Introduction
Worldwide, bladder cancer is the 7th most common
cancer in men and the 17th most common cancer in women (1). Notably, in the developed world, bladder
cancer ranks as the 4th and 9th most common cancer in men and
women, respectively (2). An estimated
375,000 bladder cancer cases are reported each year around the
world, with 68,810 novel cases and ~14,100 mortalities reported in
the United States in 2013 (3).
Transitional cell carcinoma is the most frequently occurring type
of bladder cancer. Other bladder cancer types consist of
adenocarcinoma, squamous cell carcinoma and small cell bladder
cancer (4,5). Notably, the incidence of bladder cancer
increases with age, particularly in men (6). Bladder cancer arises as a result of
multistep alterations, among which metastasis is crucial (7). Epidemiological studies have demonstrated
that several environmental factors may contribute to bladder cancer
risk, including smoking, chronic inflammation, radiation exposure,
anticancer drugs and aromatic amines, which are contained in dyes
(6,8).
The treatment of bladder cancer is based on multiple parameters,
including the extent of the disease, bladder cancer stage and the
results of a bladder cystoscopy, which evaluates the tumor
(9). The currently available
treatments are not effective and the mechanisms of initiation and
progression of bladder cancer remain unresolved due to a lack of
effective early diagnostic tools and clinical prognostic markers
(10). Nevertheless, previous studies
have identified a few markers that are associated with bladder
cancer progression, including the tumor stage, grade, invasion,
growth and metastasis (11). However,
additional molecular targets that accurately predict bladder cancer
progression are urgently required.
The matrix metalloproteinases (MMPs), a family of
zinc-dependent endopeptidases with proteolytic activity against
extracellular matrix components (12), are involved in numerous physiological
processes, including tissue remodeling, embryonic development and
reproduction (13). However, the
overexpression of MMPs is also observed in several diseases
(14). The levels of particular MMPs,
including the interstitial collagenase MMP-1, stromelysin-1 MMP-3,
gelatinases MMP-2 and MMP-9 and stromelysin-3 MMP-11, are increased
in tumor tissues, and promote the invasion of malignant cells,
regulate tumor growth and metastasis and are associated with a poor
overall survival rate (15). MMP-9
has multiple substrates; however, collagen type IV, the main
component of basement membranes, is the most crucial MMP-9
substrate in a tumor microenvironment (16). The proteolytic activity of MMP-9
against collagen type IV not only promotes invasion and metastasis,
but also releases matrix-bound growth factors and other signaling
molecules to promote growth signaling, angiogenesis and an
inflammatory response (9,17). Previous studies have revealed that
MMP-9 is involved in the pathogenesis of bladder cancer (3,9,15,18);
however, alternative studies fail to establish an association
between MMP-9 expression and bladder cancer, leading to the
conclusion that MMP-9 may not be an effective marker for bladder
cancer detection (19,20). Due to the conflicting results of
previous studies, the present study investigated the association
between MMP-9 and the pathogenesis of bladder cancer using a
retrospective meta-analysis.
Materials and methods
Literature search
The present study conducted a systematic literature
search of studies published prior to October 2014, using PubMed
(National Center for Biotechnology Information, U.S. National
Library of Medicine, Bethesda, MD, USA), EBSCO Industries, Inc.
(Birmingham, AL, USA), Ovid (New York, NY, USA), SpringerLink
(Berlin, Germany), Wiley (London, UK), Web of Science (New York,
NY, USA), Wanfang (Beijing, China), China National Knowledge
Infrastructure (Beijing, China) and Chongqing VIP Information Co.,
Ltd (Chongqing, China) databases. The literature search was
restricted to retrieving studies published in Chinese and English.
The following search keywords were used: Matrix metalloproteinase;
MMP; bladder cancer; urinary bladder neoplasms; neoplasms; bladder;
malignant tumor of urinary bladder; cancer of bladder; bladder
tumors; and urinary bladder cancer.
Study selection criteria
Published studies were selected based on the
following inclusion criteria: The studies were case-control
studies; the case group contained pathology-verified bladder cancer
patients and the control group consisted of healthy individuals;
the detection methods were enzyme-linked immunosorbent assays
(ELISA) and immunohistochemistry (IHC); and the end results were
defined as expression rates and protein levels of MMP-9 in bladder
cancer tissues. The exclusion criteria were as follows: Studies
that were letters, reviews and meta-analyses; studies that were not
associated with MMP-9 and bladder cancer; non-human studies; and
studies with incomplete data. When there were multiple studies
written by the same author, the study with the most applicable
information and largest number of cases was used.
