Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies in humans; worldwide, the incidence of HCC is
increasing, and HCC is the third leading cause of cancer-associated
mortality (1). There has been
considerable investigation concerning the molecular mechanism
underlying HCC, and accumulating evidence has demonstrated that
deregulating oncogenes and tumor suppressor genes are key to the
development and progression of HCC (2). Therefore, the development of effective
molecular targets and agents is urgently required for the treatment
of HCC.
It has been well-established that the protein kinase
B (AKT) signaling pathway is key in various types of human cancer,
including HCC (3,4). An inhibition of AKT signaling
effectively suppresses HCC growth (5,6).
Phosphatase and tensin homolog (PTEN) encodes a major lipid
phosphatase, which signals through the AKT pathway (7). PTEN acts as an inhibitor of AKT
signaling, and is frequently downregulated or mutated in human
cancers (7,8). Therefore, upregulation of PTEN activity
leads to a reduction in AKT pathway activity, and an inhibition of
PTEN may result in the activation of AKT signaling (9).
Phosphatidylinositol 3,4,5-trisphosphate Rac
exchanger 2 (PREX2) is a 183 kDa protein that activates the small
guanosine triphosphatase Rac, and is capable of mediating Rac
signaling downstream of G protein-coupled receptors and AKT
(10). A previous study demonstrated
that PREX2 regulates Purkinje cell dendrite morphology and motor
coordination (11), and it has been
reported to inhibit the activity of PTEN by binding directly to
PTEN via the guanine nucleotide exchange factor domain of PTEN
(12). Furthermore, PREX2 may work in
combination with a phosphatidylinositol-4,5-bisphosphate 3-kinase
(PIP3), catalytic subunit alpha mutant to promote proliferation and
transformation of cells, which is growth-factor independent
(12). Knockout of PREX2 suppresses
the activity of AKT and inhibits cell growth with intact PTEN
(12). Previously, PREX2 has been
suggested to play a role in several human cancers, including
melanoma, gastric cancer and neuroblastoma (13–15). In
addition, chemokine ligand 9 was identified to promote HCC cell
invasion through the upregulation of PREX2, suggesting that PREX2
is involved in the regulation of HCC cell invasion (16). However, the role of PREX2 in the
regulation of HCC cell proliferation and migration remains
unclear.
The present study aimed to investigate the exact
role of PREX2 in the regulation of HCC cell proliferation and
migration, as well as the underlying molecular mechanism.
Materials and methods
Reagents
Dulbecco's minimum essential medium (DMEM),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
fetal bovine serum (FBS), Lipofectamine® 2000 and Trizol reagent
were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). RevertAid First Strand cDNA Synthesis kit was purchased from
Fermentas (Thermo Fisher Scientific, Inc.). iQ™ SYBR® Green
Supermix was purchased from Bio-Rad Laboratories, Inc. (Hercules,
CA, USA). The following primary rabbit anti-human antibodies were
used: PREX2 polyclonal (catalog no., ab121462), phosphorylated
(p)-PTEN monoclonal (catalog no., ab109454), PTEN monoclonal
(catalog no., ab32199), p-AKT monoclonal (catalog no., ab81283),
AKT monoclonal (catalog no., ab32505) and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal
(catalog no., ab128915) antibodies. All antibodies, including mouse
anti-rabbit secondary polyclonal immunoglobulin G horseradish
peroxidase antibody (catalog no., ab6728), were purchased from
Abcam (Cambridge, MA, USA). An enhanced chemiluminescence (ECL) kit
was purchased from Pierce Biotechnology, Inc. (Rockford, IL, USA).
PREX2 small interfering (si) RNA was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). PREX2 plasmid was purchased
from DNASU Plasmid Repository (Tempe, AZ, USA).
Tissue specimens
The current study was approved by the Ethics
Committee of Yuhuangding Hospital (Yantai, Shandong, China). A
total of 15 primary HCC tissues and matched non-tumorous adjacent
specimens were collected from 15 patients undergoing liver
resection at the Department of Hepatobiliary Surgery at Yuhuangding
Hospital. Written informed consent was obtained from each patient.
The histomorphology of all the samples were confirmed by the
Department of Pathology at Yuhuangding Hospital. HCC tissues were
immediately snap-frozen in liquid nitrogen following surgical
removal.
