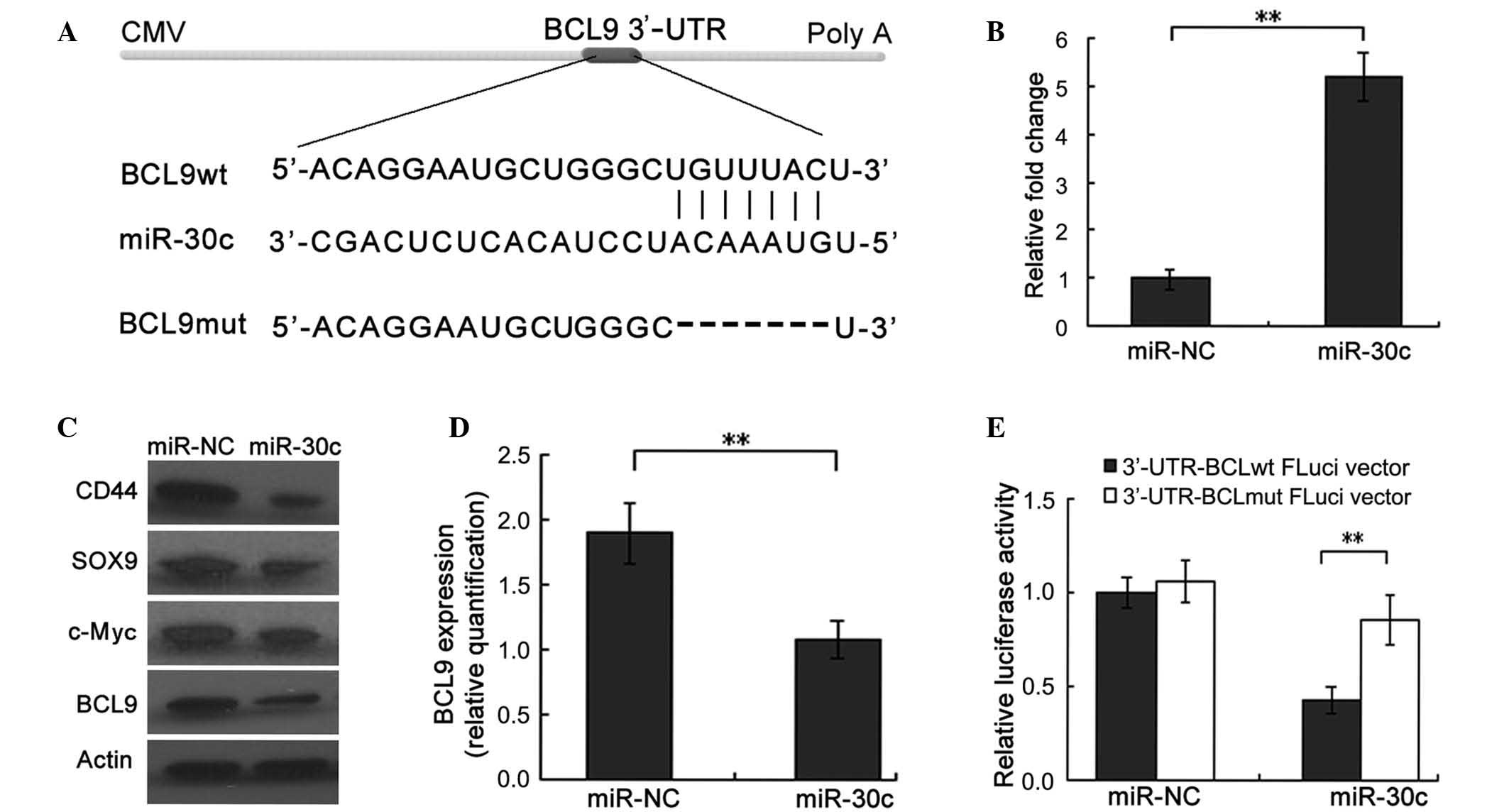
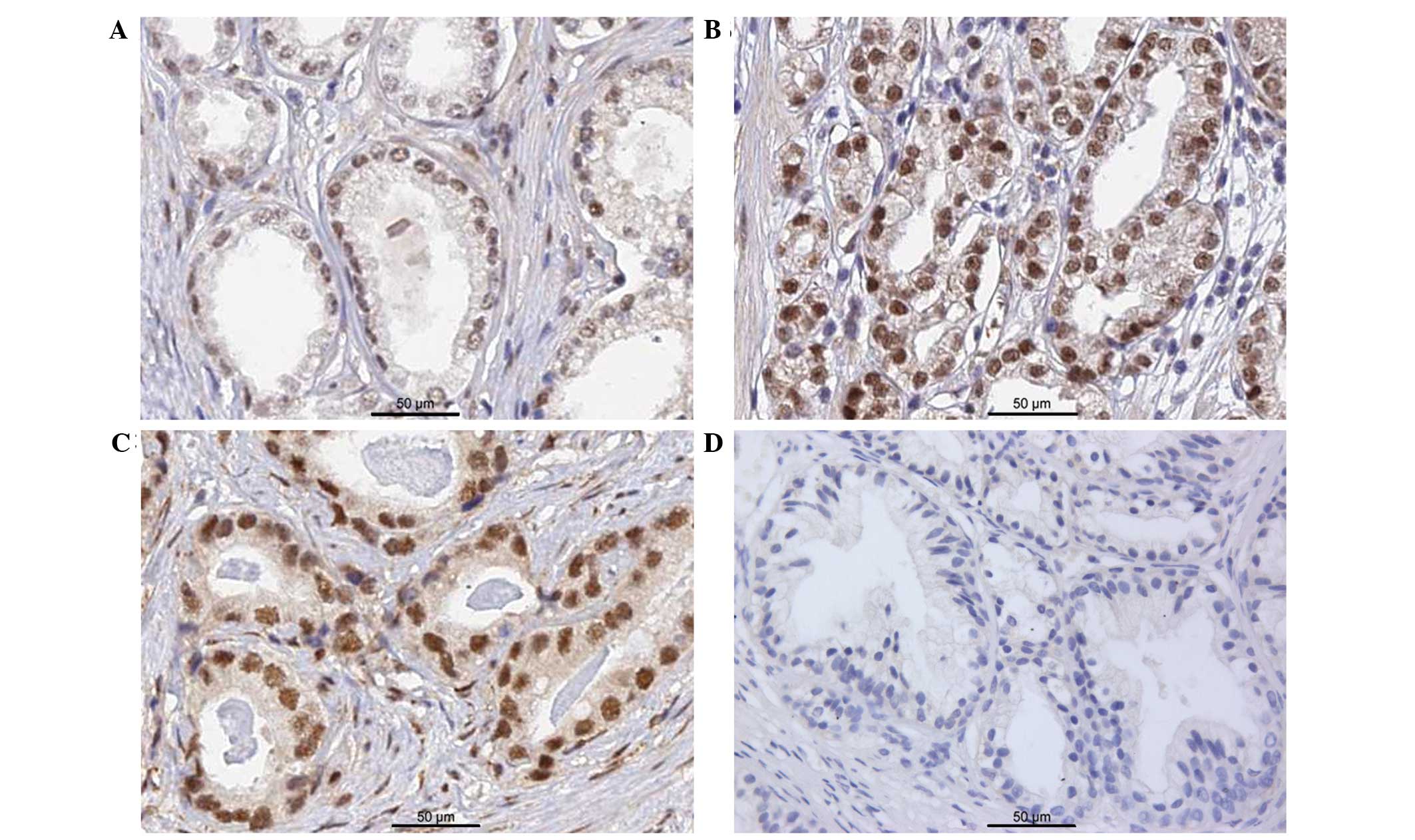
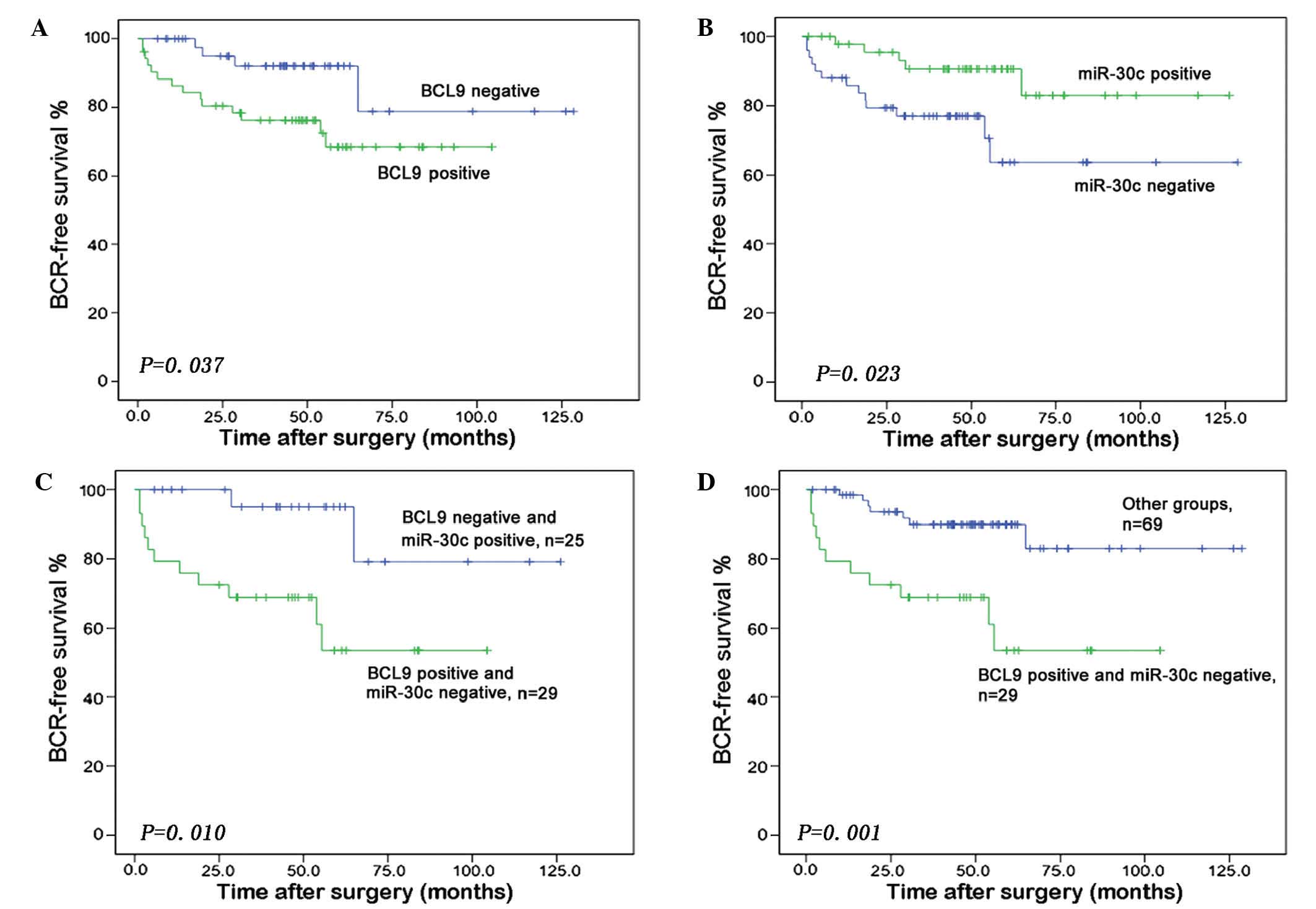
|
1
|
Kakehi Y: Watchful waiting as a treatment
option for localized prostate cancer in the PSA era. Jpn J Clin
Oncol. 33:1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heng HH, Bremer SW, Stevens JB, Ye KJ, Liu
G and Ye CJ: Genetic and epigenetic heterogeneity in cancer: A
genome-centric perspective. J Cell Physiol. 220:538–547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Q, Song R, Ortogero N, Zheng H, Evanoff
R, Small CL, Griswold MD, Namekawa SH, Royo H, Turner JM, et al:
The RNase III enzyme DROSHA is essential for microRNA production
and spermatogenesis. J Biol Chem. 287:25173–25190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plaisier CL, Pan M and Baliga NS: A
miRNA-regulatory network explains how dysregulated miRNAs perturb
oncogenic processes across diverse cancers. Genome Res.
