Introduction
Breast cancer is one of the most commonly occurring
malignant tumors in women. Approximately 1.38 million new breast
cancer cases were estimated to be diagnosed in 2008 (23% of all
cancers) worldwide, making it the second most common type of cancer
(1). In China, rates of breast cancer
in 2005 were 33 cases per 100,000 individuals in urban areas and
12.23 cases per 100,000 individuals in rural areas, with an
increased prevalence in younger patients in rural areas (2). The incidence of ductal carcinoma was
reportedly constant between 1987 and 1999 (3); however, another study reported that the
occurrence of in situ ductal carcinoma of the breast had
increased dramatically since 1983 4). Due to the increase in the
prevalence of breast cancer, research has focused on the expression
of various genes and proteins that are specific to invasive ductal
breast carcinoma. Lee et al (5) identified four unique gene expression
profiles that were able to regulate tumor progression. Similarly,
Wojnar et al (6) reported that
the expression of metallothionein may have a significant role in
the proliferation of tumors.
Breast cancer is considered to be a disease that is
associated with tumor stem cells (TSCs). Due to the potential for
infinite proliferation and tumor development in vitro and
in vivo, TCSs are considered to be the origin of tumor
metastasis and recurrence. These cells may be useful markers for
tumor-free survival in patients with cancer (7,8). TSCs are
a small group of cells present in the tumor tissue that possess the
properties of stem cells. They have the ability to self-replicate
and proliferate, and are responsible for the formation, recurrence,
metastasis and drug resistance of tumors (9). The growth and metastasis of a tumor is
dependent on angiogenesis, however, the association between TSCs
and tumor angiogenesis remains to be elucidated. A previous study
reported that a cell subpopulation in breast cancer tissues that
demonstrated aldehyde dehydrogenase (ALDH) activity may possess the
properties of stem cells (10).
Ginestier et al (10)
identified ALDH1 as a marker of progenitor cells of normal breast
and breast cancer tissues (10). By
contrast, CD133, a TSC marker for various types of cancer, was
demonstrated to promote angiogenesis in CD133+ glioblastoma stem
cells (11). CD133 is a TSC marker
that has a significant role in breast, liver and colon cancer
(12–14), and is additionally a marker for the
separation and identification of TSCs (15). Wright et al (14) identified the breast cancer stem
cell properties of CD133.
The present study aimed to investigate the
association between TSC-like cells in breast cancer and tumor
angiogenesis. The results of the present study revealed that TSCs
may be associated with tumor angiogenesis.
Materials and methods
Patients
Stem-like cells from the breast tissues of 120
patients with invasive ductal breast carcinoma were collected by
surgical resection. Patient disease was diagnosed and confirmed
histologically at The First Affiliated Hospital of Zhengzhou
University (Zhengzhou, Henan, China) between January 2009 and
December 2010. No patients received radiotherapy or chemotherapy
prior to surgery. The protocol of the present study was approved by
the Zhengzhou University Ethics Committee (Zhengzhou, Henan,
China). Written informed consent was obtained from all patients
prior to their enrollment in the current study.
Diagnosis criteria and parameters
assessed
The histological classification of invasive breast
cancer was based on the Nottingham modified Bloom and Richardson
system (16). Estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor-2 (Her-2) expression was determined prior to commencement
of the present study. Antibodies and Streptavidin-Peroxidase (SP)
Immunohistochemistry kits were purchased from Fuzhou Maixin Biotech
Co., Ltd. (Fuzhou, Fujian, China), Beijing Zhongshanjinqiao
Biological Technology Co., Ltd. (Beijing, China) and Epitomics
(Burlingame, CA, USA), and tests were performed according to the
manufacturer's protocol. The results of the histological diagnosis,
classification and immunohistochemistry of all patients were
examined by two independent, experienced pathologists, who were
blinded to the clinical data.
Immunohistochemistry
The immunohistochemical detection of ALDH1 and CD133
utilized the double staining SP method (17). The Double Staining kit was obtained
from Fuzhou Maixin Biotech Co., Ltd. Briefly, tissue samples were
paraffin-embedded (Leica Biosystems, Beijing, China), cut into 4-µm
thick slices, and gradually dewaxed and hydrated; the tissue
sections were washed with xylene (twice for 10 min; Leica
Biosystems), 100% ethanol (twice for 5 min; Leica Biosystems), 95%
ethanol (2 min), 80% ethanol (2 min), 70% ethanol (2 min),
distilled water (5 min) and phosphate-buffered saline (PBS; 3 times
for 3 min; Fuzhou Maixin Biotech Co., Ltd). A high-pressure method
was used for antigen retrieval in ethylenediaminetetraacetic acid
solution (Leica Biosystems) at pH 8.0 for 15 min (18). The specimens were incubated with
rabbit anti-ALDH1 monoclonal antibody (catalog no., ab52492;
dilution, 1:150; Abcam, Cambridge, MA, USA) overnight at 4°C, and
were additionally processed using the
5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium color
system (Fuzhou Maixin Biotech Co., Ltd.)to produce dark blue
positive signals, according to the manufacturer's instructions. The
specimens were washed for 5 min in PBS 3 times, incubated with
double staining enhancement solution (Fuzhou Maixin Biotech Co.,
Ltd.) for 15 min and rabbit anti-CD133 monoclonal antibody (catalog
no., ZA-0426; ready to use; Zhongshanjinqiao Biological Technology
Co., Ltd.) at 37°C for 1 h, processed routinely and
3,3′-diaminobenzidine (DAB; Fuzhou Maixin Biotech Co., Ltd.)
staining was applied to produce red positive signals.
