Introduction
Osteosarcoma is the most common primary malignant
bone tumor, and remarkable advances have been made in its treatment
over the past several decades (1).
The standard treatment for metastatic osteosarcoma is systemic
combination chemotherapeutics (2);
however, it would be beneficial if cancer patients could elicit
tumor-specific immunity that would control or slow the growth of
residual tumor cells. A combination of chemotherapy and
immunotherapeutic strategies represents a challenging task, because
chemotherapy is generally considered to be immunosuppressive
(3).
The majority of chemotherapeutic agents induce tumor
cell death by apoptosis, a process that has long been regarded as
non-immunogenic (4). However, recent
evidence indicates that certain anticancer drugs, such as
anthracyclines, induce an immunogenic type of apoptosis that
stimulates the engulfment of apoptotic bodies by dendritic cells
(DCs) and the activation of cytotoxic CD8+ T cells
through cross-priming (5).
Anthracyclines serve major roles in the treatment of leukemia,
lymphoma, breast, uterine, ovarian malignancies and sarcoma.
Despite its side effects, doxorubicin (ADM) induces immunogenic
cell death (ICD) in mouse tumor cells (6). Within hours after the initiation of ICD,
pre-apoptotic tumor cells translocate calreticulin (CRT) and heat
shock protein (HSP)70 from the endoplasmic reticulum to the cell
surface, together with other molecules (7). One critical feature of DCs that is
required to induce efficient antitumor response is the capacity to
cross-present tumor-associated antigens (Ag) and to cross-prime
cytotoxic T cells, which is a process requiring appropriate
activation. In addition, cells release the late apoptosis marker
high mobility group box 1 (HMGB1) into the extracellular matrix;
HMGB1 can bind Toll-like receptors (8). The release of this protein appears to be
required for optimal presentation of antigens from dying tumor
cells, T-cell priming by DCs, and subsequent T-cell-mediated
elimination of the tumor.
The present study therefore hypothesized that the
antitumor effect of chemotherapeutics may be enhanced by the
induction of ICD through the activation of cytotoxic T-lymphocytes
(CTLs) by DCs. The aim of the present study was to investigate
whether DCs enhance cell-mediated immunity in combination with ADM,
and in turn induce the expression of CRT and HSP70 on the apoptotic
cell surface and release HMGB1, and whether the combination of
immunotherapy with anticancer agents has an antitumor effect in
osteosarcoma mouse models.
Materials and methods
Cell lines
LM8 cells, a murine osteosarcoma line, derived from
Dunn osteosarcoma, were provided by the Riken BioResource Center
(Saitama, Japan). The cells were maintained in complete medium
consisting of RPMI 1640 (Sigma-Aldrich, Tokyo, Japan) supplemented
with 10% heat-inactivated fetal bovine serum (Wako Pure Chemical
Industries, Ltd., Osaka, Japan), 100 µg/ml streptomycin
(Sigma-Aldrich), and 100 U/ml penicillin (Sigma-Aldrich). The cells
were cultured at 37°C in 5% CO2.
Immunofluorescence in cultured
cells
LM8 osteosarcoma cells were seeded at a density of
1×105 cells/slide on LAB-TEK II Chamber Slides (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and fixed with 4%
paraformaldehyde (Wako Pure Chemical Industries, Ltd.). For
immunofluorescent staining of membrane CRT and HSP70, cells were
blocked with 10% BSA (Wako Pure Chemical Industries, Ltd.) in PBS,
and incubated with rabbit anti-mouse polyclonal CRT and monoclonal
HSP70 antibodies (catalog no., ab2907 and ab181606; Abcam, Tokyo,
Japan; 1:500 diluted in PBS with 1.5% BSA) followed by FITC-labeled
donkey anti-rabbit polyclonal immunoglobulin (Ig)G secondary
antibody [ab6798; Abcam; 1:500 diluted in PBS with 1.5% goat serum
(Sigma-Aldrich)]. Digital images were captured using a BIOREVO
microscope equipped with a confocal microscopy system (BZ-9000,
Keyence, Japan).
HMGB1 measurement
Quantification of HMGB1 in the supernatants was
assessed by Quantikine® (R&D Systems, Inc., Minneapolis, MN,
USA) according to the manufacturer's protocol using Skanlt software
for Multiskan™ FC plate reader (Thermo Fisher Scientific,
Inc.).
Membrane and subcellular fractionation
and immunoblot analysis
Membrane, cytoplasmic, and nuclear fractions were
extracted using a Global Protein Fractionation kit, according to
the manufacturer's instructions (St. Louis Biosciences, St. Louis,
MO, USA), with minor modifications. Briefly, two cytoplasmic and
nuclear fractions were extracted using NE-PER Nuclear and
Cytoplasmic Extraction Reagents (Pierce™; Thermo Fisher Scientific,
Inc.). Protein (15 µg) was resolved on a precast 10% Tris-HCl
Criterion 10-well gel (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) at 200 V (300 mAmp) for 30 min. The gel was wet-transferred to
a PVDF membrane for 1 h, and blocked with PBST containing 5%
instant dry non-fat milk for 30 min at room temperature. The
following antibodies were obtained from Cell Signal Technology
(Danvers, MA, USA): Rabbit monclonal anti-mouse CRT (catalog no.,
12238), polyclonal HSP70 (catalog no., 4876), polyclonal HMGB1
(catalog no., 3935), monclonal IgG NF-κB (catalog no., 3017),
polyclonal IκB-α (catalog no., 9242) and polyclonal α-Tubulin
(catalog no., 2144). The antibodies were diluted at 1:1000 and were
incubated for 1 h at room temperature. Immunocomplexes were
visualized with horseradish peroxidase-conjugated anti-rabbit IgG
antibodies (GE Healthcare, Tokyo, Japan), developed the blots using
ECL Plus system (GE Healthcare) with a ChemiDoc camera (ImageQuant
LAS 4000mini; GE Healthcare). The quantification of western blot
signals was performed by the densitometry with ImageQuant TL
version 8.1 software (GE Healthcare). All western blot experiments
were repeated at least three times.