Statistical analysis
Odds ratio (OR) at 95% confidence intervals (CI)
were calculated using the fixed or random effects model. The
significance of the overall effect size was estimated by the Z-test
(21). Forest plots were created to
demonstrate the differences in the OR at 95% CI between groups. The
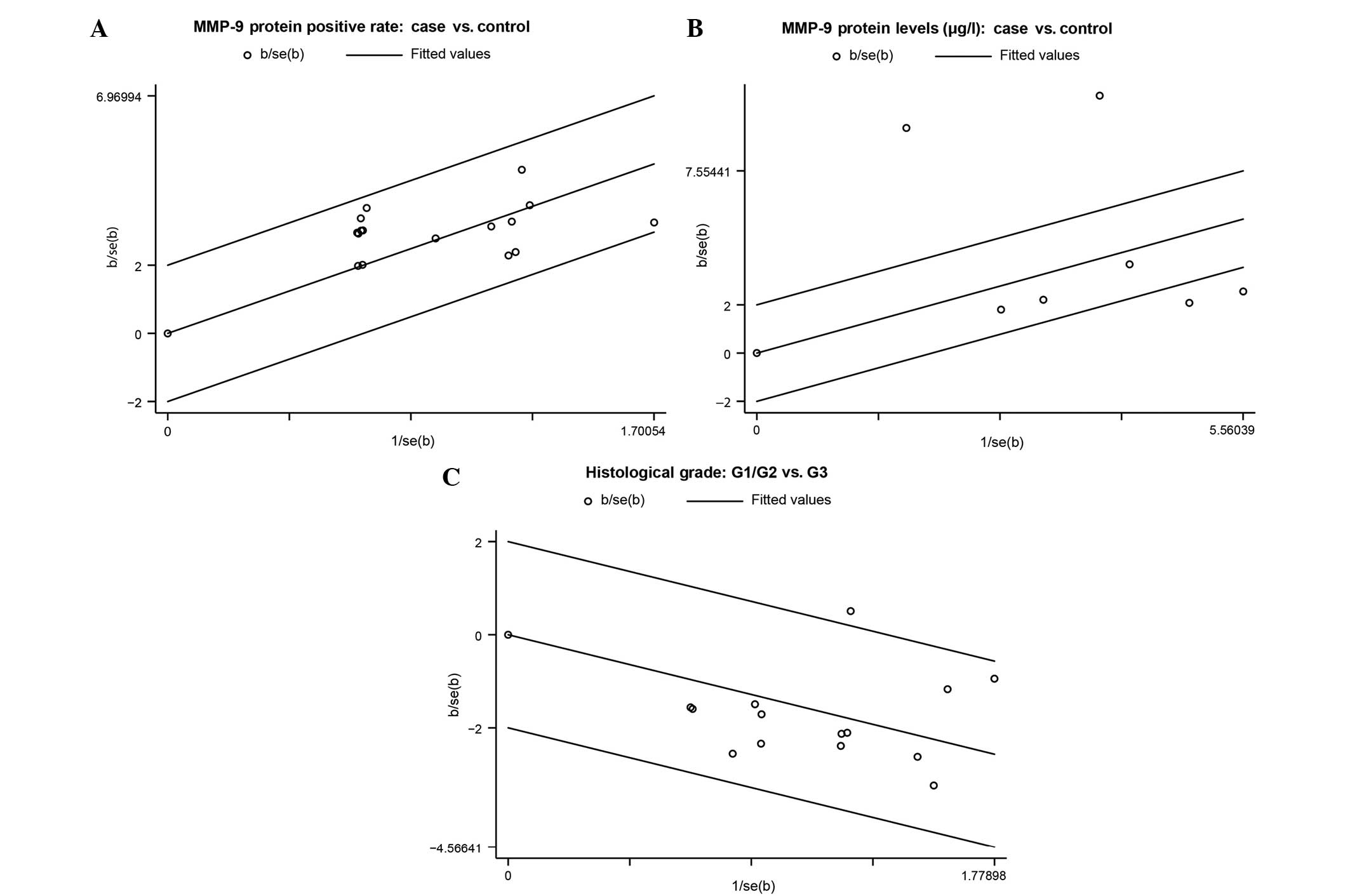
heterogeneity was assessed using a Galbraith radial plot (22) and Cochran's Q-test (21); heterogeneity was regarded as
Ph<0.05. A Galbraith radial plot is a scatter plot of
standardized estimates against reciprocals of standard errors,
possibly with respect to a transformed scale, designed so that the
original estimates may be compared and interpreted. Heterogeneity
is indicated if the dot lies beyond the 95% CI, otherwise no
heterogeneity existed. In addition, I2 test was used to
evaluate the degree of heterogeneity (24). If there was heterogeneity among
studies (Ph<0.05 or I2>50%), a random
effects model was applied; otherwise a fixed effects model was used
(25). Sensitivity analysis was
conducted to estimate the effects of a single study on the overall
results. Publication bias was evaluated by classic fail-safe N,
funnel plots and Egger's test (26–28). All
statistical analyses were performed using Comprehensive
Meta-Analysis software version 2.0 (Biostat, Inc., Englewood, NJ,
USA).
Results
Baseline characteristics
The present literature search initially retrieved
358 relevant articles, 132 of which were studies published in
Chinese and 253 were studies published in English. In total, 299 of
the studies were not included, as follows: Duplicates, n=8; letters
or reviews, n=12; non-human studies, n=15; and not relevant to the
present study topic, n=264. The full text of the remaining studies
(n=86) was reviewed. Additional studies were excluded as they were
not case-control or cohort studies (n=19), were irrelevant to the
present study (n=4) or possessed incomplete data (n=1). Overall, 23
case-control studies (15,18–20,29–47),
published between 1998 and 2014, were selected for meta-analysis,
and consisted of 1,040 bladder cancer patients and 244 healthy
control individuals. Among the 23 studies, the study subjects in 3
studies were African, 14 studies enrolled Asian subjects and 6
studies enrolled Caucasian subjects. All sample sources from the
study subjects were tissues, and the detection methods consisted of
ELISA and IHC. Baseline characteristics of the studies are
presented in Table I.
 | Table I.Characteristics of studies on the
levels of MMP-9 protein expression in bladder cancer patients and
healthy control individuals, and the expression levels of MMP-9 in
patients with a histological G1/G2 grade tumor compared with
patients with a G3 grade tumor. |
Table I.
Characteristics of studies on the
levels of MMP-9 protein expression in bladder cancer patients and
healthy control individuals, and the expression levels of MMP-9 in
patients with a histological G1/G2 grade tumor compared with
patients with a G3 grade tumor.