Cell culture
Human HCC HepG2, LH86, LMH and PLHC-1 cell lines,
and normal human liver THLE-3 cell line were obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in DMEM with 10% FBS at 37°C in a humidified
incubator containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the tissue samples
using Trizol reagent, according to the manufacturer's protocol. For
mRNA detection, RevertAid First Strand cDNA Synthesis kit was used
to convert RNA into complementary DNA, according to the
manufacturer's protocol. RT-qPCR was performed using iQ SYBR Green
Supermix and an Applied Biosystems 7500 thermocycler (Thermo Fisher
Scientific, Inc.). The specific primer pairs were as follows: PREX2
sense, 5′-GGACTCCACGGAAACACAGTG-3′ and anti-sense,
5′-GTGAGACTGCCATTCCTCTAAAA-3′; GAPDH, as a reference, sense,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and anti-sense,
5′-GCCATCACGCCACAGTTTC-3′. Independent experiments were repeated
three times. The relative expression levels of mRNA were analyzed
by use of the 2−∆∆Cq method.
Transfection of HCC cells
Lipofectamine 2000 was used to perform cell
transfection, according to the manufacturer's protocol. For PREX2
functional analysis, HCC cells were transfected with PREX2 siRNA or
pcDNA3.1-PREX2 plasmid.
Western blot analysis
Tissues and cell lines were solubilized in cold RIPA
lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). The proteins were separated using 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene difluoride membrane (Thermo Fisher Scientific Inc.).
The polyvinylidene difluoride membrane was incubated with phosphate
buffered saline containing 5% milk overnight at 4°C. Subsequently,
the polyvinylidene difluoride membrane was incubated with
Tris-buffered saline and Tween 20 containing 5% milk at room
temperature for 3 h, and incubated with rabbit anti-human PREX2
polyclonal (dilution, 1:200) and rabbit anti-human p-PTEN
(dilution, 1:500), PTEN (dilution, 1:100), p-AKT, (dilution,
1:100), AKT (dilution, 1:200) and GAPDH (dilution, 1:100)
monoclonal antibodies at room temperature for 3 h. Following the
incubation, the membrane was incubated again with rabbit anti-mouse
secondary antibody (dilution, 1:10,000) at room temperature for 1
h. An ECL kit was used to perform chemiluminescence detection. The
relative protein expression was analyzed by Image-Pro® Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA),
represented as the density ratio vs. GAPDH.
Cell proliferation assay
MTT assay was used to measure cell proliferation.
The cells were cultured in a 96-well plate (10,000 cells/well),
each well had 100 µl fresh serum-free medium with 0.5 g/l MTT.
Following an incubation at 37°C for 0, 24, 48 and 72 h, the medium
was removed by aspiration and 50 µl dimethyl sulfoxide
(Sigma-Aldrich, St. Louis, MO, USA) was added to each well.
Following an incubation at 37°C for 10 min, the A492 of each sample
was measured using a plate reader (SM800 Microplate Reader;
Shanghai Utrao Medical Instrument Co., Ltd., Shanghai, China).
Scratch assay
The cells were collected by incubation with 0.5%
trypsin (Thermo Fisher Scientific Inc.) and re-suspended in DMEM
containing 10% FBS. Each well of a 24-well plate was seeded with
5×104 cells. When the cells reached ~100% confluence
following culturing at 37°C in an atmosphere of 5% CO2,
the bottom of the wells were scratched with a 10 µl pipette tip.
The cells were washed with serum-free medium and cultured in DMEM
medium containing 10% FBS at 37°C in an atmosphere of 5%
CO2 for 36 h. Images of the cells were captured (DP26
Digital Camera for Microscopy; Olympus Corporation, Tokyo,
Japan).