Animals
A total of 1×106 LM8 cells were
hypodermically implanted into the subcutaneous gluteal region of 24
female C3H mice 6–8 weeks old. The C3H mice were purchased from
Sankyo Labo, Inc. (Toyama, Japan) and housed in a specific
pathogen-free animal facility at Oita University (Oita, Japan).
The animal experimental protocol was approved by the
Ethics Review Committee for Animal Experimentation of Oita
University (Oita, Japan), and all mice used in the present study
were anesthetized with ketamine/xylazine or isoflurane/oxygen for
experiments and euthanized with cervical dislocation under
anesthesia. All efforts were made to minimize suffering.
Generation of DCs
Bone marrow-derived DCs were generated as described
by Lutz and Rössner (9) with minor
modifications. Briefly, erythrocyte-depleted mouse bone marrow
cells obtained from flushed femur bone marrow cavities
(1×106 cells/ml) were cultured in complete medium with
20 ng/ml granulocyte macrophage colony-stimulating factor (GM-CSF;
PeproTech EC Ltd., London, UK), recombinant mouse GM-CSF (100
ng/ml; PeproTech EC Ltd.) and IL-4 (R&D Systems Europe, Ltd.,
Abingdon, UK) at 25 ng/ml (U/ml) in 10-cm tissue culture dishes at
37°C in an atmosphere containing in 5% CO2.
Flow cytometry
For flow cytometric analysis, DCs were counted with
a FACSVerse™ Flow Cytometer (Becton Dickinson, San Jose, CA, USA)
and stained with fluorochrome-conjugated antibodies (BD
Pharmingen™; BD Biosciences, Tokyo, Japan) for the following
markers: APC hamster anti-mouse monoclonal cluster of
differentiation (CD)11c (catalog no., 550261), PE hamster
anti-mouse monoclonal CD80 (catalog no., 553769) and APC rat
anti-mouse monoclonal CD86 (catalog no., 558703). The antibodies
diluted at 1:400 and were stained for 1 h at room temperature.
CD11c was used as a marker for all DCs regardless of the degree of
maturation, whereas CD80, CD86 are markers for DCs. Data analysis
was performed with FACSuite™ version 1.0.3 software (Becton
Dickinson).
Therapy protocol
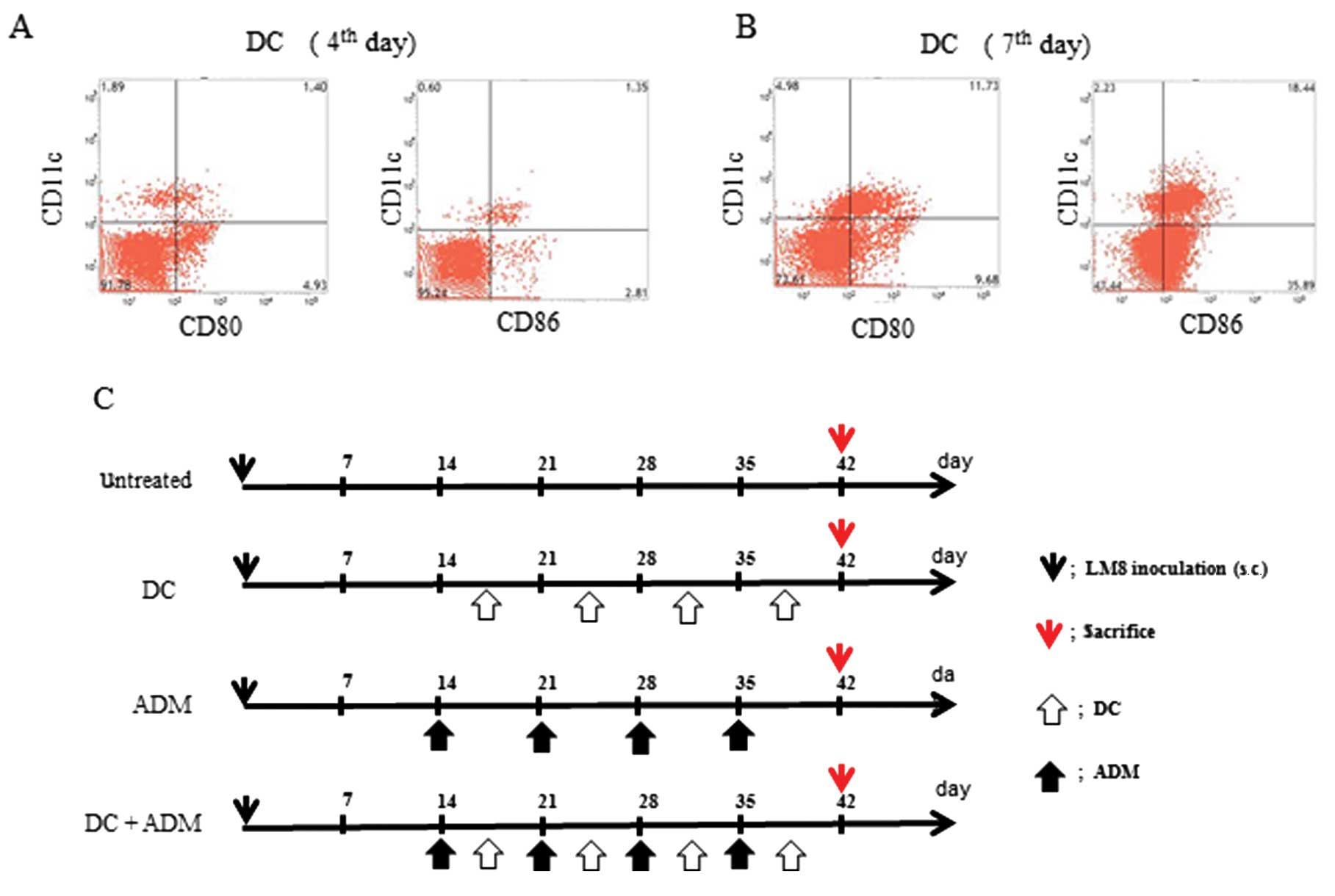
All the animals developed tumors. The maturation of
the DCs were assessed at day 4 (Fig.