|
|
|
|
|
| Gender, n | Positive MMP-9
expression, n | Histological grade
of tumors, n | MMP-9 levels,
µg/l |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author, year
(Ref.) | Country | Diagnosis | Detection
method | Mean (range) age,
years | Male | Female | Patients | Controls | G1/G2 | G3 | Patients | Controls | Ref. |
|---|
| Zhao et al,
2014 | China | BUCC | IHC | 62.2 (37–81) | 48 | 12 | 60 | 15 | 47 | 13 | 0 | 0 | (29) |
| Eissa et al,
2013 | Egypt | BUCC | IHC | 61.5 (26–83) | 35 | 11 | 46 | 20 | 36 | 10 | 0 | 0 | (15) |
| Ramón de Fata et
al, 2013 | Spain | BC | ELISA | NA | NA | NA | 0 | 0 | 0 | 0 | 31 | 11 | (41) |
| Gunes et al,
2013 | Turkey | BC | ELISA | 57.0 (40–67) | 57 | 33 | 0 | 0 | 0 | 0 | 90 | 40 | (18) |
| Zheng et al,
2012 | China | BTCC | IHC | 62.7 (30–84) | 24 | 16 | 40 | 10 | 28 | 12 | 0 | 0 | (42) |
| Urquidi et
al, 2012 | USA | BC | ELISA | 69.5 (22–90) | 55 | 9 | 0 | 0 | 0 | 0 | 64 | 63 | (19) |
| Goodison et
al, 2012 | USA | BC | ELISA | 69.5 (22–90) | 55 | 9 | 0 | 0 | 0 | 0 | 64 | 41 | (20) |
| Chen et al,
2011 | China | BUCC | IHC | 57.9 (37–76) | 53 | 26 | 79 | 18 | 58 | 21 | 0 | 0 | (39) |
| Zhang et al,
2010 | China | BC | IHC | (43–72) | 44 | 10 | 54 | 15 | 38 | 16 | 0 | 0 | (36) |
| Zhu et al,
2009 | China | BTCC | IHC | 62.7 (–84) | 24 | 16 | 40 | 10 | 28 | 12 | 0 | 0 | (47) |
| Zhu et al,
2009 | China | BTCC | IHC | (38–72) | 32 | 14 | 46 | 14 | 35 | 11 | 0 | 0 | (33) |
| Zhang et al,
2009 | China | BTCC | IHC | 59.2 (35–76) | 52 | 13 | 65 | 10 | 45 | 20 | 0 | 0 | (34) |
| Eissa et al,
2007 | Egypt | BC | ELISA | 57.0 (25–82) | 104 | 50 | 154 | 30 | 0 | 0 | 0 | 0 | (31) |
| Tang et al,
2007 | China | BTCC | ELISA | 59.0 (27–78) | 65 | 21 | 86 | 10 | 62 | 24 | 0 | 0 | (45) |
| Zhong et al,
2006 | China | BTCC | ELISA | 56.4 (31–76) | 31 | 7 | 0 | 0 | 0 | 0 | 38 | 16 | (46) |
| Wu et al,
2005 | China | BTCC | IHC | 64.5 (31–80) | 48 | 8 | 56 | 5 | 40 | 16 | 0 | 0 | (43) |
| Sun et al,
2005 | China | BTCC | ELISA | (32–80) | 54 | 14 | 68 | 10 | 48 | 20 | 38 | 20 | (44) |
| Wang et al,
2004 | China | BC | IHC | 58.0 (26–84) | 50 | 15 | 65 | 19 | 45 | 20 | 0 | 0 | (38) |
| Guo et al,
2004 | China | BTCC | IHC | 57.2 | 44 | 10 | 54 | 10 | 37 | 17 | 0 | 0 | (37) |
| Eissa et al,
2003 | Egypt | BC | ELISA | 58.0 (30–78) | NA | NA | 73 | 21 | 22 | 38 | 0 | 0 | (32) |
| Nutt et al,
2003 | UK | BC | ELISA | NA | NA | NA | 28 | 12 | 0 | 0 | 0 | 0 | (40) |
| Guan et al,
2003 | China | BTCC | ELISA | 62.0 | 37 | 15 | 0 | 0 | 0 | 0 | 52 | 32 | (35) |
| Bianco et
al, 1998 | USA | BC | NA | (40–89) | 51 | 14 | 26 | 15 | 10 | 16 | 0 | 0 | (30) |
Association between MMP-9 and bladder
cancer
The results of the Galbraith radial plot and
Cochran's Q-test demonstrated that the OR estimates for all
included studies were within the 95% CI of the OR from the main
analysis, suggesting no heterogeneity existed in the rate of MMP-9
protein expression between bladder cancer patients and healthy
control individuals (case vs. control, Ph=0.448 and
I2=0.430%; histological G1/G2 vs. G3 grade,
Ph=0.357 and I2=8.58%). Therefore, a fixed
effects model was applied. The MMP-9 protein levels demonstrated
heterogeneity between bladder cancer patients and healthy control
individuals, with 4 studies beyond the 95% CI
(Ph<0.001 and I2=95.212%). Therefore, a
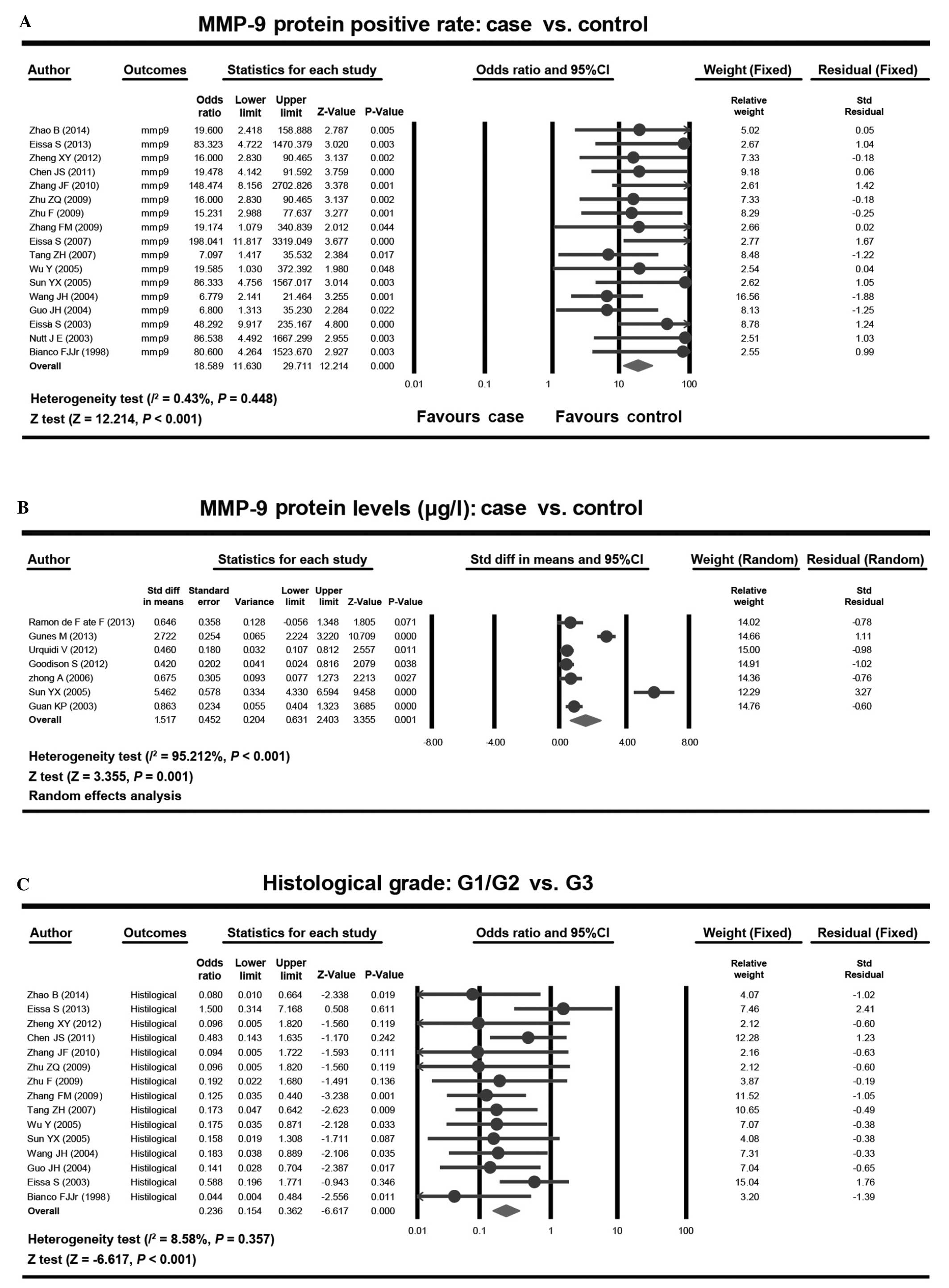
random effects model was used (Fig.
