma
overlaying small cell carcinoma of the rectum. In the present
study, 1 tumor was composed of small cell carcinoma and
moderately-differentiated adenocarcinoma, suggesting that small
cell carcinoma may be derived from stem cells, which has been
previously reported in other studies (12–14).
The majority of previous studies concerning CT and
MRI results in cases of extrapulmonary small cell carcinoma of the
rectum appear to be case reports. Spiliopoulou et al
(15) reported a case in which CT
confirmed the presence of a large rectal mass extending from the
anorectal junction to the mid-rectum, with multiple enlarged
perirectal and mesorectal lymph nodes. Ihtiyar et al
(2) reported a case in which
abdominal tomography revealed the presence of a large rectal
neoplasm with multiple lesions, which were possibly metastatic, in
the right and left lobes of the liver, and massive enlargement of
the peri-aortic and peri-iliac lymph nodes. Joshua et al
(4) reported a case in which CT
demonstrated the presence of a rectal tumor originating from the
anterior wall of the rectum, extending into the peri-rectal fat and
seminal vesicles. Liver and lymph-node involvement has been
observed in 70–80% of patients in the early stages of
extrapulmonary small cell carcinoma (16). A meta-analysis by Brenner et al
(9) suggested that extrapulmonary
small cell carcinoma arising from the gastrointestinal tract
exhibits a common pattern of spread: First to the liver, followed
by metastasis to the lymph nodes and bone marrow. In the present
study, 3 patients presented with multiple liver metastases, 2
patients with multiple pulmonary metastases, 1 patient with
multiple bone metastases and 1 patient with lymph nodes metastases
in the perirectal fat spaces, inguinal region, hepatic hilar
region, retroperitoneal, mediastinum and hilus pulmonis. Therefore,
together, these results indicate that small cell carcinoma of the
rectum is more likely to metastasize to the liver, pulmonary, lymph
nodes and bone.
Due to the high incidence of distant metastases,
surgery has been recommended as the primary palliative treatment
for extrapulmonary small cell carcinoma; however, a multimodality
approach with combined chemotherapy and radiation therapy has been
advocated to improve patient survival time (5). Spiliopoulou et al (15) divided extrapulmonary small cell
carcinoma of the rectum into two major groups, limited disease or
extensive disease, based on whether the disease extent could be
covered by an acceptable radiotherapy portal. The treatment options
for extensive disease are systemic chemotherapy or supportive care.
The treatment approach for limited disease is not clear; certain
physicians suggest local treatment using surgery or radiotherapy,
while others suggest multimodality approaches or chemotherapy alone
(15). In an additional study of 81
patients with extrapulmonary small cell carcinoma, the majority of
patients presented with limited disease, and the combination of
chemotherapy and radiation therapy was observed to be as effective
as surgery (17). It has also been
suggested that local control of extrapulmonary small cell carcinoma
of the rectum may be achieved with multidrug chemotherapy and
radiation therapy, without the requirement for radical surgery
(18). Surgery should only be
performed on tumors of small dimensions, and a range of
non-surgical treatments should be administered to patients with
advanced-stage disease (19). In the
present study, 1 patient (stage IVB), who underwent an exploratory
laparotomy, sigmoidostomy and post-operative chemotherapy,
succumbed to the carcinoma ~2 months after discharge from hospital.
Another patient (stage IVB), who received no treatment, succumbed
~1 month after discharge from hospital, while the remaining 2
patients were lost to follow-up.
In conclusion, small cell carcinoma of the rectum is
most likely to metastasize to the liver, pulmonary, lymph nodes and
bone. Distinguishing features identified by CT and MRI include the
thickening of the rectal wall, the presence of soft-tissue tumors,
tumor local invasion, lymphadenopathy and distant metastasis.
Pre-operative CT and MRI are required as an aid in selecting the
correct treatment management and for the prognosis assessment of
patients.
Acknowledgements
The authors would like to thank Professor Lizhi Liu
(Medical Imaging and Minimally Invasive Interventional Center, Sun
Yat-sen University Cancer Center; State Key Laboratory of Oncology
in South China; Collaborative Innovation Center for Cancer
Medicine, Guangzhou, China) for editing the original
manuscript.