Statistical analysis
All data are presented as the mean ± standard
deviation. One-way analysis of variance were used to analyze the
data using SPSS software version 17 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
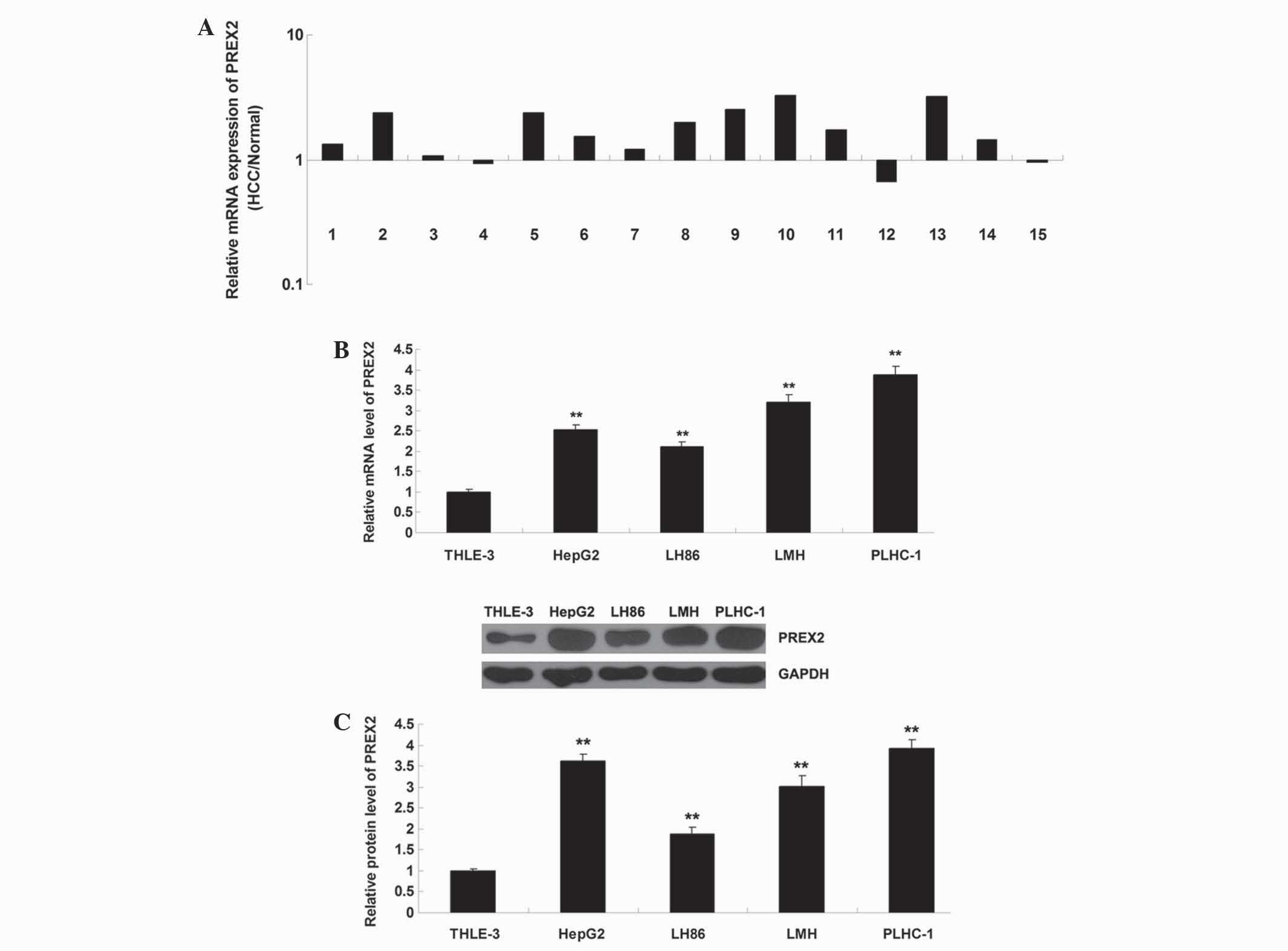
PREX2 was upregulated in HCC
tissues
The present study examined the expression of PREX2
in HCC tissues and human HCC HepG2, LH86, LMH and PLHC-1 cell
lines. As revealed in Fig. 1A, the
expression of PREX2 was increased in HCC tissues compared with the
normal matched adjacent tissues. Fig. 1B
and C demonstrate that the mRNA and protein expression level of
PREX2 was also significantly increased in the HCC cell lines
compared with the normal human liver THLE-3 cells. These findings
suggest that deregulation of PREX2 is implicated in HCC. Since
PLHC-1 cells demonstrated the most significant upregulation of
PREX2, the present study used this cell line in subsequent
experiments.