1A) and day 7 (Fig. 1B) using
flow cytometry. The following 4 groups were established (Fig. 1C): i) Untreated (control; n=6); ii)
DCs were injected twice a week into the subcutaneous contralateral
gluteal region (DC; n=6); iii) intraperitoneal injection of ADM
(ADM; 6 mg/kg of body weight; n=6); and (iv) DCs were injected
twice a week into the subcutaneous contralateral gluteal region and
intraperitoneal injection of ADM (6 mg/kg of body weight) was
performed twice per week (DC+ADM; n=6). All experiments were
performed under the guidelines for animal experiments as stipulated
by the Oita University Graduate School of Medical Science.
Tumor volume
Tumor volumes were measured using the micro-CT
apparatus (R_mCT) which allows us to obtain high-resolution CT
images in small living animals. The I-view-R (J. Morita Mfg Corp,
Kyoto, Japan) was used as the viewer, and diagnosis was made with
slice images viewed in all directions. Tumor volumes were estimated
using the following formula: Tumor volume = (length ×
width2)/2.
Immunohistochemistry
Immunohistochemistry was used to measure the levels
of CD11c, as a marker of DCs; CD8, as a marker of cytotoxic T
lymphocytes; and CRT and HMGB1, inside tumor lesions. Primary tumor
lesions were resected and all the samples were fixed in a frozen
section. Six samples per mouse were cut into 10-µm-thick slices.
The tissue sections were incubated overnight at room temperature
with primary rabbit anti-mouse polyclonal CD8-α (catalog no.,
sc-7188; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
monoclonal CD11c (catalog no., ab52632; Abcam), polyclonal CRT,
monoclonal HSP70 and monoclonal HMGB1 (catalog no., ab79823;
Abcam), which were diluted at 1:200 in Ab Diluent (Dako ChemMate,