The detection of CD34, CD105 and vascular
endothelial growth factor (VEGF) was achieved using the single
staining SP method, high-pressure antigen retrieval and DAB
staining to produce brown positive signals. Mouse anti-CD105
monoclonal antibody (catalog no., ZM-0297; dilution, 1:100), murine
anti-CD34 monoclonal antibody (catalog no., ZM-0046; dilution;
1:100) and rabbit anti-VEGF monoclonal antibody (catalog no.,
ZA-0580; ready to use) were purchased from Beijing Zhongshanjinqiao
Biological Technology Co., Ltd.
Evaluation of immunohistochemical
results
ALDH1-positive cells exhibited cytoplasm staining
with a blue-black color, and CD133-positive cells possessed a red
cell membrane and partially red cytoplasm. The cells were divided
into the following four phenotypes at the cellular level, according
to the staining of ALDH1 and CD133: i) ALDH1+/CD133+ phenotype with
blue cytoplasm and red membrane; ii) ALDH1+/CD133- phenotype with
blue cytoplasm and non-stained membrane; iii) ALDH1-/CD133+
phenotype with non-stained cytoplasm and red membrane; and iv)
ALDH1-/CD133- phenotype with non-stained cytoplasm and
membrane.
Each specimen was classified for ALDH1+/CD133+
dominance at the tissue level by semiquantitative scoring, as
described by Currie et al (19). Briefly, tumor cells were scored
semiquantitatively according to the percentage of ALDH1+/CD133+
cells (0, 0%; 1, 1%; 2, 1–10%; 3, 10–33%; 4, 33–66%; 5, 66–100%)
and intensity of staining of ALDH1+/CD133+ cells (0, negative; 1,
weak; 2, moderate; and 3, strong). The composite scores (score
range, 0–8) of the specimens were subsequently calculated as
follows: composite score = percentage + intensity. Tumors with ALDH
and CD133 composite scores of ≥6 were defined as ALDH1+/CD133+
tumors.
Microvessels were identified by monoclonal antibody
staining, which revealed the localization of CD34 in the membrane
and cytoplasm of endothelial cells and CD105 in the membrane only
of endothelial cells. A single endothelial cell or cell cluster
stained brown was considered to be a blood vessel, and blood
vessels with a thick muscle layer or with a diameter >8 red
blood cells were excluded from the vessel count. A total of five
areas with high vascular density were localized on each slide
following visualization under a low-power lens (magnification,
×40), and the blood vessels in each area were subsequently counted
under a high-power lens (magnification, ×400) (Olympus CX31;
Olympus Corporation China, Beijing, China). The microvessel density
(MVD) was defined as the mean of the vessel count totals for the
five areas of high vascular density.
Cytoplasm-localized VEGF staining, indicated by
brown positive signals, was analyzed using the semiquantitative
Shimizu scoring system (20).
Percentage scores of 0, 1, 2 and 3 indicated the portion of
positive cells stained of 0, <1/3, 1/3–2/3 and >2/3,
respectively. Intensity scores of 0, 1, 2 and 3 indicated the
conditions of no staining, yellow staining, light brown staining
and dark brown staining, respectively. Composite scores (score
range, 0–6) were used to evaluate VEGF staining as follows: 0–2,
negative (−); 3, weak positive (+); 4–5; medium positive (++) and
6, strong positive (+++). A composite score >3 was considered to
indicate VEGF expression.
Statistical analysis
SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA) was utilized for data processing and statistical analysis.
Count data were presented as a percentage or proportion indicating
the association between the ALDH1+/CD133+ tumor phenotype and other
clinicopathological parameters, and this was analyzed using the χ2
test. Continuous variables were expressed as the mean ± standard
deviation, and statistical analysis was performed using the
independent samples t-test. Kendall rank correlation
analysis was used for analysis of the co-expression of VEGF in
ALDH1+/CD133+ phenotype tumor cells. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
All patients were women, with a mean age of
48.68±10.94 years (range, 24–72 years). The patients belonged to
the following categories, according to the Nottingham modified
Bloom and Richardson system (16):
Grade I (n=21; 17.5%); grade II (n=78; 65%); and grade III (n=21;
17.5%). A total of 60 patients (50%) were found to have lymph node
metastasis. In addition, 74 patients (61.7%) were ER+ and 46
(38.3%) were ER-, 43 patients (35.8%) were PR+ and 77 (64.2%) were
PR-, and 22 patients (18.3%) were Her-2 (0), 36 (30%) were Her-2
1+, 28 (23.3%) were Her-2 2+ and 34 (28.3%) were Her-2 3+ (Table I).
 | Table I.Correlation between ALDH1+/CD133+
stem-like cells in invasive ductal breast carcinoma and
clinicopathological features. |
Table I.