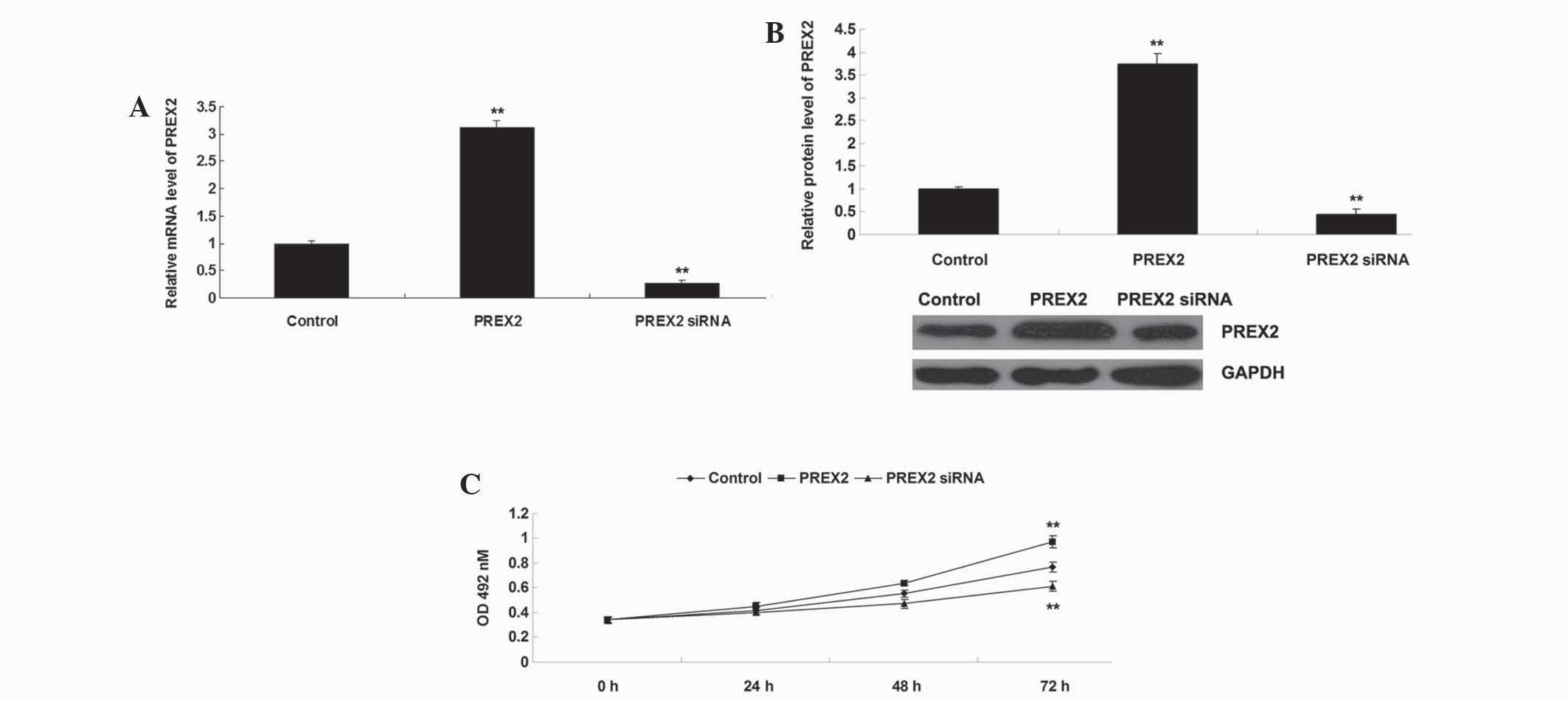
Upregulation of PREX2 promoted the
proliferation of HCC cells
The present study investigated the role of PREX2 in
the regulation of HCC proliferation in vitro in more detail.
The current study transfected PLHC-1 cells with PREX2 plasmid or
PREX2 siRNA to modulate the expression level of PREX2. Following
transfection, RT-qPCR and western blot analysis were used to
determine the mRNA and protein levels of PREX2 in PLHC-1 cells. The
results demonstrated that the mRNA and protein levels of PREX2 were
upregulated in PLHC-1 cells transfected with PREX2 plasmid, and
reduced in PLHC-1 cells transfected with PREX2 siRNA compared with
the control cells (control cells, non-transfected PLHC-1 cells;
Fig. 2A and B).
Subsequently, an MTT assay was performed to
determine cell proliferation in PLHC-1 cells transfected with PREX2
plasmid or PREX2 siRNA. As revealed in Fig. 2C, the proliferation of PLHC-1 cells
was significantly reduced following knockdown of PREX2, and the
proliferation was notably increased following overexpression of
PREX2 compared with the control cells. Therefore, this suggests
that PREX2 serves an oncogenic role in the regulation of HCC cell
proliferation.