Dako, Japan). The secondary antibody for CD11c and CRT was
FITC-labeled donkey anti-rabbit polyclonal IgG (catalog no.,
A-21206; Thermo Fisher Scientific, Inc.) and for CD8, HSP70 and
HMGB1 was Rhodamine red-labeled goat anti-rabbit polyclonal IgG
(catalog no., R-6394; Thermo Fisher Scientific, Inc.). The
secondary antibodies were diluted at 1:300 in Ab diluent and added
for 60 min at room temperature in the dark. Digital images were
captured using BIOREVO microscope equipped with a confocal
microscopy system (BZ-9000).
Cytokine evaluations
Serum interferon (IFN)-γ and interleukin (IL)-12
release in mice were measured by enzyme-linked immunosorbent assay
(ELISA) using Quantikine® (R&D Systems, Inc.) according to the
manufacturer's protocol and a Skanlt for Multiskan FC plate
reader.
Statistical analysis
The difference among the four groups was determined
using a non-repeated measures analysis of variance and the Scheffe
test. All analyses were conducted using SPSS 18.0 software (SPSS,
Inc., Chicago, IL, USA). Results were expressed as the mean ±
standard deviation, and P<0.05 was considered to indicate a
statistically significant difference.
Results
ADM induced ICD in LM8 cells in
vitro
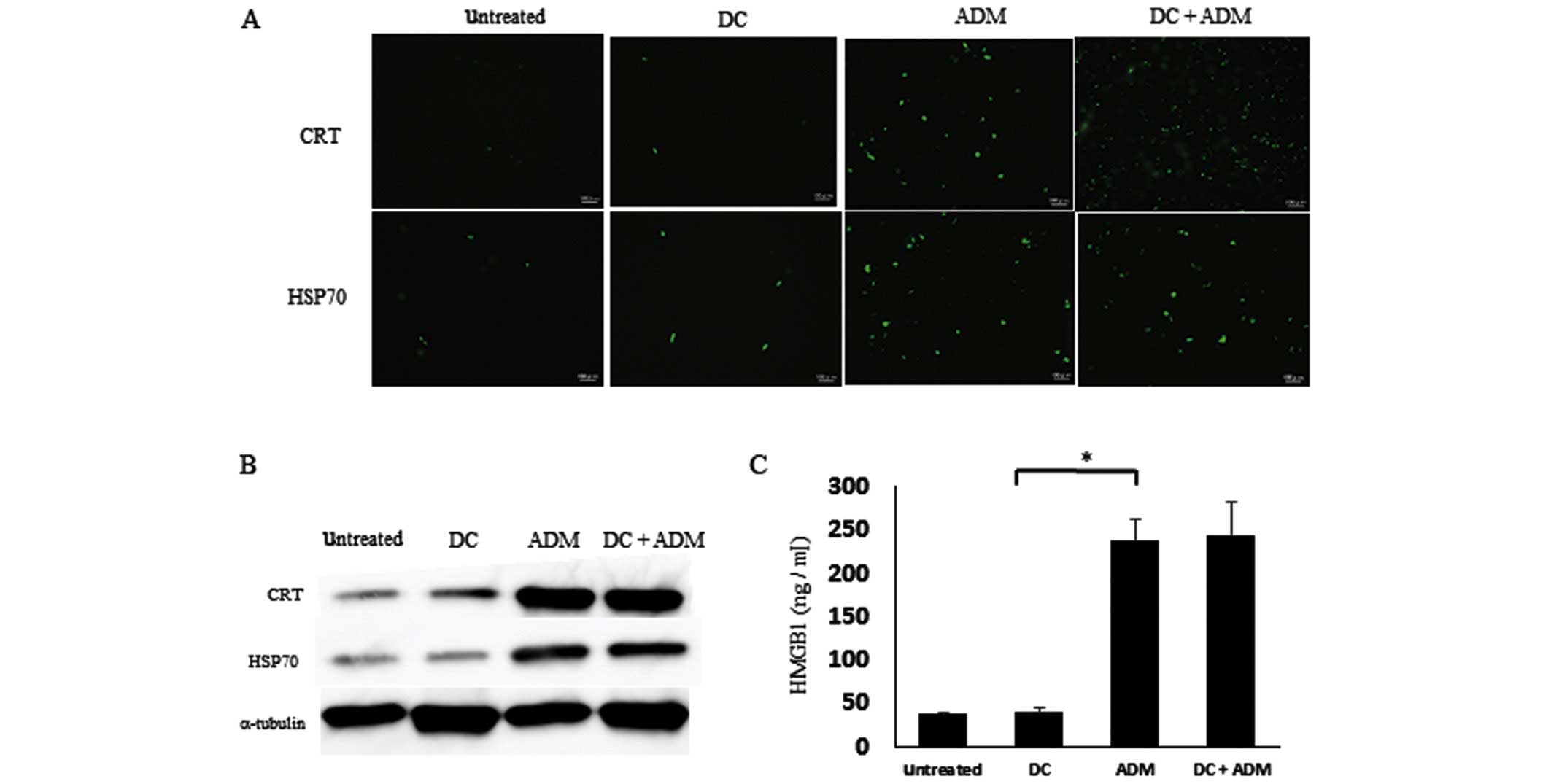
To verify the ability of ADM to induce ICD, the
expression of CRT and HSP70 on the cell surface and the release of
HMGB1 in the supernatant was evaluated by immunofluorescence. ADM
treatment markedly increased the expression of CRT and HSP 70 on
the cell membrane (Fig. 2A). The
protein expression levels of CRT and HSP70 on the cell surface was
also increased in ADM-treated cells (Fig.
2B). In addition, the amount of HMGB1 in the cell culture