1). Meta-analysis results demonstrated that the expression
rates of MMP-9 in bladder cancer patients were significantly
increased compared with the healthy control individuals (OR,
18.589; 95% CI, 11.630–29.711; P<0.001). MMP-9 protein levels in
patients with bladder cancer were markedly higher compared with the
healthy control individuals, which was at a statistically
significant level [standardized mean difference (SMD), 1.517; 95%
CI, 0.631–2.403; P=0.001]. Furthermore, among histological grades,
the expression rates of MMP-9 in tumor histological G1/G2 grade was
significantly decreased compared with G3 grade (OR, 0.236; 95% CI,
0.154–0.362; P<0.001; Fig. 2).
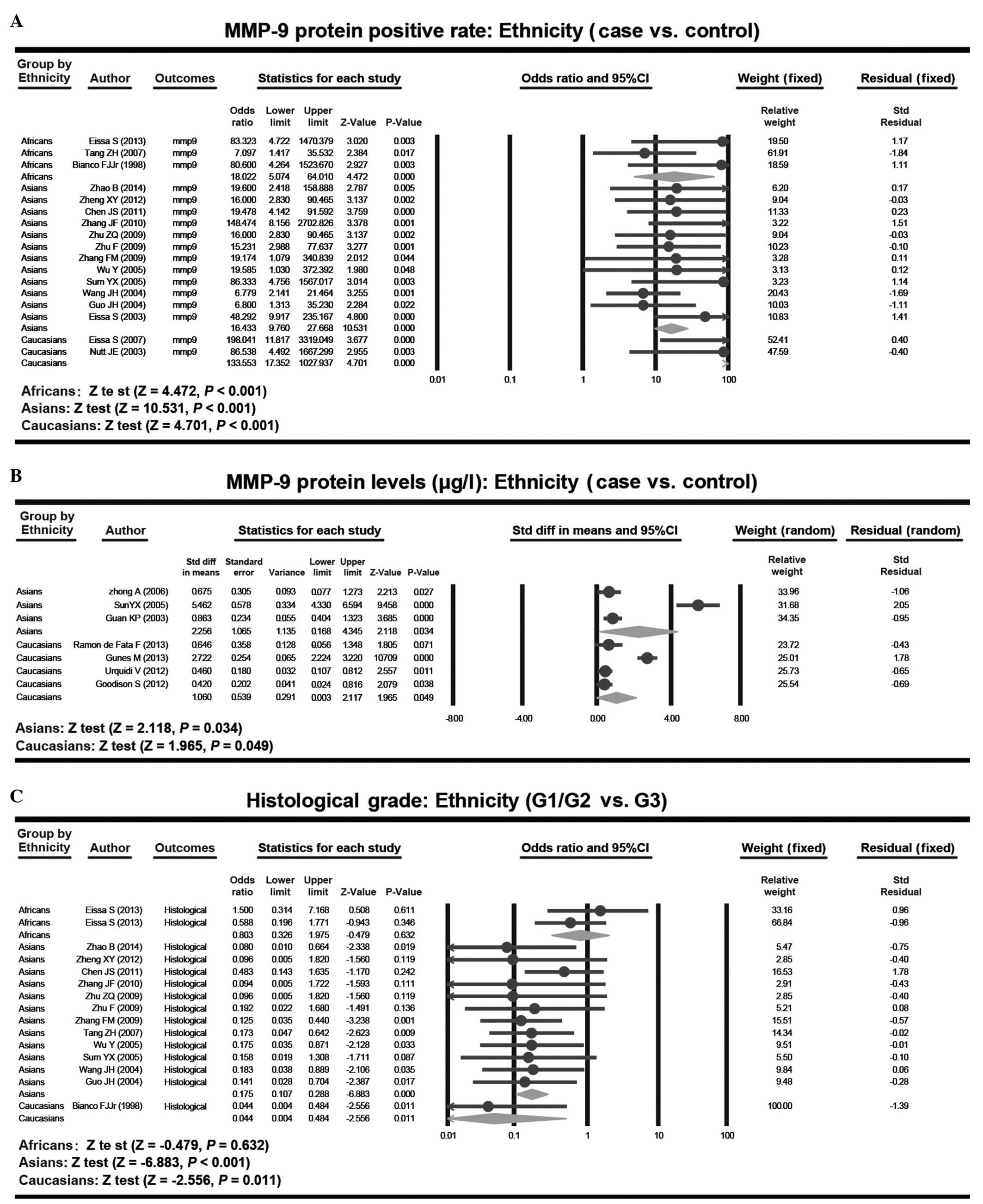
Subgroup analysis based on ethnicity demonstrated that the rates of
MMP-9 protein expression between bladder cancer patients and
healthy controls were significantly different in African (OR,
18.022; 95% CI, 5.074–64.010; P<0.001), Asian (OR, 16.433; 95%
CI, 9.760–27.668; P<0.001) and Caucasian patients (OR, 133.553;
95% CI, 17.352–1027.937; P<0.001). The MMP-9 protein levels in
bladder cancer patients and healthy controls were significantly
different in Asian (SMD, 2.256; 95% CI, 0.168–4.345; P=0.034) and
Caucasian (SMD, 1.060; 95% CI, 0.003–2.117; P=0.049) study
subjects. Furthermore, the expression rates of MMP-9 between G1/G2
and G3 tumors demonstrated significant differences in Asian (OR,
0.175; 95% CI, 0.107–0.288; P<0.001) and Caucasian patients (OR,
0.044; 95% CI, 0.004–0.484; P=0.011), while there was no clear
difference in African patients (OR, 0.803; 95% CI, 0.326–1.975;
P=0.632; Fig. 3).