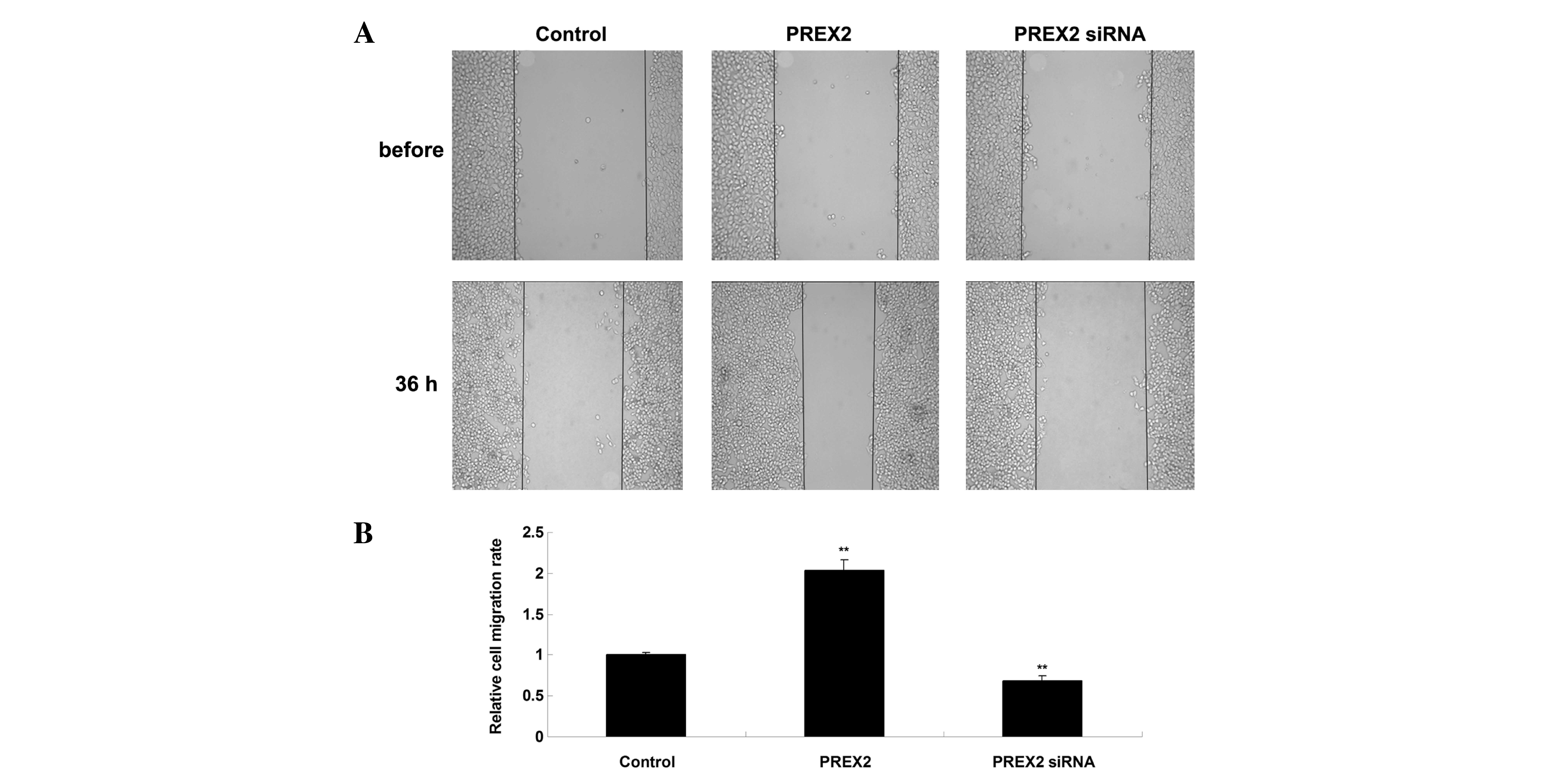
Upregulation of PREX2 enhanced HCC
cell migration
A scratch assay was performed to determine cell
migration in PLHC-1 cells transfected with PREX2 plasmid or PREX2
siRNA (Fig. 3A). Following the
inhibition of PREX2 expression, the migration of PLHC-1 cells was
significantly reduced compared with the control cells (Fig. 3B). Following overexpression of PREX2,
the migratory capacity of PLHC-1 cells was notably upregulated
compared with the control cells (Fig.
3B). Accordingly, this suggests that PREX2 may serve an
oncogenic role in the regulation of HCC cell migration.
PREX2 inhibited PTEN activity and
activated AKT signaling in HCC cells
PREX2 inhibits the activity of PTEN, and activates
AKT signaling (16). Accordingly, the
present study investigated the activity of PTEN and AKT signaling
pathways in PLHC-1 cells transfected with PREX2 plasmid or PREX2
siRNA by western blot analysis (Fig.