22:2302–2314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kent OA and Mendell JT: A small piece in
the cancer puzzle: microRNAs as tumor suppressors and oncogenes.
Oncogene. 25:6188–6196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He HC, Han ZD, Dai QS, Ling XH, Fu X, Lin
ZY, Deng YH, Qin GQ, Cai C, Chen JH, et al: Global analysis of the
differentially expressed miRNAs of prostate cancer in Chinese
patients. BMC Genomics. 14:7572013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rane JK, Scaravilli M, Ylipää A, Pellacani
D, Mann VM, Simms MS, Nykter M, Collins AT, Visakorpi T and
Maitland NJ: MicroRNA Expression Profile of Primary Prostate Cancer
Stem Cells as a Source of Biomarkers and Therapeutic Targets. Eur
Urol. 67:7–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ling XH, Han ZD, Xia D, He HC, Jiang FN,
Lin ZY, Fu X, Deng YH, Dai QS, Cai C, et al: MicroRNA-30c serves as
an independent biochemical recurrence predictor and potential tumor
suppressor for prostate cancer. Mol Biol Rep. 41:2779–2788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu F, Deng H, Yao H, Liu Q, Su F and Song
E: Mir-30 reduction maintains self-renewal and inhibits apoptosis
in breast tumor-initiating cells. Oncogene. 29:4194–4204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez I, Cazalla D, Almstead LL, Steitz
JA and DiMaio D: miR-29 and miR-30 regulate B-Myb expression during
cellular senescence. Proc Natl Acad Sci USA. 108:522–527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong Z, Xia Y, Wang P, Liu B and Chen Y:
Low expression of microRNA-30c promotes invasion by inducing
epithelial mesenchymal transition in non-small cell lung cancer.
Mol Med Rep. 10:2575–2579. 2014.PubMed/NCBI
|
|
12
|
Yun SI, Kim HH, Yoon JH, Park WS, Hahn MJ,
Kim HC, Chung CH and Kim KK: Ubiquitin specific protease 4
positively regulates the WNT/β-catenin signaling in colorectal
cancer. Mol Oncol. 9:1834–1851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carotenuto M, De Antonellis P, Liguori L,
Benvenuto G, Magliulo D, Alonzi A, Turino C, Attanasio C, Damiani
V, Bello AM, et al: H-Prune through GSK-3β interaction sustains
canonical WNT/β-catenin signaling enhancing cancer progression in
NSCLC. Oncotarget. 5:5736–5749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kramps T, Peter O, Brunner E, Nellen D,
Froesch B, Chatterjee S, Murone M, Züllig S and Basler K:
Wnt/wingless signaling requires BCL9/legless-mediated recruitment
of pygopus to the nuclear beta-catenin-TCF complex. Cell.
109:47–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Townsley FM, Cliffe A and Bienz M: Pygopus
and Legless target Armadillo/beta-catenin to the nucleus to enable
its transcriptional co-activator function. Nat Cell Biol.
6:626–633. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willis TG, Zalcberg IR, Coignet LJ,
Wlodarska I, Stul M, Jadayel DM, Bastard C, Treleaven JG, Catovsky
D, Silva ML, et al: Molecular cloning of translocation
t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21.