supernatant was significantly increased after ADM treatment alone
(Fig. 2C; P=0.00043).
Ligand administration induced DC
activation
CRT, HSP70, and HMGB1 ligands were administered to
DCs in culture to verify the ability of these ligands to activate
DC function. DCs treated with CRT (Fig.
3A), HSP (Fig. 3B) and HMGB1
(Fig. 3C) and HMGB1 ligands were
activated, as compared to untreated DCs, as assessed by flow
cytometric analysis. Thirty minutes after CRT (Fig. 3D), HSP70 (Fig. 3E) and HMGB1 (Fig. 3F) ligand treatment, the amount of
NF-κB in the nuclear fraction was higher than the amount of NF-κB
in the cytoplasmic fraction and IκB in whole lysate, as assessed by
western blot analysis. Thus, CRT, HSP, and HMGB1 ligands could
activate DCs function in vitro.
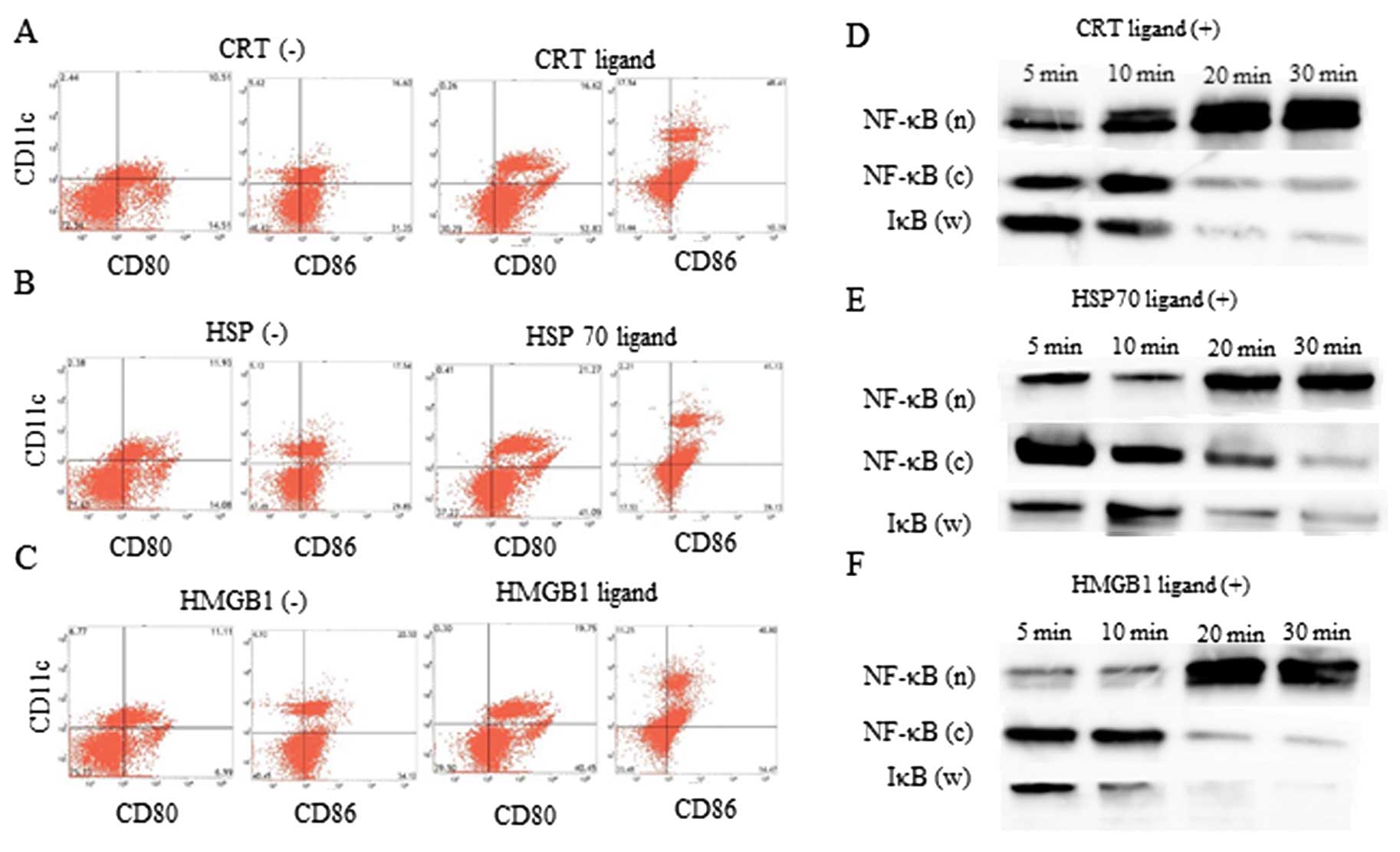 | Figure 3.DC activation status examined using
flow cytometry. The change in CD80 and CD86 expression on DCs with
or without the (A) CRT ligand, (B) HSP70 ligand, and (C) HMGB1
ligand administration. NF-κB expression in nuclear (n) and
cytoplasmic (c) fraction, and IκB expression of whole lysate (w) up
to 30 min after treatment with the (D) CRT ligand, (E) HSP70
ligand, and (F) HMGB1 ligand. DC, dendritic cell; CRT,
calreticulin; ADM, doxorubicin; HSP70, heat shock protein 70;
HMGB1, high mobility group box 1. |
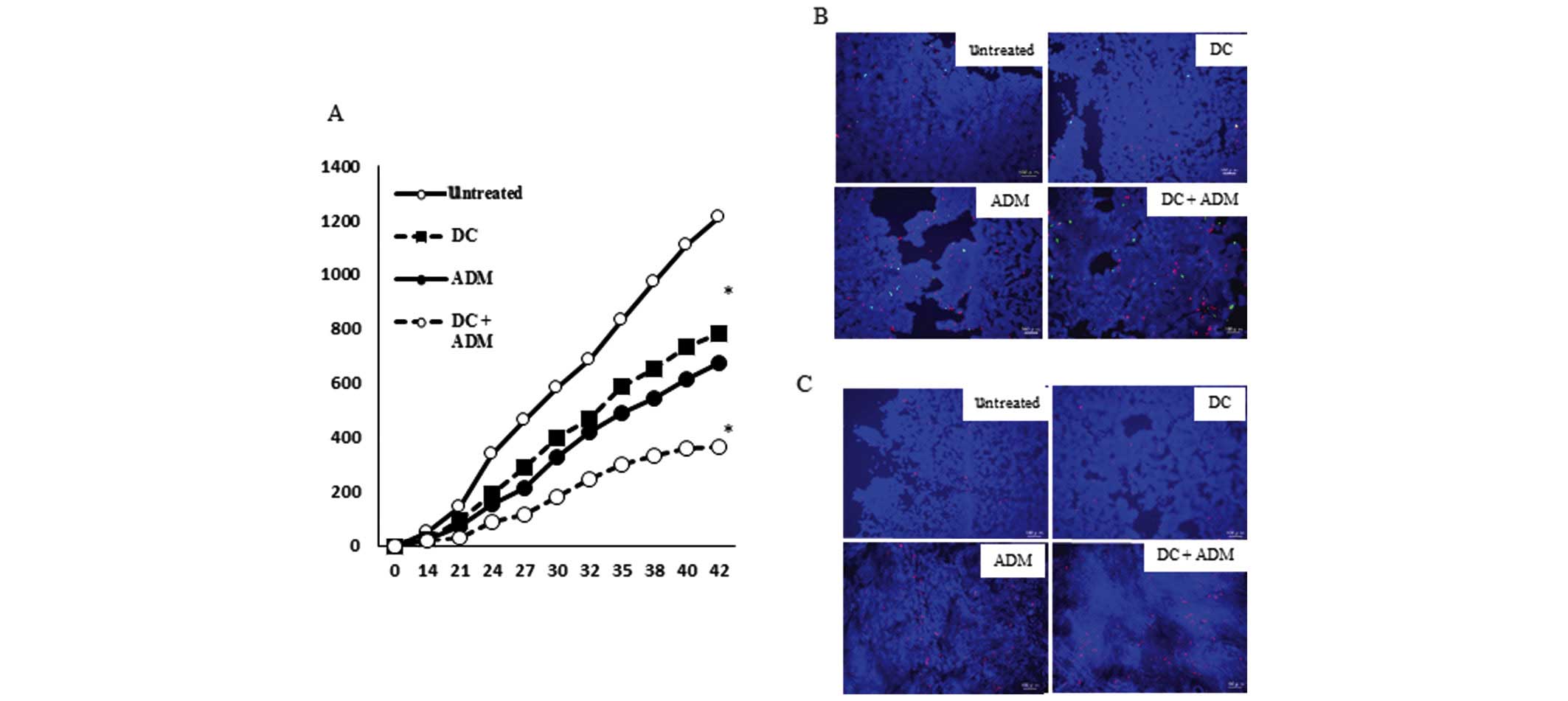
Tumor volume of the tumor lesion
A total of 42 days after the tumor cell inoculation,
the volume of the metastatic lesion in mice that received a DC
injection in combination with ADM treatment (366.67±14.95
mm3) was significantly lower (P<0.01) than that
observed in mice that received DCs (782.41±13.66 mm3) or
ADM alone (677.69±26.01 mm3; Fig. 4A).