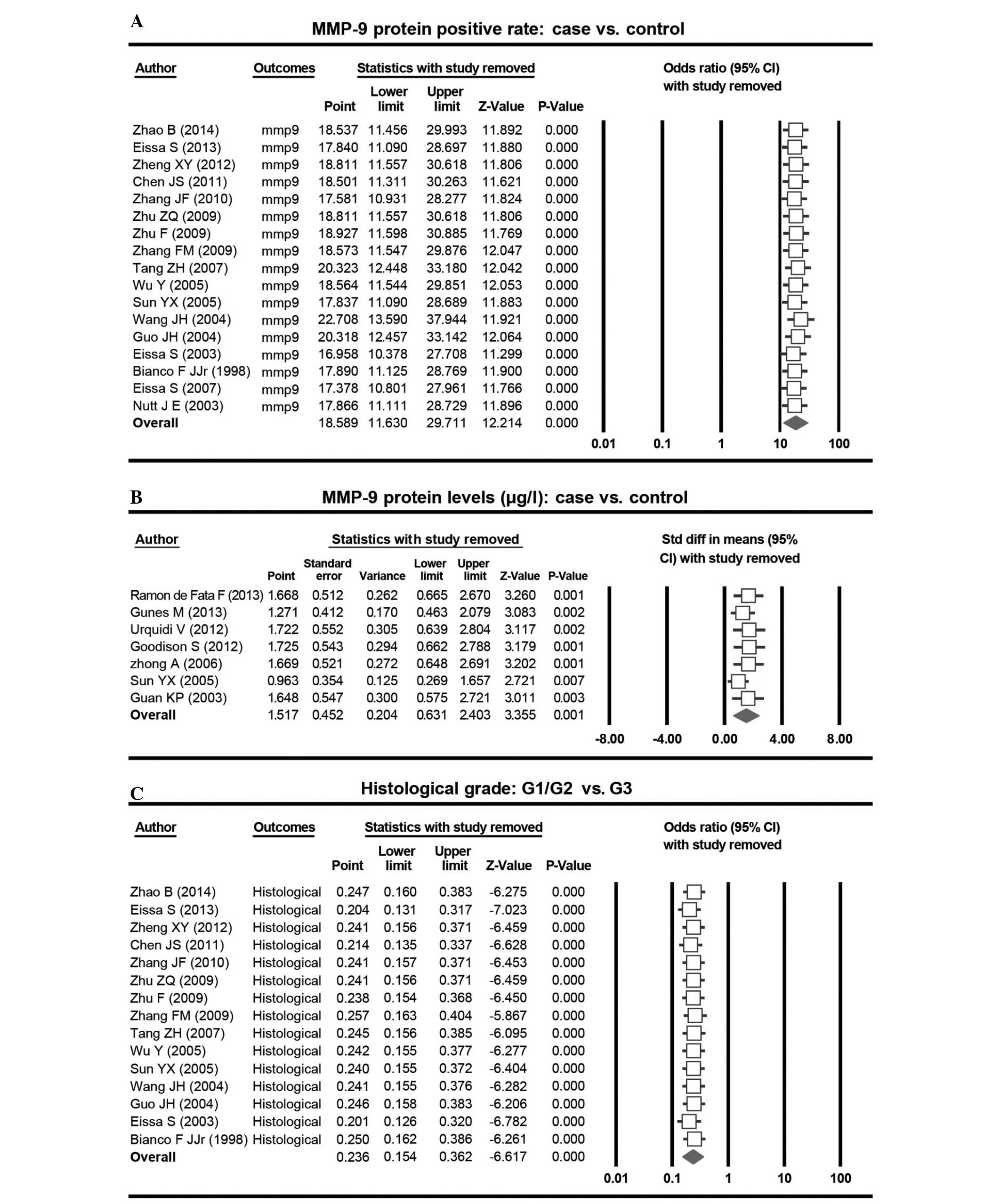
The results of the sensitivity analysis suggested
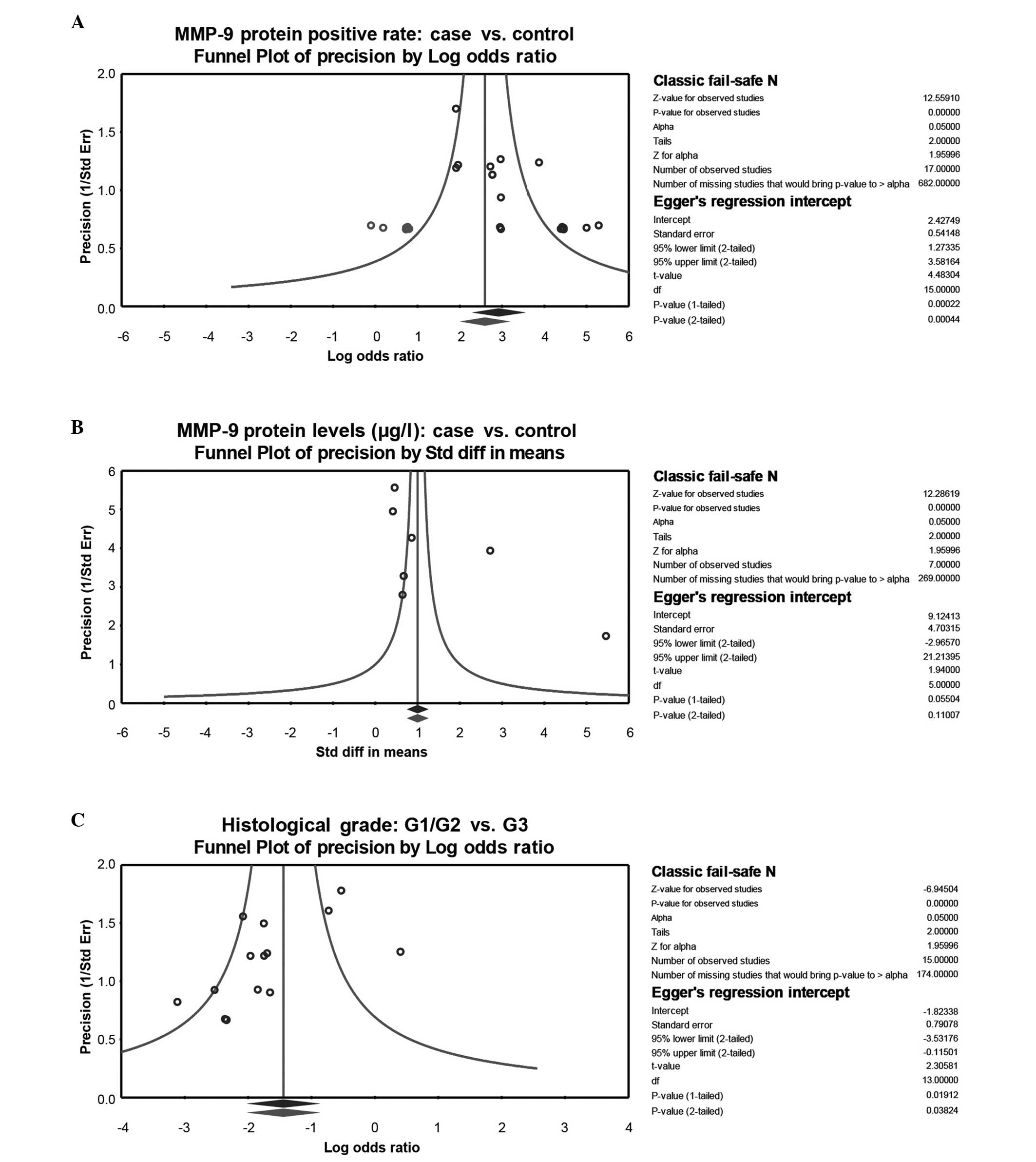
that no study had a significant effect on the polled OR (Fig. 4). The funnel plot demonstrated no
evidence of clear asymmetry, suggesting no publication bias
regarding the MMP-9 protein levels in bladder cancer patients and
healthy individuals. This was additionally confirmed by classic
fail-safe N and the Egger's test (P=0.11). The funnel plot, classic
fail-safe N and Egger's test demonstrated the existence of a
publication bias regarding the expression rates of MMP-9 in bladder
cancer patients compared with the expression rates in the healthy
control individuals (P<0.05). Furthermore, publication bias in
the selected studies, regarding the expression rates of MMP-9 in
histology G1/G2 tumor grade and G3 grade, were identified
(P<0.05; Fig. 5).
Discussion
In the developed world, bladder cancer is the 4th
most common malignancy among men (2).
Epidemiological studies have demonstrated that several
environmental factors may increase the risk of bladder cancer,
including smoking, chronic inflammation, radiation exposure,
anticancer drugs and aromatic amines, which are contained in dyes
(2,6).
The present study conducted a meta-analysis to
investigate the association between MMP-9 and bladder cancer. The
present results revealed that the expression rates of MMP-9 in
bladder cancer patients were significantly increased compared with
the expression rates in the healthy control individuals, suggesting
that the MMP-9 protein is associated with the pathogenesis of
bladder cancer. Furthermore, MMP-9 protein levels in patients with
bladder cancer were significantly increased compared with the
protein levels in the healthy control individuals, suggesting that
the MMP-9 protein may be associated with a risk of bladder cancer.
MMPs degrade various components of the extracellular matrix, which
is important in numerous biological processes, including
embryogenesis and tissue repair (48). However, the degradation of the
extracellular matrix has severe adverse consequences in
pathological conditions; for instance, the increased expression and
activity of MMP-2 have been reported in a variety of pathological
cardiovascular conditions, including hypertension (49–51).
Previous studies indicate that MMPs may not only be involved in
promoting invasion and metastasis, but are vital in the majority of
steps leading to cancer development (52–54). Tumor
cells overproduce MMP-9, leading to the degradation of type IV
collagen and the basement membrane, which disrupts tissue
architecture and function (55). The
present findings are consistent with previous studies, which
suggested that MMP-9 plays a role in bladder cancer (56,57). The
present study identified that the expression rates of MMP-9 in
bladder cancer patients was significantly increased compared with
healthy control individuals (6,9).
The role of MMPs in tumor progression, angiogenesis
and in various inflammatory diseases is well documented (54,58). MMP-9
is mainly expressed by macrophages, neutrophils and mast cells
(41). The inflammatory response is
often associated with advanced neoplasia, which may also induce the
overproduction of MMP-9 (59). The