4A). The findings demonstrated that p-PTEN expression levels
were increased in PLHC-1 cells transfected with PREX2 plasmid,
indicating that the activity of PTEN was reduced. By contrast, the
activity of PTEN was upregulated in PLHC-1 cells following
knockdown of PREX2. Furthermore, the level of p-AKT was increased
in PLHC-1 cells with an overexpression of PREX2, indicating that
the activity of the AKT signaling pathway was upregulated (Fig. 4C; P<0.01). By contrast, knockdown
of PREX2 downregulated the activity of the AKT signaling pathway in
PLHC-1 cells (Fig. 4C; P<0.01).
Therefore, these findings indicate that PREX2 inhibits PTEN
activity and activates AKT signaling in HCC cells.
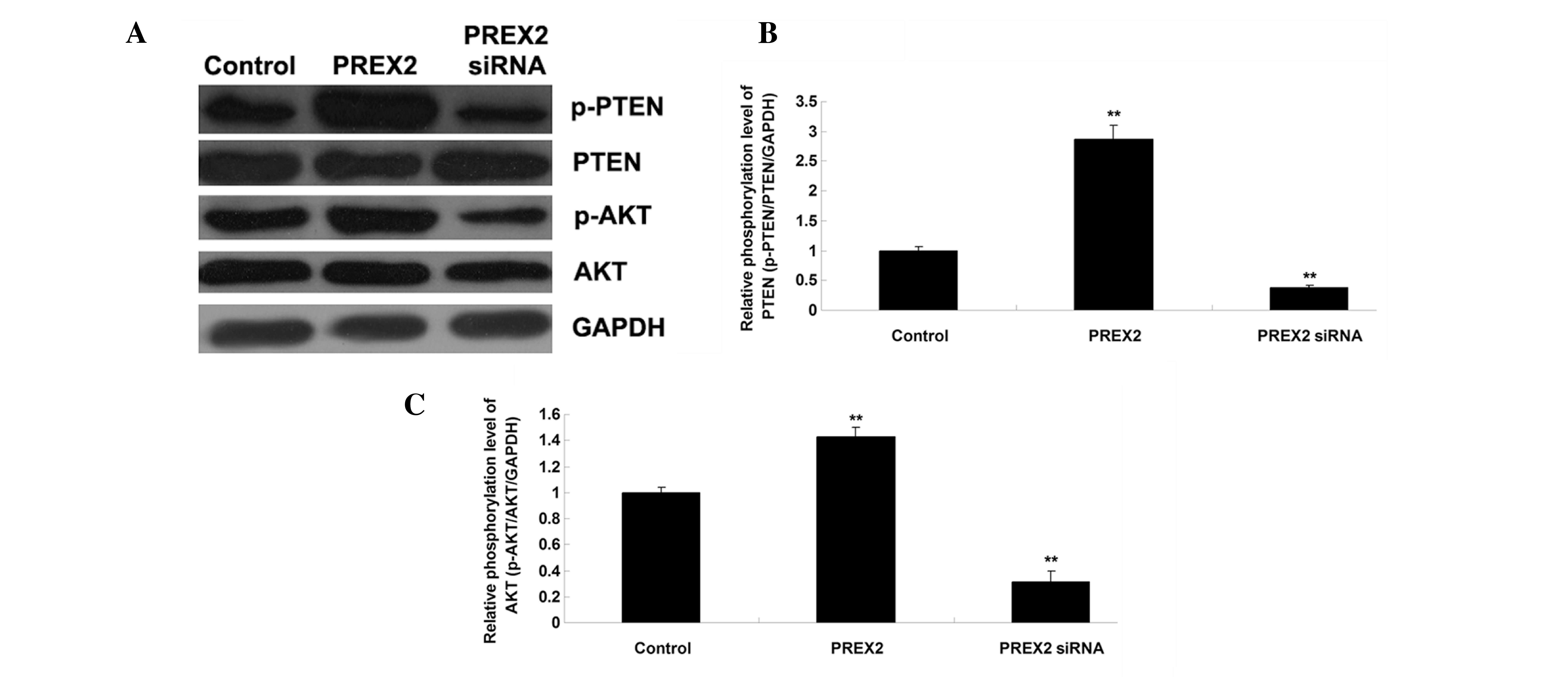 | Figure 4.(A) Western blot analysis was
performed to determine the protein expression of p-PTEN, PTEN,
p-AKT, and AKT in human hepatocellular carcinoma PLHC-1 cells
transfected with PREX2 plasmid or PREX2 siRNA. GAPDH was used as a
reference. Relative phosphorylation level of (B) PTEN and (C) AKT.