Correlation between ALDH1+/CD133+
stem-like cells in invasive ductal breast carcinoma and
clinicopathological features.
| Clinicopathological
features | n | Positive, n (%) | Negative, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.258 |
| ≤45 | 53 | 11
(20.8) | 42 (79.2) |
|
|
>45 | 67 | 20
(29.9) | 47 (70.1) |
|
| Diameter, cm |
|
|
| 0.962 |
| ≤2 | 43 | 11
(25.6) | 32 (74.4) |
|
|
>2 | 77 | 20
(26.0) | 57 (74.0) |
|
| Estrogen
receptor |
|
|
| 0.028 |
|
Positive | 74 | 14
(18.9) | 60 (81.1) |
|
|
Negative | 46 | 17
(37.0) | 29 (63.0) |
|
| Progesterone
receptor |
|
|
| 0.074 |
|
Positive | 43 |
7 (16.3) | 36 (83.7) |
|
|
Negative | 77 | 24
(31.2) | 53 (68.8) |
|
| Human epidermal
growth factor receptor-2 |
|
|
|
0.632 |
| 0 | 22 |
6 (27.3) | 16 (72.7) |
|
| 1+ | 36 |
9 (25.0) | 27 (75.0) |
|
| 2+ | 28 |
5 (17.9) | 23 (82.1) |
|
| 3+ | 34 | 11
(32.4) | 23 (67.6) |
|
| Lymphatic
metastasis |
|
|
| 0.835 |
|
Positive | 60 | 16
(26.7) | 44 (73.3) |
|
|
Negative | 60 | 15
(25.0) | 45 (75.0) |
|
| Gradea |
|
|
| 0.153 |
| I | 21 | 2
(9.5) | 19 (90.5) |