present results suggest that the serum MMP-9 level is an important
molecular biomarker, which may identify the initiation and predict
the progression of bladder cancer. In addition to the elevated
MMP-9 levels identified in bladder cancer tissue, the present study
observed that the MMP-9 levels were significantly increased in
patients with higher grade tumors. Therefore, MMP-9 is associated
with the clinicopathological features of bladder cancer.
The present study also conducted subgroup analysis
based on ethnicity. The present results revealed that the rate of
MMP-9 protein expression between bladder cancer patients and
healthy control individuals was significantly different in African,
Asian and Caucasian patients. However, the MMP-9 protein levels in
bladder cancer patients and healthy control individuals were
significantly different between Asian and Caucasian study subjects,
but not between Asian or Caucasian and African study subjects. The
expression rate of MMP-9 between G1/G2 and G3 grade tumors was
similar, which may be due to the small sample size in the present
meta-analysis.
There are limitations in the present meta-analysis
that should be acknowledged. First, the sample size was relatively
small; therefore the present results lacked sufficient statistical
power to fully investigate the association between MMP-9 expression
and the pathogenesis of bladder cancer. Second, of the 23 eligible
studies, the majority of studies (n=14) were performed using the
Chinese population, therefore indicating selection bias. Additional
studies using a larger sample size are required to provide a more
accurate statistical analysis that is representative of the world
population.
In conclusion, the results of the present
meta-analysis demonstrated that MMP-9 is associated with the
clinicopathological features of bladder cancer, suggesting that
MMP-9 may be used in combination with other tumor-specific markers,
which may improve the sensitivity of diagnosis and treatment of
bladder cancer.
Glossary
Abbreviations
Abbreviations:
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
MMP
|
matrix metalloproteinase
|
|
IHC
|
immunohistochemistry
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Menashe I, Figueroa JD, Garcia-Closas M,
Chatterjee N, Malats N, Picornell A, Maeder D, Yang Q,
Prokunina-Olsson L, Wang Z, et al: Large-scale pathway-based
analysis of bladder cancer genome-wide association data from five
studies of European background. PLoS One. 7:e293962012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar B, Koul S, Petersen J, Khandrika L,
Hwa JS, Meacham RB, Wilson S and Koul HK: p38 mitogen-activated
protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer
by modulation of MMP-2 and MMP-9 activity. Cancer Res. 70:832–841.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gui Y, Guo G, Huang Y, et al: Frequent
mutations of chromatin remodeling genes in transitional cell
carcinoma of the bladder. Nat Genet. 43:875–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samplaski MK, Heston W, Elson P,
Magi-Galluzzi C and Hansel DE: Folate hydrolase (prostate-specific
membrane [corrected] antigen) 1 expression in bladder cancer
subtypes and associated tumor neovasculature. Mod Pathol.