**P<0.01 vs. control. p, phosphorylated; PTEN, phosphatase and
tensin homolog deleted on chromosome 10 AKT, protein kinase B;
PREX2, phosphatidylinositol-3,4,5-trisphosphate Rac exchanger 2;
siRNA, small interfering RNA; Control, non-transfected human
hepatocellular carcinoma PLHC-1 cells; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Discussion
The identification of effective molecular targets is
urgently required for the treatment of HCC (17). The present study demonstrated that the
expression of PREX2 was increased in HCC tissues compared with
matched normal adjacent tissues. In addition, PREX2 was
significantly upregulated in HCC cell lines compared with a normal
liver cell line. The present in vitro study revealed that
PREX2 promoted the proliferation and migration of HCC cells by
modulating PTEN-AKT signaling.
PREX2 is a guanine nucleotide exchange factor that
acts on the guanosine triphosphatase Rac, and PREX2 has been
identified as an inhibitor of PTEN (18). PTEN negatively regulates intracellular
levels of (PIP3) in cells and functions as a tumor suppressor gene
by negatively regulating the AKT signaling pathway (19,20).
Therefore, PREX2-induced inactivation of PTEN may lead to an
accumulation of PIP3, which may increase the activity of the
downstream AKT signaling pathway (12).
As the AKT signaling pathway acts as a key regulator
in tumorigenesis, PREX2 may be crucial in human cancers (2,21). In an
independent extension cohort of 107 human melanomas, whole-genome
sequencing identified PREX2 as a significantly mutated gene with a
mutation frequency of ~14% (15).
These mutations consisted of non-synonymous PREX2 mutations and
truncations, which were distributed along the entire length of the
PREX2 gene. Furthermore, ectopic expression of mutant PREX2, which
was present in patients with melanomas, accelerated the tumor
formation of immortalized human melanocytes in vivo
(15). In addition, overexpression of
PREX2 has been revealed to be associated with the poor overall
survival of patients with breast cancer (22). The present study identified that the
expression of PREX2 was upregulated in HCC tissues and cell lines
compared with adjacent normal tissues and normal liver cell lines.
Lan et al (16) demonstrated
that high mRNA levels of PREX2 were associated with poor
differentiation, portal vein invasion and metastasis of cells and
qualitative hepatitis B surface antigen in 45 HCC tissue specimens.
In addition, the authors demonstrated that the inhibition of PREX2
by siRNA reduced the invasion ability of liver cancer cells
(16). Therefore, PREX2 may be
critical in HCC development.
PREX2 is involved in several types of human cancer,
including HCC; however, the exact role of PREX2 in the regulation
of HCC cell proliferation and migration has not been elucidated. In
the present study, HCC cells were transfected with PREX2-specific
siRNA or PREX2 plasmid to downregulate or upregulate PREX2
expression, respectively. It was observed that knockdown of PREX2
significantly suppressed the proliferation and migration of HCC
cells, and overexpression of PREX2 notably enhanced HCC cell
proliferation and migration. Consequently, the evidence in the
present study suggests that PREX2 may play a promoting role in the
regulation of HCC growth and metastasis. It has been reported that
PREX2 stimulates cell proliferation by inhibiting PTEN and
stimulating downstream AKT signaling (23). Chen et al (14) revealed that knockdown of PREX2
inhibited the proliferation, migration and invasion of
neuroblastoma cells by activating PTEN and suppressing the AKT
signaling pathway. Similarly, Guo et al (13) reported that an inhibition of PREX2
suppressed the proliferation and clonogenicity of gastric cancer
cells, and induced cell apoptosis and a cell cycle arrest at G1-S
phase, by activating PTEN and inhibiting AKT activity. The current
study identified that inhibiting the PREX2 expression increased the
activity of PTEN and reduced the activity of AKT, and an
overexpression of PREX2 upregulated the PTEN activity and
inactivated AKT, suggesting that PREX2 promotes the proliferation
and migration in HCC cells by modulating PTEN-AKT signaling.
In summary, the present study demonstrated that
PREX2 was upregulated in HCC tissues and cell lines and exerted
promoting effects on the proliferation and migration of HCC cells
by inhibiting PTEN activity, and therefore activating AKT
signaling. Consequently, PREX2 may be a potential target for the
treatment of HCC.