|
| II | 78 | 22
(28.2) | 56 (71.8) |
|
|
III | 21 |
7 (33.3) | 14 (66.7) |
No significant associations were
observed between ALDH1+/CD133+ tumor cells and clinicopathological
features
The 120 invasive ductal breast carcinoma tissue
specimens demonstrated differential distribution patterns of the
cells in the ALDH1+/CD133+, ALDH1+/CD133-, ALDH1-/CD133+ and
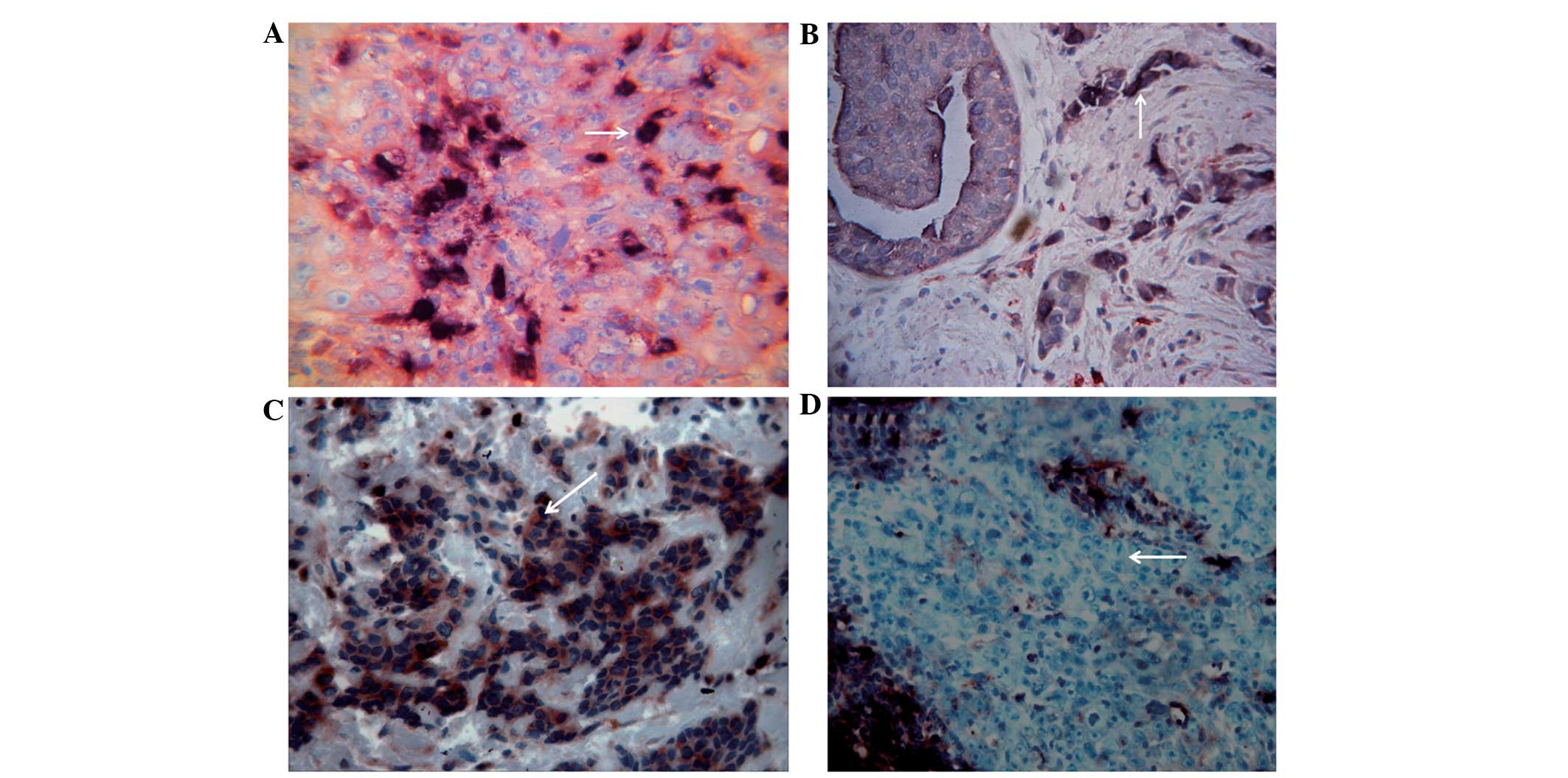
ALDH1-/CD133- phenotypes. The ALDH1+/CD133+ phenotype was
distributed in the center and towards the edge of the tumor nest
and infiltrating strands, and appeared as randomly scattered cells
or small clusters (Fig. 1A). The
ALDH1+/CD133- phenotype demonstrated a scattered distribution
(Fig. 1B), and the ALDH1-/CD133+ and
ALDH1-/CD133- phenotypes had a sheet-like or nested distribution
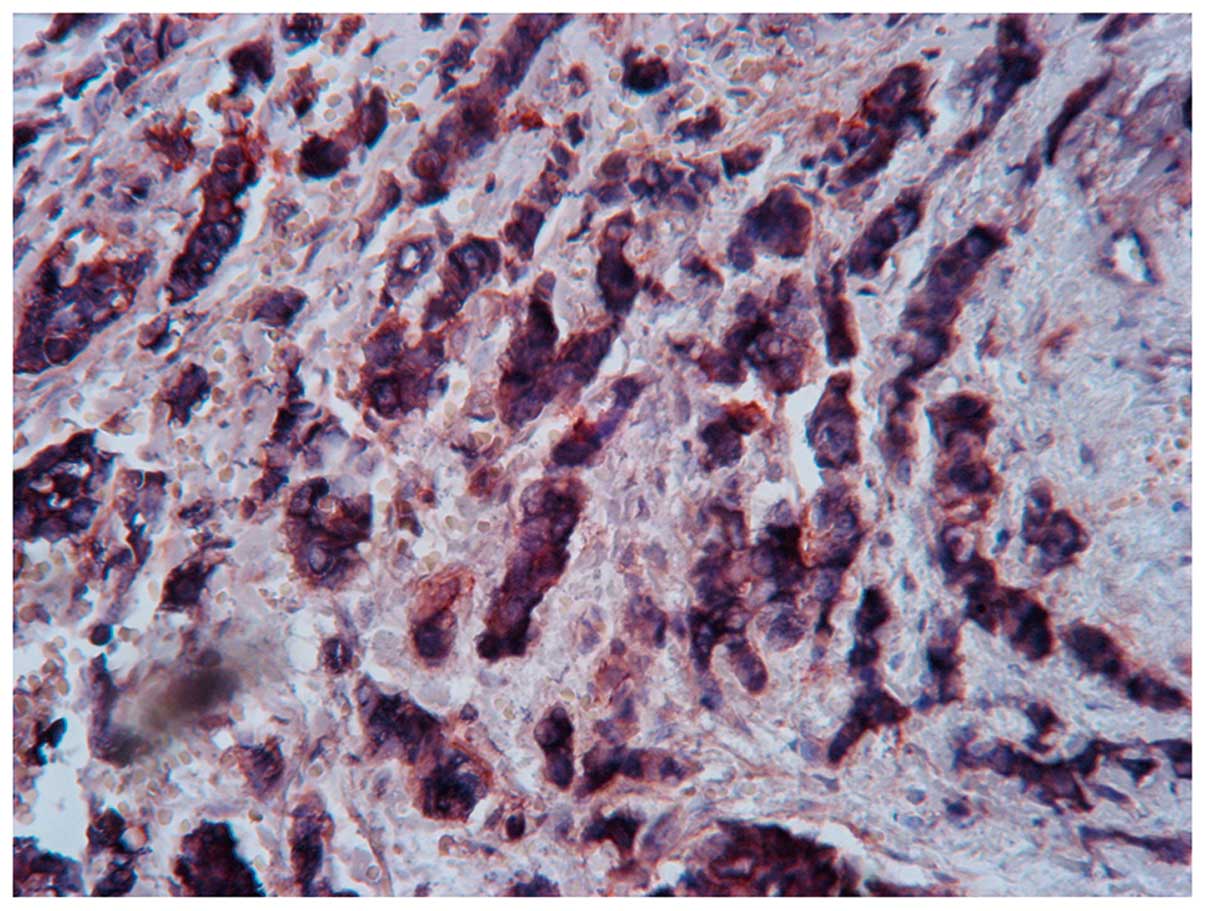
(Fig. 1C and D). ALDH1+/CD133+ tumor
cells were identified in 31 specimens (Fig. 2). A total of 43 patients (35.8%)
possessed ALDH1+/CD133+ stem-like cells with a diameter of ≤2 cm,
and 77 patients (64.2%) had cells of >2 cm diameter (Table I).