24:1521–1529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan Y, Liang H, Li T, Li M, Li R, Qin X
and Li S: The MMP-1, MMP-2, and MMP-9 gene polymorphisms and
susceptibility to bladder cancer: A meta-analysis. Tumour Biol.
35:3047–3052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Said N, Frierson HF, Sanchez-Carbayo M,
Brekken RA and Theodorescu D: Loss of SPARC in bladder cancer
enhances carcinogenesis and progression. J Clin Invest.
123:751–766. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuo W, Zhang L, Cai L, Zhu B and Chen Z:
XRCC1 Arg399Gln polymorphism and bladder cancer risk: Updated
meta-analyses based on 5767 cases and 6919 controls. Exp Biol Med
(Maywood). 238:66–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Offersen BV, Knap MM, Horsman MR,
Verheijen J, Hanemaaijer R and Overgaard J: Matrix
metalloproteinase-9 measured in urine from bladder cancer patients
is an independent prognostic marker of poor survival. Acta Oncol.
49:1283–1287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacobs BL, Lee CT and Montie JE: Bladder
cancer in: 2010 How far have we come? CA Cancer J Clin. 60:244–272.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burger M, Oosterlinck W, Konety B, Chang
S, Gudjonsson S, Pruthi R, Soloway M, Solsona E, Sved P, Babjuk M,
et al: International Consultation on Urologic Disease-European
Association of Urology Consultation on Bladder Cancer: 2012 ICUD
EAU International Consultation on Bladder Cancer 2012:
Non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol.
63:36–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
13
|
Zitka O, Kukacka J, Krizkova S, Huska D,
Adam V, Masarik M, Prusa R and Kizek R: Matrix metalloproteinases.
Curr Med Chem. 17:3751–3768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Surgucheva I, Chidambaram K, Willoughby DA
and Surguchov A: Matrix metalloproteinase 9 expression: New
regulatory elements. J Ocul Biol Dis Infor. 3:41–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eissa S, Badr S, Elhamid SA, Helmy AS,
Nour M and Esmat M: The value of combined use of survivin mRNA and
matrix metalloproteinase 2 and 9 for bladder cancer detection in
voided urine. Dis Markers. 34:57–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gunes M, Kemik AS, Pirincci N, Gecit I,
Taken K, Yuksel MB, Kaba M and Eryilmaz R: Preoperative levels of
matrix metalloproteinase-7 and −9 and tissue inhibitor of matrix
metalloproteinase-1 relation to pathologic parameters in bladder
carcinoma patients. Asian Pac J Cancer Prev. 14:873–876. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urquidi V, Chang M, Dai Y, Kim J, Wolfson
ED, Goodison S and Rosser CJ: IL-8 as a urinary biomarker for the
detection of bladder cancer. BMC Urol. 12:122012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goodison S, Chang M, Dai Y, Urquidi V and
Rosser CJ: A multi-analyte assay for the non-invasive detection of
bladder cancer. PLoS One. 7:e474692012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Manning AK and Dupuis J: A method
of moments estimator for random effect multivariate meta-analysis.
Biometrics. 68:1278–1284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jackson D, White IR and Riley RD:
Quantifying the impact of between-study heterogeneity in
multivariate meta-analyses. Stat Med. 31:3805–3820. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sterne JA and Egger M: Funnel plots for
detecting bias in meta-analysis: Guidelines on choice of axis. J
Clin Epidemiol. 54:1046–1055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wikstrom EA, Naik S, Lodha N and Cauraugh
JH: Balance capabilities after lateral ankle trauma and
intervention: A meta-analysis. Med Sci Sports Exerc. 41:1287–1295.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Egger M, Smith Davey G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao B, Wang Q, Lei Y, Zhang G, Li R and
Song Z: Study on the expression of MMP-9 and CD34 in bladder
urothelial carcinoma and their correlation. J Clin Res.
31:1518–1521. 2014.(In Chinese).
|
|
30
|
Bianco FJ Jr, Gervasi DC, Tiguert R,
Grignon DJ, Pontes JE, Crissman JD, Fridman R and Wood DP Jr:
Matrix metalloproteinase-9 expression in bladder washes from
bladder cancer patients predicts pathological stage and grade. Clin
Cancer Res. 4:3011–3016. 1998.PubMed/NCBI
|
|
31
|
Eissa S, Ali-Labib R, Swellam M, Bassiony
M, Tash F and El-Zayat TM: Noninvasive diagnosis of bladder cancer
by detection of matrix metalloproteinases (MMP-2 and MMP-9) and
their inhibitor (TIMP-2) in urine. Eur Urol. 52:1388–1396. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eissa S, Labib RA, Mourad MS, Kamel K and
El-Ahmady O: Comparison of telomerase activity and matrix
metalloproteinase-9 in voided urine and bladder wash samples as a
useful diagnostic tool for bladder cancer. Eur Urol. 44:687–694.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu F, Zhang Y, Yan R, Zhang HQ and Zhang
YJ: Correlation of expressions of MMP-2 and MMP-9 in the
transitional cell carcinoma of bladder with tremor invasion and
metastasis. Zhong Liu Yan Jiu Yu Lin Chuang. 21:680–682. 2009.(In
Chinese).
|
|
34
|
Zhang FM, Gao XM, Li SS, Li SB, Liu Y and
Xu H: Expressions of MMP-9 and VEGF in 65 cases with bladder
transitional cell carcinoma and their significance. Zhong Liu Xue
Za Zhi. 15:1032–1034. 2009.(In Chinese).
|
|
35
|
Guan KP, Ye HY, Yan Z, Wang Y and Hou SK:
Serum levels of endostatin and matrix metalloproteinase-9
associated with high stage and grade primary transitional cell
carcinoma of the bladder. Urology. 61:719–723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang JF, Sun LJ, Luo B, Hao J, Song BL
and Liu P: Expression of survivin, MMP-9 and PTEN proteins in
bladder carcinoma. Xian Dai Yu Fang Yi Xue. 37:4681–4682. 2010.(In
Chinese).