ADM induced ICD in mice tumor
tissues
The ability of ADM to induce the expression of ICD
markers in tumors in vivo was then determined. Mice
receiving ADM injections demonstrated a marked increase in CRT and
HSP70 expression on the cell membrane (Fig. 4B). The release of HMGB1 in the tumor
tissues was further analyzed. ADM induced release of HMGB1 in all
tested mice (Fig. 4C).
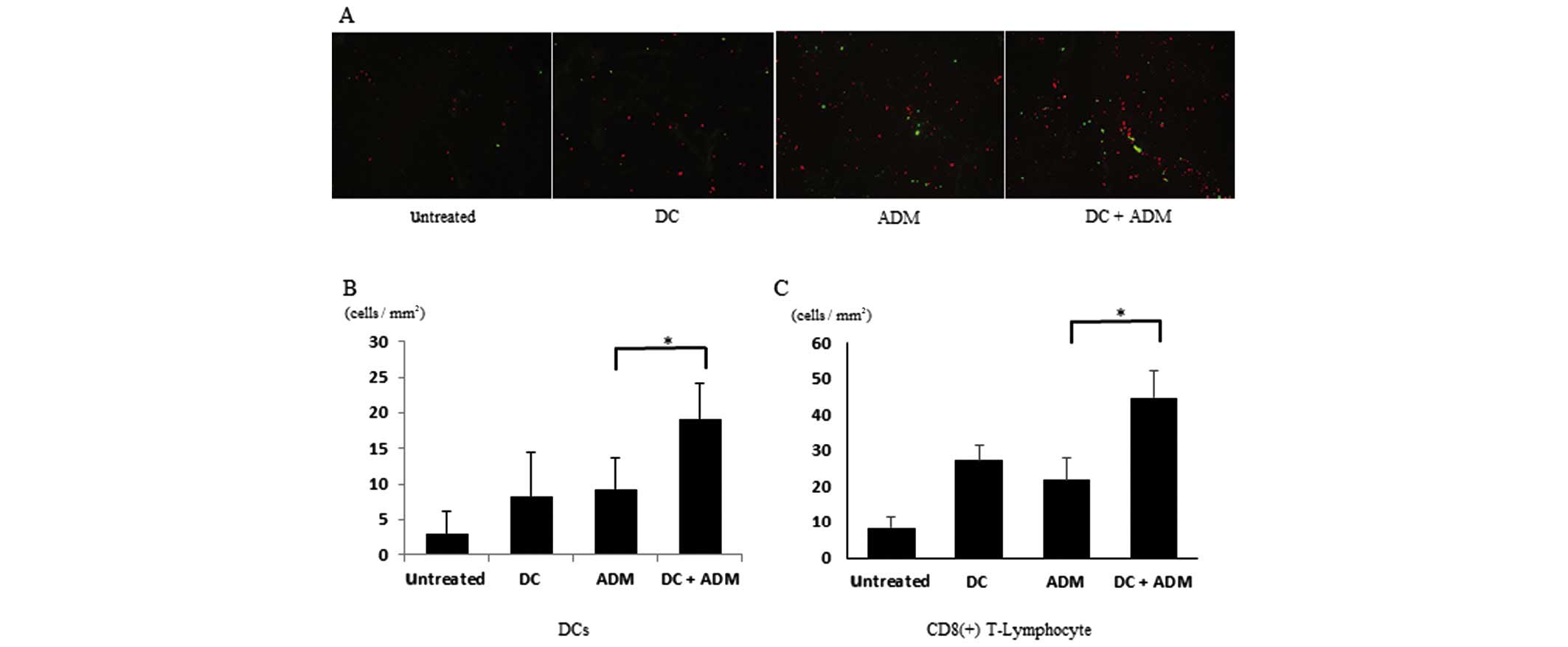
Infiltration of CD8+
T-lymphocytes and DCs within the tumor
CD8+ T-lymphocytes gathered within the
primary tumor in ADM-treated mice (Fig.
5A). DCs were also recruited to the metastatic area in the
ADM-treated groups compared with the non-ADM-treated groups. The
number of CD8+ T-lymphocytes per unit area was
significantly higher (P<0.01) in the mice that received DCs
combined with ADM (44.78±7.44 cells/mm2) than in those
that received DCs (27.38±4.31 cells/mm2) or ADM alone
(21.74±6.43 cells/mm2; Fig.
5B). The number of CD11C+ cells per unit area was
significantly higher (P<0.01) in the mice that received DCs
combined with ADM (19.23±5.032 cells/mm2) than in those
that received DCs (8.34±6.22 cells/mm2) or ADM alone
(9.32±4.324 cells/mm2; Fig.
5C).