A correlation was identified between
ALDH1+/CD133 tumor cells and expression of VEGF and MVD
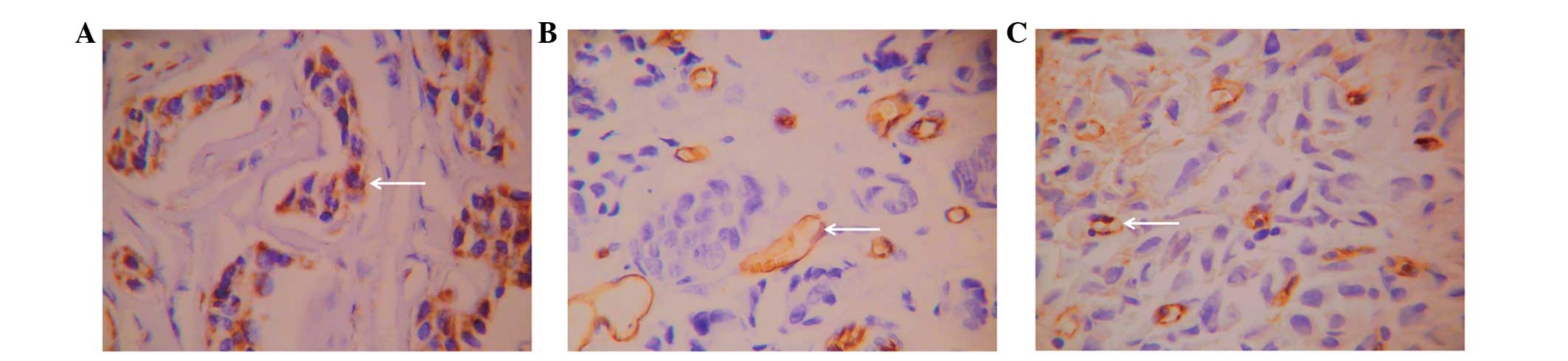
VEGF-positive cells demonstrated a sheet-like or
nested distribution (Fig. 3A) and
were observed in 68.3% (82/120) of patients. CD34 was expressed in
microvessels and large vessels in normal and tumor tissues and
demonstrated clear boundaries with other tissues (Fig. 3B). CD105 exhibited relatively high
expression in single or small clusters of endothelial cells in
microvessels, low expression in large vessels and no expression in
normal tissues (Fig. 3C). The novel
vascular endothelial cells exhibited an irregular arrangement, thin
vessel walls, an unclear lumen and clear boundaries with other
tissues. The mean microvessel density of the 120 patients with
invasive ductal breast carcinoma marked by CD34 was 26.93±9.02,
while that marked by CD105 was 17.63±8.59. Positive correlations
were noted between ALDH1+/CD133+ tumor cells and VEGF expression,
CD34-marked microvessel density and CD105-marked microvessel
density (P<0.05; Table II).
 | Table II.Correlation between VEGF expression
and vessel-associated factors in ALDH1+/CD133+ stem-like cells in
invasive ductal breast carcinoma. |
Table II.
Correlation between VEGF expression
and vessel-associated factors in ALDH1+/CD133+ stem-like cells in
invasive ductal breast carcinoma.