|
|
37
|
Guo JH, Wang HR, Wang J, Zhu J and Song
HY: Expression of matrix metalloproteinase-9 and its correlation
with tumor interstitial microvascular density and tumor metastasis
in transitional cell carcinoma of the bladder. Zhong Guo Ai Zheng
Za Zhi. 14:116–118. 2004.(In Chinese).
|
|
38
|
Wang JH, Chen ZF, Chen WB and Shi Z:
Expression of MMP-9 and TIMP-1 in bladder cancer and its
significance. Xian Dai Mi Niao Wai Ke Za Zhi. 9:140–141. 2004.(In
Chinese).
|
|
39
|
Chen JS, Yang WM, Xu YM and Wang SL: The
expression of MMP-9 and cyclin E in bladder urothelial carcinoma
and their clinical significance. Xian Dai Mi Niao Wai Ke Za Zhi.
26:726–731. 2011.(In Chinese).
|
|
40
|
Nutt JE, Durkan GC, Mellon JK and Lunec J:
Matrix metalloproteinases (MMPs) in bladder cancer: The induction
of MMP9 by epidermal growth factor and its detection in urine. BJU
Int. 91:99–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Ramón Fata F, Ferruelo A, Andrés G,
Gimbernat H, Sánchez-Chapado M and Angulo JC: The role of matrix
metalloproteinase MMP-9 and TIMP-2 tissue inhibitor of
metalloproteinases as serum markers of bladder cancer. Actas Urol
Esp. 37:480–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng XY, Pang X, Wang FJ and Zhu ZQ:
Expression of CXCR4 and MMP-9 in bladder transitional cell
carcinoma tissue. Xian Dai Sheng Wu Yi. 12:5255–5260. 2012.(In
Chinese).
|
|
43
|
Wu Y and Zeng FQ: Expression of
MMP-9/TIMP-1 in bladder and the clinical significance transitional
cell carcinoma. Xian Dai Mi Niao Wai Ke Za Zhi. 20:577–580.
2005.(In Chinese).
|
|
44
|
Sun YX, Zhang ZC, Sun LQ and Gao HW:
Correlation of content and expression of matrix metalloproteinases
in transitional cell carcinoma of bladder with tumor invasion and
metastasis. Ji Lin Da Xue Xue Bao (Yi Xue Ban). 31:127–129.
2005.(In Chinese).
|
|
45
|
Tang ZH, Guo SX, Cai QF and Chen TL:
Expressions of MMP-9 and TIMP-1 and their relationship with the
invasion and metastasis of bladder cancer. Guo Ji Mi Niao Xi Tong
Za Zhi. 27:148–152. 2007.(In Chinese).
|
|
46
|
Zhong A, Yang WM and Ye ZQ: The expression
of urinary MMP2 and MMP9 in the bladder transitional cell carcinoma
and their correlations to the tumor invasion and metastasis. Chuang
Wai Ke Za Zhi. 14:655–657. 2006.(In Chinese).
|
|
47
|
Zhu ZQ, Zheng XY, Zhu QH, Wen JH and Fan
CH: Expression and clinical significance of CXCR4 and MMP-9 in
bladder cancer. Zhong Guo Xian Dai Yi Xue Za Zhi. 19:337–340.
2009.(In Chinese), 343.
|
|
48
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3:a0050582011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jacob-Ferreira AL, Lacchini R, Gerlach RF,
Passos CJ, Barbosa F Jr and Tanus-Santos JE: A common matrix
metalloproteinase (MMP)-2 polymorphism affects plasma MMP-2 levels
in subjects environmentally exposed to mercury. Sci Total Environ.
409:4242–4246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Castro MM, Rizzi E, Rodrigues GJ, Ceron
CS, Bendhack LM, Gerlach RF and Tanus-Santos JE: Antioxidant
treatment reduces matrix metalloproteinase-2-induced vascular
changes in renovascular hypertension. Free Radic Biol Med.
46:1298–1307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ceron CS, Castro MM, Rizzi E, Montenegro
MF, Fontana V, Salgado MC, Gerlach RF and Tanus-Santos JE:
Spironolactone and hydrochlorothiazide exert antioxidant effects
and reduce vascular matrix metalloproteinase-2 activity and
expression in a model of renovascular hypertension. Br J Pharmacol.
160:77–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
O-Charoenrat P and Khantapura P: The role
of genetic polymorphisms in the promoters of the matrix
metalloproteinase-2 and tissue inhibitor of metalloproteinase-2
genes in head and neck cancer. Oral Oncol. 42:257–267. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Srivastava P, Mandhani A, Kapoor R and
Mittal RD: Role of MMP-3 and MMP-9 and their haplotypes in risk of
bladder cancer in North Indian cohort. Ann Surg Oncol.
17:3068–3075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sand JM, Larsen L, Hogaboam C, Martinez F,
Han M, Larsen Røssel M, Nawrocki A, Zheng Q, Karsdal MA and Leeming
DJ: MMP mediated degradation of type IV collagen alpha 1 and alpha
3 chains reflects basement membrane remodeling in experimental and
clinical fibrosis - validation of two novel biomarker assays. PLoS
One. 8:e849342013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Reis ST, Leite KR, Piovesan LF,
Pontes-Junior J, Viana NI, Abe DK, Crippa A, Moura CM, Adonias SP,
Srougi M and Dall'Oglio MF: Increased expression of MMP-9 and IL-8
are correlated with poor prognosis of Bladder Cancer. BMC Urol.
12:182012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kumar B, Koul S, Petersen J, Khandrika L,
Hwa JS, Meacham RB, Wilson S and Koul HK: p38 mitogen-activated
protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer
by modulation of MMP-2 and MMP-9 activity. Cancer Res. 70:832–841.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stankovic S, Konjevic G, Gopcevic K, Jovic
V, Inic M and Jurisic V: Activity of MMP-2 and MMP-9 in sera of
breast cancer patients. Pathol Res Pract. 206:241–247. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zamilpa R, Ramirez TA, Dai Q, Dayah T,
Nguyen N, Zhang J, Ahuja SS, D'Armiento, Jin YF and Lindsey ML:
MMP-9 overexpression in macrophages regulates the post-myocardial
infarction inflammatory response through SCYE1. FASEB J. 26(Suppl):
399.22012.
|