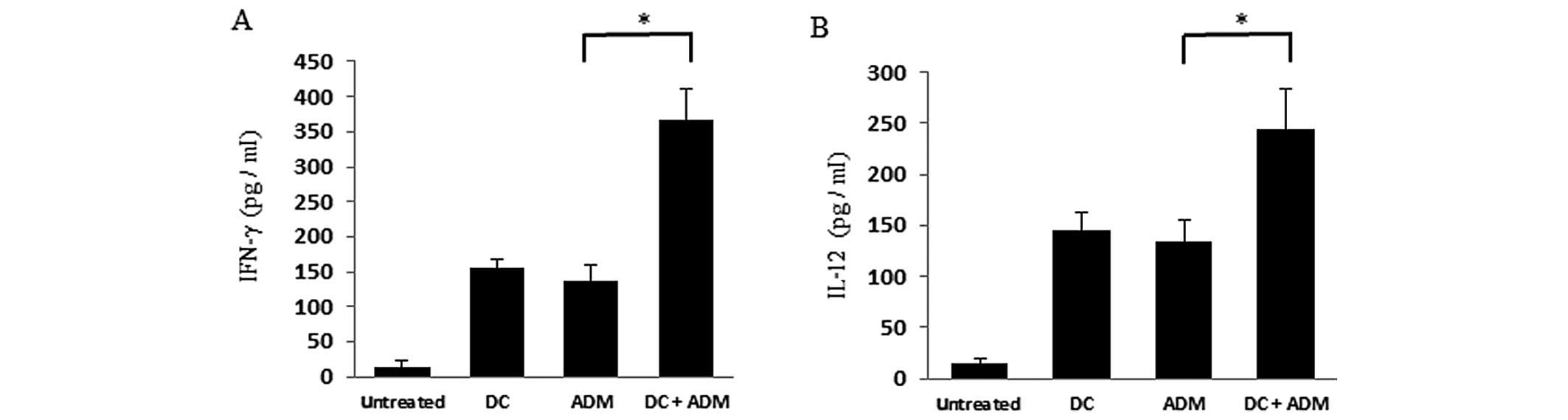
Cytokine release
Mice treated with both DCs and ADM displayed higher
serum IFN-γ levels (365.64±45.32 pg/ml; P<0.01) than those
received DCs (155.53±13.01 pg/ml) or ADM treatment alone
(138.37±28.11 pg/ml; Fig. 6A). Mice
treated with both DCs and ADM displayed higher serum IL-12 levels
(243.52±39.92 pg/ml; P<0.01) than those that received DCs
(145.43±16.38 pg/ml) or ADM treatment alone (133.72±21.29 pg/ml;
Fig. 6B).
Discussion
The majority of osteosarcoma patients are treated
with a combination of surgery and chemotherapy. Despite recent
advances in locally, curative therapy, chemotherapeutic treatments
for metastatic disease are often ineffective. Therefore, the
development of novel therapeutic options is of great interest. The
present authors have previously investigated the effect of
immunotherapy using DCs on cell death (10); and the combination of DCs with
anti-regulatory T cells (Tregs) antibodies, such as
anti-transforming growth factor β (TGF-β) (11) or anti-cytotoxic T-Lymphocyte Antigen 4
(CTLA-4) (12).
Certain chemotherapeutic regimens trigger cancer
cell death through stimulating endogenous immune responses
(13–15). Identification of potent activating
signals expressed by immunogenic tumor cells would significantly
contribute to understanding the interaction between tumor cells and
the immune system and would facilitate the design of more effective
immunotherapeutic strategies.
ICD elicited by chemotherapeutics is characterized
by a series of events that include pre-apoptotic surface
translocation of CRT and HSP70, and the release of HMGB1 into the
extracellular milieu. These proteins can bind to DCs and enhance
adaptive antitumor responses (6,16). The
present study initially examined whether ADM could induce ICD in a
mouse osteosarcoma cell line. Increased expression of CRT and HSP70
indicated that ADM treatment may translocate immunogenic factors to
the cell membrane. ADM also induced the release of HMGB1, as shown
by ELISA. Further, ICD-related ligands, including CRT, HSP70 and
HMGB1, were secreted in LM8 cells, at least in these culture
conditions.
In the present study, the interaction between
immature DCs and immunogenic tumor cells led to increased tumor
cell uptake and induced moderate expression of
maturation-associated markers on DCs, such as CD80 and CD86.
Similar to published studies by Zitvogel et al (15), the rate of phagocytosis in the present
study correlated with the intensity of CRT expression and, to a
lesser degree, with HSP expression (17). In addition, DC activation through
ligand ligation can be evaluated through NK-κB expression in the
nucleus (18). A higher percentage of
mature DCs was observed when DCs were cocultured with ADM-treated
LM8 cells. NF-κB expression in the DC nuclear fraction was
increased, and cytoplasmic NF-κB and IκB significantly decreased
within 30 min. Thus, one may conclude that ICD-related ligands
induced DC activation in the present study.
The effect of ADM-induced ICD in vivo was
analzed in the present study using a mouse model of osteosarcoma.
Normal mice with tumors that formed from injected LM8 osteosarcoma
cells were treated with DCs generated from bone marrow, and ADM was
administered. CRT and HSP70 translocation to the cell surface and
HMGB1 release was observed after ADM treatment. Combination
treatment with DCs and ADM resulted in smaller lesions. Thus, ADM
administered via the tail vein induced ICD in the established
tumors tissues, and DCs may cooperate at the tumor site through
activation by ICD ligands.
Upon exposure to ADM, the expression of CRT and
HSP70 expression on the surface of dying cells may be a marker for
ICD, which may deliver an activating stimulus to DCs. HMGB1 is
actively secreted from inflammatory cells or released from necrotic
cells (19,20), and it signals through TLR2, TLR4, and
RAGE in DCs (21,22). DCs should migrate to the site of
release, and activate to transmit information to CTLs. To evaluate
whether tumor cells expressing ICD markers could recruit DCs and
CD8+ T cell following DC infiltration, the ability of
tumor cell-loaded DCs to activate tumor cell-specific T cell
responses was further evaluated. In mice treated with DCs and ADM,
the number of CD8+ T cells and DCs inside the tumor
tissue increased compared with mice treated with DCs or ADM alone.
This is consistent with the previous results demonstrating that
tumor lesion volumes were significantly reduced after combination
therapy. Taken together these data suggest that ICD factors may
facilitate the activity of DCs and CTLs in the tumor.