| Vessel-associated
factors | Positive
(n=31) | Negative
(n=89) | P-value |
|---|
| Positive percentage
of VEGF, n (%) | 26
(83.9) | 56
(62.9)c |
<0.05 (χ2 test) |
| MVD
(CD34)a, mean ± SD |
28.78±10.55 | 25.90±8.34 | <0.05 (t
test) |
| MVD
(CD105)b, mean ± SD | 21.29±9.06 | 16.35±8.10 | <0.01 (t
test) |
VEGF and ALDH1+/CD133+ cancer cell
phenotypes are co-expressed
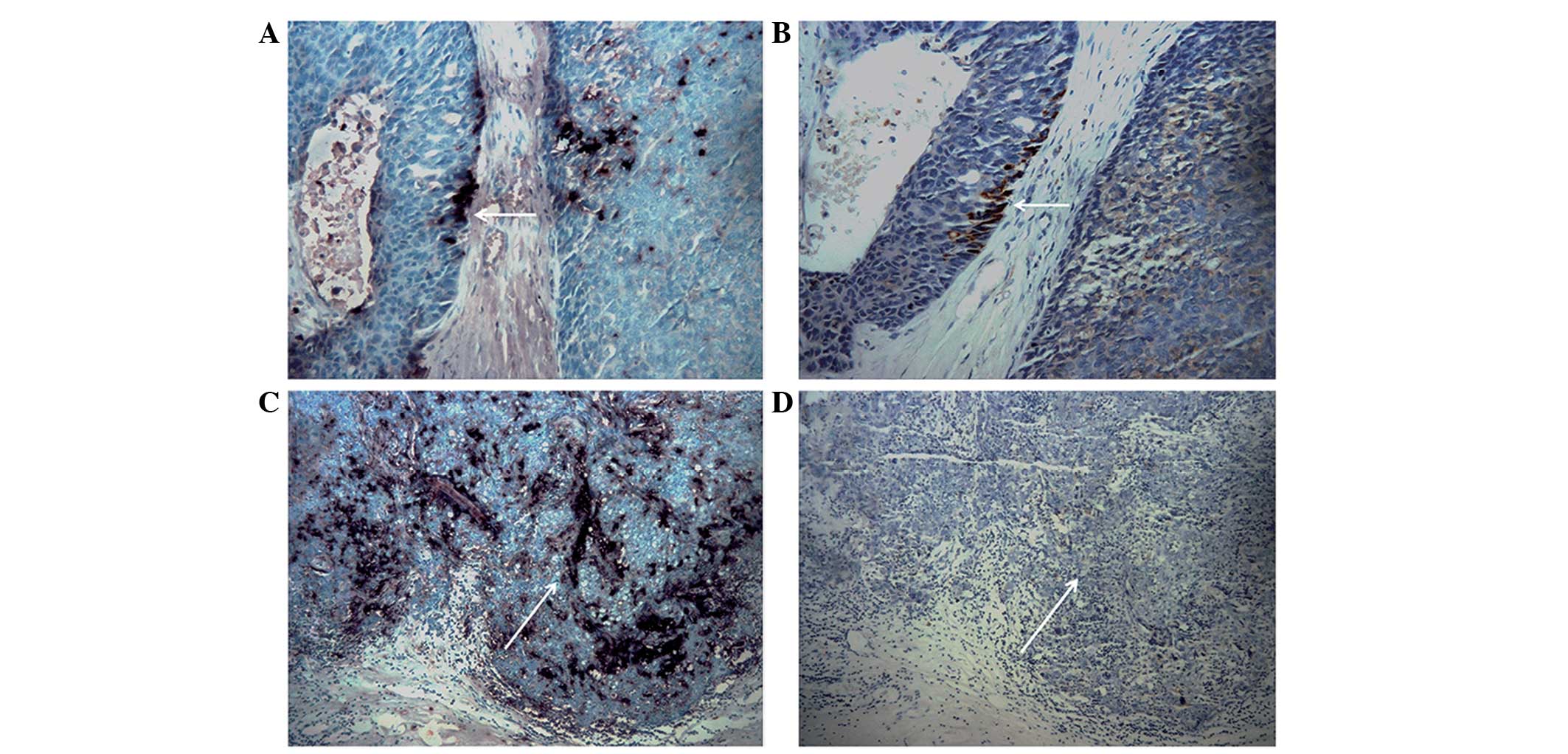
By comparing two consecutive sections of the same
tissue, 16 specimens of invasive breast cancer with an invasive
ALDH1+/CD133+ phenotype were revealed to exhibit VEGF expression,
accounting for 51.6% (16/31) of the tumors with ALDH1+/CD133+ cells
(Fig. 4). Statistical analysis
suggested a correlation between the ALDH1+/CD133+ tumor phenotype
and co-expression of VEGF (P=0.020; Table III).
 | Table III.Expression of vascular endothelial
growth factor in ALDH1+/CD133+ phenotype tumor cells. |
Table III.
Expression of vascular endothelial
growth factor in ALDH1+/CD133+ phenotype tumor cells.