References
|
1
|
Zhu AX: Molecularly targeted therapy for
advanced hepatocellular carcinoma in: 2012 Current status and
future perspectives. Semin Oncol. 39:493–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Psyrri A, Arkadopoulos N, Vassilakopoulou
M, Smyrniotis V and Dimitriadis G: Pathways and targets in
hepatocellular carcinoma. Expert Rev Anticancer Ther. 12:1347–1357.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimitrova V and Arcaro A: Targeting the
PI3K/AKT/mTOR signaling pathway in medulloblastoma. Curr Mol Med.
15:82–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohta K, Hoshino H, Wang J, Ono S, Iida Y,
Hata K, Huang SK, Colquhoun S and Hoon DS: MicroRNA-93 activates
c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by
directly inhibiting PTEN and CDKN1A. Oncotarget. 6:3211–3224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Hu C, Cheng J, Chen B, Ke Q, Lv Z,
Wu J and Zhou Y: MicroRNA-145 suppresses hepatocellular carcinoma
by targeting IRS1 and its downstream Akt signaling. Biochem Biophys
Res Commun. 446:1255–1260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang HY, Yang SL, Liang HF and Li CH: HBx
protein promotes oval cell proliferation by up-regulation of cyclin
D1 via activation of the MEK/ERK and PI3K/Akt pathways. Int J Mol
Sci. 15:3507–3518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carnero A and Paramio JM: The
PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front Oncol.
4:2522014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eng C: PTEN: One gene, many syndromes. Hum
Mutat. 22:183–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donald S, Hill K, Lecureuil C, Barnouin R,
Krugmann S, Coadwell John W, Andrews SR, Walker SA, Hawkins PT,
Stephens LR and Welch HC: P-Rex2, a new guanine-nucleotide exchange
factor for Rac. FEBS Lett. 572:172–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Donald S, Humby T, Fyfe I, Segonds-Pichon
A, Walker SA, Andrews SR, Coadwell WJ, Emson P, Wilkinson LS and
Welch HC: P-Rex2 regulates Purkinje cell dendrite morphology and
motor coordination. Proc Natl Acad Sci USA. 105:4483–4488. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fine B, Hodakoski C, Koujak S, Su T, Saal
LH, Maurer M, Hopkins B, Keniry M, Sulis ML, Mense S, et al:
Activation of the PI3K pathway in cancer through inhibition of PTEN
by exchange factor P-REX2a. Science. 325:1261–1265. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo B, Liu L, Yao J, Ma R, Chang D, Li Z,
Song T and Huang C: miR-338-3p suppresses gastric cancer
progression through a PTEN-AKT axis by targeting P-REX2a. Mol
Cancer Res. 12:313–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Pan M, Han L, Lu H, Hao X and Dong
Q: miR-338-3p suppresses neuroblastoma proliferation, invasion and
migration through targeting PREX2a. FEBS Lett. 587:3729–3737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berger MF, Hodis E, Heffernan TP, Deribe
YL, Lawrence MS, Protopopov A, Ivanova E, Watson IR, Nickerson E,
Ghosh P, et al: Melanoma genome sequencing reveals frequent PREX2
mutations. Nature. 485:502–506. 2012.PubMed/NCBI
|
|
16
|
Lan X, Xiao F, Ding Q, Liu J, Liu J, Li J,
Zhang J and Tian DA: The effect of CXCL9 on the invasion ability of
hepatocellular carcinoma through up-regulation of PREX2. J Mol
Histol. 45:689–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hung CH, Chiu YC, Chen CH and Hu TH:
MicroRNAs in hepatocellular carcinoma: Carcinogenesis, progression,
and therapeutic target. Biomed Res Int. 2014:4864072014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hodakoski C, Hopkins BD, Barrows D, Mense
SM, Keniry M, Anderson KE, Kern PA, Hawkins PT, Stephens LR and
Parsons R: Regulation of PTEN inhibition by the pleckstrin homology
domain of P-REX2 during insulin signaling and glucose homeostasis.
Proc Natl Acad Sci USA. 111:155–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salmena L, Carracedo A and Pandolfi PP:
Tenets of PTEN tumor suppression. Cell. 133:403–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trotman LC, Niki M, Dotan ZA, Koutcher JA,
Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van
Dyke T, et al: Pten dose dictates cancer progression in the
prostate. PLoS Biol. 1:E592003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oishi N, Yamashita T and Kaneko S:
Molecular biology of liver cancer stem cells. Liver Cancer.
3:71–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pandiella A and Montero JC: Molecular
pathways: P-Rex in cancer. Clin Cancer Res. 19:4564–4569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leslie NR: P-REX2a driving tumorigenesis
by PTEN inhibition. Sci Signal. 2:pe682009. View Article : Google Scholar : PubMed/NCBI
|