DCs loaded with tumor cells killed by ADM
efficiently stimulated the activation of cell-mediated immunity by
increasing serum IFN-γ and IL-12 levels. These cells also induced
significantly higher secretion of cytotoxic cytokines compared with
non-immunogenic tumor cells, which may be relevant for the design
of cancer immunotherapy studies. The results of the present study
revealed that stimulating DCs using ADM-treated tumor cells could
enhance cell-mediated immunity.
Despite the continuous introduction of novel drugs
and improved chemotherapy protocols, it is likely that, at some
point, chemotherapy will reach its limits, and clinical efficacy
will plateau. A combination of treatment modalities classically
surgery with chemo- or radiotherapy has been a standard strategy
for cancer treatment. Many clinical studies based on the
combination of chemotherapy and immunotherapy have demonstrated
variable responses (23). Indeed,
chemotherapy may be either immunostimulatory or immunosuppressive,
depending on the dosage and the timing of administration, and may
synergize with immunotherapy approaches in vivo (24). Chemotherapy and immunotherapy should
not be considered antagonizing forms of therapy; rather, it is
conceivable that their combination could substantially improve the
prognosis of cancer patients.
Acknowledgements
The authors thank Dr Hidekatsu Iha for helpful
discussions regarding the present study.
References
|
1
|
Stiller CA, Bielack SS, Jundt G and
Steliarova-Foucher E: Bone tumours in European children and
adolescents, 1978–1997. Report from the Automated Childhood Cancer
Information System project. Eur J Cancer. 42:2124–2135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milosević DB: The different level of
immunological recovery after chemotherapy in leukemia and lymphoma
patients. J Exp Clin Cancer Res. 20:517–522. 2001.PubMed/NCBI
|
|
4
|
Tesniere A, Panaretakis T, Kepp O, Apetoh
L, Ghiringhelli F, Zitvogel L and Kroemer G: Molecular
characteristics of immunogenic cancer cell death. Cell Death
Differ. 15:3–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Apetoh L, Mignot G, Panaretakis T, Kroemer
G and Zitvogel L: Immunogenicity of anthracyclines: Moving towards
more personalized medicine. Trends Mol Med. 14:141–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy.
Nature. 13:1050–1059. 2007.
|
|
7
|
Fucikova J, Kralikova P, Fialova A,
Brtnicky T, Rob L, Bartunkova J and Spísek R: Human tumor cells
killed by anthracyclines induce a tumor-specific immune response.
Cancer Res. 71:4821–4833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sancho D, Joffre OP, Keller AM, Rogers NC,
Martínez D, Hernanz-Falcón P, Rosewell I and Sousa Reise C:
Identification of a dendritic cell receptor that couples sensing of
necrosis to immunity. Nature. 458:899–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lutz MB and Rössner S: Factors influencing
the generation of murine dendritic cells from bone marrow: The
special role of fetal calf serum. Immunobiology. 212:855–862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawano M, Nishida H, Nakamoto Y, Tsumura H
and Tsuchiya H: Cryoimmunologic antitumor effects enhanced by
dendritic cells in osteosarcoma. Clin Orthop Relat Res.
468:1373–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawano M, Itonaga I, Iwasaki T, Tsuchiya H
and Tsumura H: Anti-TGF-β antibody combined with dendritic cells
produce antitumor effects in osteosarcoma. Clin Orthop Relat Res.
470:2288–2294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawano M, Itonaga I, Iwasaki T and Tsumura
H: Enhancement of antitumor immunity by combining anti-cytotoxic T
lymphocyte antigen-4 antibodies and cryotreated tumor lysate-pulsed
dendritic cells in murine osteosarcoma. Oncol Rep. 29:1001–1006.
2013.PubMed/NCBI
|
|
13
|
Zitvogel L, Kepp O and Kroemer G: Immune
parameters affecting the efficacy of chemotherapeutic regimens. Nat
Rev Clin Oncol. 8:151–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Locher C, Conforti R, Aymeric L, Ma Y,
Yamazaki T, Rusakiewicz S, Tesnière A, Ghiringhelli F, Apetoh L,
Morel Y, et al: Desirable cell death during anticancer
chemotherapy. Ann N Y Acad Sci. 1209:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zitvogel L, Kepp O, Senovilla L, Menger L,
Chaput N and Kroemer G: Immunogenic tumor cell death for optimal
anticancer therapy: The calreticulin exposure pathway. Clin Cancer
Res. 16:3100–3104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michaud M, Sukkurwala AQ, Di Sano F,
Zitvogel L, Kepp O and Kroemer G: Synthetic induction of
immunogenic cell death by genetic stimulation of endoplasmic
reticulum stress. Oncoimmunology. 3:e282762014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoshino K and Kaisho T: Nucleic acid
sensing toll-like receptors in dendritic cells. Curr Opin Immunol.
20:408–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Vishnubhakat JM, Bloom O, Zhang M,
Ombrellino M, Sama A and Tracey KJ: Proinflammatory cytokines
(tumor necrosis factor and interleukin 1) stimulate release of high
mobility group protein-1 by pituicytes. Surgery. 126:389–392. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JS, Svetkauskaite D, He Q, Kim JY,
Strassheim D, Ishizaka A and Abraham E: Involvement of toll-like
receptors 2 and 4 in cellular activation by high mobility group box
1 protein. J Biol Chem. 279:7370–7377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apetoh L, Ghiringhelli F, Tesniere A,
Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP,
Delaloge S, et al: The interaction between HMGB1 and TLR4 dictates
the outcome of anticancer chemotherapy and radiotherapy. Immunol
Rev. 220:47–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nowak AK, Lake RA and Robinson BW:
Combined chemoimmunotherapy of solid tumours: Improving vaccines?
Adv Drug Deliv Rev. 58:975–990. 2006. View Article : Google Scholar : PubMed/NCBI
|