| ALDH1+/CD133+
tumor | Positive (n=82), n
(%) | Negative (n=38), n
(%) | P-value |
|---|
| Positive
(n=31) | 16 (51.6) | 15 (48.4) | 0.020 |
| Negative
(n=89) | 66 (74.2) | 23 (25.8) |
|
Discussion
The present study investigated the correlation
between TSC-like cells in breast cancer and tumor angiogenesis, and
the results revealed that TSCs may be associated with tumor
angiogenesis. The growth of tumor cells requires an adequate supply
of oxygen and nutrients (21), and
rapid growth leads to the development of hypoxia in the interior of
tumors (22). Tumor angiogenesis is
one of the compensating mechanisms that provide increased oxygen
and nutrients to tumors (23). TSCs
have been reported to have a significant role in the development of
various types of cancer (24,25). As a marker for breast cancer stem
cells, cell sorting based on ALDH1 is easier and more efficient
compared with cell sorting based on CD44+/CD24-/low, offering a
novel target and strategic direction for future studies
investigating breast cancer stem cells (10). The expression of CD133 has been
observed to increase during hypoxia (26). CD133+ glioma stem-like cells (GSCs)
exhibit enhanced secretion of VEGF under hypoxic conditions, and
these cells secrete increased levels of VEGF compared with CD133-
GSCs under hypoxic and normal conditions (27). Previous studies have suggested that
hypoxia may assist with maintenance of the phenotype of TSCs and
may promote self-renewal of TSCs; however, hypoxia may additionally
inhibit TSC differentiation into mature tumor cells (28,29). Ping
et al (30) demonstrated that
CD133+ GSCs were located close to capillaries and induced
production of VEGF via activation of the phosphoinositide 3-kinase
(PI3K)/Akt signaling pathway. The inhibition of PI3K/Akt or
extracellular signal-regulated kinase 1/2 signaling reduced
hypoxia-induced CD133 expression, indicating the significance of
the aforementioned signaling pathways in the stem cell response to
hypoxia (29). A total of 11
ALDH+/CD133+ ovarian cancer stem cells were required to form tumors
in mice, which is markedly reduced compared with the 1,000
ALDH+/CD133- ovarian cancer stem cells required to generate a
tumor, which indicates the increased tumorigenic capacity of
ALDH+/CD133+ ovarian cancer stem cells (31). The correlation between ALDH1-positive
cells and angiogenesis remains to be elucidated.
In order to investigate the correlation between
TSC-like cells in invasive ductal breast carcinoma and tumor
angiogenesis, the expression of VEGF was evaluated in various
phenotypes of stem-like cells. The ALDH1+/CD133+ tumor phenotype
and co-expression of VEGF were found to be correlated in the
present study. CD34 and CD105 were selected as markers of tumor
vessels in the present study. CD34 is a pan-endothelial marker, and
its antibody is able to bind to vascular endothelial cells;
however, it lacks the specificity to recognize tumor vascular
endothelial cells (32). CD105 is a
marker for neovascular endothelial cells that is extensively
expressed in proliferating endothelial cells and demonstrates no or
reduced expression in normal vascular endothelial cells, making it
useful for tumor diagnosis, treatment and prognosis predictions
(33). A previous study reported that
CD105 possessed increased specificity compared with CD34 as a
marker for neovascular tumors (34).
The results of the present study revealed that ALDH1+/CD133+
stem-like cells were positively correlated with CD34 MVD and CD105
MVD.
The small sample size was one of the key limitations
of the present study, with regard to the high prevalence of
invasive ductal breast carcinoma worldwide. Therefore, the results
of the present study require validation via studies with a larger
patient cohort.
In conclusion, the present study revealed a
correlation between breast cancer stem cells and tumor
angiogenesis. However, the mechanisms involved have yet to be fully
elucidated. The present results may provide a novel target and
strategy for future studies investigating tumor growth and
metastasis.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no., 81172179; Beijing,
China).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer
in: 2008 GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Curado MP: Breast cancer in the world:
Incidence and mortality. Salud Publica Mex. 53:372–384.
2011.PubMed/NCBI
|
|
3
|
Li CI, Anderson BO, Daling JR and Moe RE:
Trends in incidence rates of invasive lobular and ductal breast
carcinoma. JAMA. 289:1421–1424. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ernster VL, Barclay J, Kerlikowske K,
Grady D and Henderson C: Incidence of and treatment for ductal
carcinoma in situ of the breast. JAMA. 275:913–918. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee S, Stewart S, Nagtegaal I, Luo J, Wu
Y, Colditz G, Medina D and Allred DC: Differentially expressed
genes regulating the progression of ductal carcinoma in situ to
invasive breast cancer. Cancer Res. 72:4574–4586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wojnar A, Pula B, Piotrowska A, Jethon A,
Kujawa K, Kobierzycki C, Rys J, Podhorska-Okolow M and Dziegiel P:
Correlation of intensity of MT-I/II expression with Ki-67 and MCM-2
proteins in invasive ductal breast carcinoma. Anticancer Res.
31:3027–3033. 2011.PubMed/NCBI
|
|
7
|
Nanashima A, Hatachi G, Tsuchiya T,
Matsumoto H, Arai J, Abo T, Murakami G, Tominaga T, Takagi K and
Nagayasu T: Clinical significances of cancer stem cells markers in
patients with intrahepatic cholangiocarcinoma who underwent
hepatectomy. Anticancer Res. 33:2107–2114. 2013.PubMed/NCBI
|
|
8
|
Madjd Z, Ramezani B, Molanae S and
Asadi-Lari M: High expression of stem cell marker ALDH1 is
associated with reduced BRCA1 in invasive breast carcinomas. Asian
Pac J Cancer Prev. 13:2973–2978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gil J, Stembalska A, Pesz KA and Sasiadek
MM: Cancer stem cells: The theory and perspectives in cancer
therapy. J Appl Genet. 49:193–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau
CK, Li ML, Tam KH, Lam CT, Poon RT and Fan ST: Identification of
local and circulating cancer stem cells in human liver cancer.