References
|
1
|
Lee SS, Ha HK, Kim AY, Kim TK, Kim PN, Yu
E, Lee MG, Myung SJ, Jung HY, Kim JH and Min YI: Primary
extrapulmonary small cell carcinoma involving the stomach or
duodenum or both: Findings on CT and barium studies. AJR Am J
Roentgenol. 180:1325–1329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ihtiyar E, Algin C, Isiksoy S and Ates E:
Small cell carcinoma of rectum: A case report. World J
Gastroenterol. 11:3156–3158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clery AP, Dockerty MB and Waugh JM:
Small-cell carcinoma of the colon and rectum. A clinicopathologic
study. Arch Surg. 83:164–172. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joshua AM, Adams D, McKenzie P, Solomon M
and Clarke SJ: Small blue cell tumors of the rectum. Case 2.
Small-cell carcinoma of the rectum. J Clin Oncol. 23:912–913. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levine MS, Pantongrag-Brown L, Buck JL,
Buetow PC, Lowry MA and Sobin LH: Small-cell carcinoma of the
esophagus: Radiographic findings. Radiology. 199:703–705. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Comprehensive Cancer Network
(NCCN): NCCN Clinical Practise Guidelines in Oncology. Rectal
Cancer. Version 2.2015. (Fort Washington, PA). NCCN. 392015.
|
|
7
|
Saclarides TJ, Szeluga D and Staren ED:
Neuroendocrine cancers of the colon and rectum. Results of a
ten-year experience. Dis Colon Rectum. 37:635–642. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong YN, Jack RH, Mak V, Henrik M and
Davies EA: The epidemiology and survival of extrapulmonary small
cell carcinoma in South East England, 1970–2004. BMC Cancer.
9:2092009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brenner B, Tang LH, Klimstra DS and Kelsen
DP: Small-cell carcinomas of the gastrointestinal tract: A review.
J Clin Oncol. 22:2730–2739. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brenner B, Shah MA, Gonen M, Klimstra DS,
Shia J and Kelsen DP: Small-cell carcinoma of the gastrointestinal
tract: A retrospective study of 64 cases. Br J Cancer.
90:1720–1726. 2004.PubMed/NCBI
|
|
11
|
Frazier SR, Kaplan PA and Loy TS: The
pathology of extrapulmonary small cell carcinoma. Semin Oncol.
34:30–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vilor M, Tsutsumi Y, Osamura RY, Tokunaga
N, Soeda J, Ohta M, Nakazaki H, Shibayama Y and Ueno F: Small cell
neuroendocrine carcinoma of the rectum. Pathol Int. 45:605–609.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mills SE, Allen MS Jr and Cohen AR:
Small-cell undifferentiated carcinoma of the colon. A
clinicopathological study of five cases and their association with
colonic adenomas. Am J Surg Pathol. 7:643–651. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Damjanov I, Amenta PS and Bosman FT:
Undifferentiated carcinoma of the colon containing exocrine,
neuroendocrine and squamous cells. Virchows Arch A Pathol Anat
Histopathol. 401:57–66. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spiliopoulou P, Panwar U and Davidson N:
Rectal small cell carcinoma: A case report and review of the
literature. Case Rep Oncol. 4:475–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sterling RK: Ectopic ACTH syndrome
associated with anorectal carcinoma. Report of a case and review of
the literature. Dig Dis Sci. 38:955–959. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galanis E, Frytak S and Lloyd RV:
Extrapulmonary small cell carcinoma. Cancer. 79:1729–1736. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robidoux A, Monté M, Heppell J and Schürch
W: Small-cell carcinoma of the rectum. Dis Colon Rectum.
28:594–596. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shirouzu K, Morodomi T, Isomoto H,
Yamauchi Y, Kakegawa T and Morimatsu M: Small-cell carcinoma of the
rectum. Clinicopathologic study. Dis Colon Rectum. 28:434–439.
1985. View Article : Google Scholar : PubMed/NCBI
|