Blood. 91:1873–1881. 1998.PubMed/NCBI
|
|
17
|
Mani M, Carrasco DE, Zhang Y, Takada K,
Gatt ME, Dutta-Simmons J, Ikeda H, Diaz-Griffero F, Pena-Cruz V,
Bertagnolli M, et al: BCL9 promotes tumor progression by conferring
enhanced proliferative, metastatic, and angiogenic properties to
cancer cells. Cancer Res. 69:7577–7586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deka J, Wiedemann N, Anderle P,
Murphy-Seiler F, Bultinck J, Eyckerman S, Stehle JC, André S,
Vilain N, Zilian O, et al: Bcl9/Bcl9l are critical for Wnt-mediated
regulation of stem cell traits in colon epithelium and
adenocarcinomas. Cancer Res. 70:6619–6628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao JJ, Lin J, Zhu D, Wang X, Brooks D,
Chen M, Chu ZB, Takada K, Ciccarelli B, Admin S, et al: miR-30-5p
functions as a tumor suppressor and novel therapeutic tool by
targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res.
74:1801–1813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia W, Eneh JO, Ratnaparkhe S, Altman MK
and Murph MM: MicroRNA-30c-2* expressed in ovarian cancer cells
suppresses growth factor-induced cellular proliferation and
downregulates the oncogene BCL9. Mol Cancer Res. 9:1732–1745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Health Organization. HO (2010)
International statistical classification of diseases and related
health problems. 10th revision. 2:2010.http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdfAccessed.
August 10–2012
|
|
22
|
International Union Against Cancer (UICC):
Urological Tumors, Prostate. TNM Classification of Malignant
Tumours (6th). Sobin LH and Wittekind Ch: (New York, NY).
Wiley-Liss. 184–187. 2002.
|
|
23
|
Montironi R, Mazzuccheli R, Scarpelli M,
Lopez-Beltran A, Fellegara G and Algaba F: Gleason grading of
prostate cancer in needle biopsies or radical prostatectomy
specimens: Contemporary approach, current clinical significance and
sources of pathology discrepancies. BJU Int. 95:1146–1152. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Y, Campisi J, Higano C, Beer TM,
Porter P, Coleman I, True L and Nelson PS: Treatment-induced damage
to the tumor microenvironment promotes prostate cancer therapy
resistance through WNT16B. Nat Med. 18:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Femia AP, Dolara P, Giannini A, Salvadori
M, Biggeri A and Caderni G: Frequent mutation of Apc gene in rat
colon tumors and mucin-depleted foci, preneoplastic lesions in
experimental colon carcinogenesis. Cancer Res. 67:445–449. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yardy GW, Bicknell DC, Wilding JL,
Bartlett S, Liu Y, Winney B, Turner GD, Brewster SF and Bodmer WF:
Mutations in the AXIN1 gene in advanced prostate cancer. Eur Urol.
56:486–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de la Roche M, Worm J and Bienz M: The
function of BCL9 in Wnt/beta-catenin signaling and colorectal
cancer cells. BMC Cancer. 8:1992008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adachi S, Jigami T, Yasui T, Nakano T,
Ohwada S, Omori Y, Sugano S, Ohkawara B, Shibuya H, Nakamura T, et
al: Role of a BCL9-related beta-catenin-binding protein, B9L, in
tumorigenesis induced by aberrant activation of Wnt signaling.
Cancer Res. 64:8496–8501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mieszczanek J, de la Roche M and Bienz M:
A role of Pygopus as an anti-repressor in facilitating
Wnt-dependent transcription. Proc Natl Acad Sci USA.
105:19324–19329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hyeon J, Ahn S, Lee JJ, Song DH and Park
CK: Prognostic Significance of BCL9 Expression in Hepatocellular
Carcinoma. Korean J Pathol. 47:130–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong K, Chen K, Han L and Li B:
MicroRNA-30b/c inhibits non-small cell lung cancer cell
proliferation by targeting Rab18. BMC Cancer. 14:7032014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bockhorn J, Yee K, Chang YF, Prat A, Huo
D, Nwachukwu C, Dalton R, Huang S, Swanson KE, Perou CM, et al:
MicroRNA-30c targets cytoskeleton genes involved in breast cancer
cell invasion. Breast Cancer Res Treat. 137:373–382. 2013.
View Article : Google Scholar : PubMed/NCBI
|