Hepatology. 47:919–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell
characteristics. Breast Cancer Res. 10:R102008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Y and Wu PY: CD133 as a marker for
cancer stem cells: Progresses and concerns. Stem Cells Dev.
18:1127–1134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frierson HF Jr, Wolber RA, Berean KW,
Franquemont DW, Gaffey MJ, Boyd JC and Wilbur DC: Interobserver
reproducibility of the Nottingham modification of the Bloom and
Richardson histologic grading scheme for infiltrating ductal
carcinoma. Am J Clin Pathol. 103:195–198. 1995.PubMed/NCBI
|
|
17
|
Mylona E, Giannopoulou I, Fasomytakis E,
Nomikos A, Magkou C, Bakarakos P and Nakopoulou L: The
clinicopathologic and prognostic significance of CD44+/CD24(−/low)
and CD44-/CD24+ tumor cells in invasive breast carcinomas. Human
Pathol. 39:1096–1102. 2008. View Article : Google Scholar
|
|
18
|
Jasani B and Rhodes A: The role and
mechanism of high-temperature antigen retrieval in diagnostic
pathology. Curr Diag Pathol. 7:153–160. 2001. View Article : Google Scholar
|
|
19
|
Currie MJ, Beardsley BE, Harris GC,
Gunningham SP, Dachs GU, Dijkstra B, Morrin HR, Wells JE and
Robinson BA: Immunohistochemical analysis of cancer stem cell
markers in invasive breast carcinoma and associated ductal
carcinoma in situ: Relationships with markers of tumor hypoxia and
microvascularity. Human Pathol. 44:402–411. 2013. View Article : Google Scholar
|
|
20
|
Shimizu M, Saitoh Y and Itoh H:
Immunohistochemical staining of Ha-ras oncogene product in normal,
benign, and malignant human pancreatic tissues. Hum Pathol.
21:607–612. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: Role of reoxygenation, free radicals, and stress granules.
Cancer cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horbinski C, Mojesky C and Kyprianou N:
Live free or die: Tales of homeless (cells) in cancer. Am J Pathol.
177:1044–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duffy JP, Eibl G, Reber HA and Hines OJ:
Influence of hypoxia and neoangiogenesis on the growth of
pancreatic cancer. Mol Cancer. 2:122003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei B, Han XY, Qi CL, Zhang S, Zheng ZH,
Huang Y, Chen TF and Wei HB: Coaction of spheroid-derived stem-like
cells and endothelial progenitor cells promotes development of
colon cancer. PloS One. 7:e390692012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin TA and Jiang WG: Evaluation of the
expression of stem cell markers in human breast cancer reveals a
correlation with clinical progression and metastatic disease in
ductal carcinoma. Oncol Rep. 31:262–272. 2014.PubMed/NCBI
|
|
26
|
Platet N, Liu SY, Atifi ME, Oliver L,
Vallette FM, Berger F and Wion D: Influence of oxygen tension on
CD133 phenotype in human glioma cell cultures. Cancer Lett.
258:286–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li
Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD and Rich JN: Stem
cell-like glioma cells promote tumor angiogenesis through vascular
endothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Panchision DM: The role of oxygen in
regulating neural stem cells in development and disease. J Cell
Physiol. 220:562–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soeda A, Park M, Lee D, Mintz A,
Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T,
Kassam AB, et al: Hypoxia promotes expansion of the CD133-positive
glioma stem cells through activation of HIF-1α. Oncogene.
28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC,
Jiang T, Lin MC, Chen JH, Wang B, Zhang R, et al: The chemokine
CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated
VEGF production and tumour angiogenesis via PI3K/AKT signalling. J
Pathol. 224:344–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Silva IA, Bai S, McLean K, Yang K,
Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds
RK, et al: Aldehyde dehydrogenase in combination with CD133 defines
angiogenic ovarian cancer stem cells that portend poor patient
survival. Cancer Res. 71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fanelli M, Locopo N, Gattuso D and
Gasparini G: Assessment of tumor vascularization:
Immunohistochemical and non-invasive methods. Int J Biol Markers.
14:218–231. 1999.PubMed/NCBI
|
|
33
|
Pufe T, Harde V, Petersen W, Goldring MB,
Tillmann B and Mentlein R: Vascular endothelial growth factor
(VEGF) induces matrix metalloproteinase expression in immortalized
chondrocytes. J Pathol. 202:367–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takase Y, Kai K, Masuda M, Akashi M and
Tokunaga O: Endoglin (CD105) expression and angiogenesis status in
small cell lung cancer. Pathol Res Pract. 206:725–730. 2010.
View Article : Google Scholar : PubMed/NCBI
|