Introduction
Neurenteric cysts (NCs) are uncommon congenital
cystic lesions of presumed ectodermal origin that rarely appear in
the cranio-spinal axis. They are characterized by cyst walls that
are lined with a simple or pseudostratified, ciliated or
non-ciliated, cuboidal or columnar epithelium, with basement
membranes that resemble those of the respiratory or intestinal
tract (1). The precise pathogenesis
of NCs remains controversial (1,2). NCs are
usually located in the spinal subdural space, particularly in the
lower cervical and upper thoracic regions (3). Intracranial NCs are relatively
infrequently reported, compared with the incidence of intraspinal
cases (4). To the best of our
knowledge, no more than 100 cases of intracranial NC have been
reported in the literature (1,4,5).
Surgery is the primary treatment for NC, and gross
total resection of the lesion with careful protection of the
surrounding structures is recommended (6). In the majority of cases, this rare
benign lesion carries a favorable prognosis following surgical
intervention (6–8). However, in some cases the remnants of
the NCs, which occur due to subtotal or partial resection caused by
adhesion between the NCs and surrounding structures, may develop
into tumor recurrences (6).
Therefore, close follow-up with radiological examination is
required, particularly for patients for whom total resection of the
lesion was not achieved.
Compared with the incidence of recurrent NCs, the
malignant transformation of NCs is extremely rare. To the best of
our knowledge, very few reports have described this unusual
phenomenon (9–16). Therefore, the demographic
characteristics, treatment and prognosis of this type of NC is not
clear. The objective of the present study was to analyze the
clinical, pathological and radiological characteristics of
malignant-transformed NCs based on the data from the present case
combined with previous literature. In addition, strict criteria for
malignant-transformed NCs were defined. Written informed consent
was obtained from the patient.
Case report
On December 2, 2008, a 58-year-old woman presented
to the Beijing Tiantan Hospital (Beijing, China), with a 5-month
history of occasional headaches, dizziness and vomiting. The
symptoms progressively worsened, and the patient began to suffer
from intermittent dysphagia and an abnormal gait. A neurological
examination revealed palsy of the left cranial IX and X nerves,
decreased sensation in the limbs on the right side and an ataxic
gait. The patient had undergone a left suboccipital retrosigmoid
craniotomy to remove a lesion in the left cerebello-pontine angle 8
years previously in a local hospital. The lesion was pathologically
diagnosed as an NC, as detailed radiological and histopathological
data were not available. Magnetic resonance imaging (MRI; MAGNETOM
Trio 3.0T superconducting magnetic resonance imager; Siemens AG,
Munich, Germany) scans revealed a cystic mass in the left side of
the foramen magnum that was located anteriolaterally to the medulla
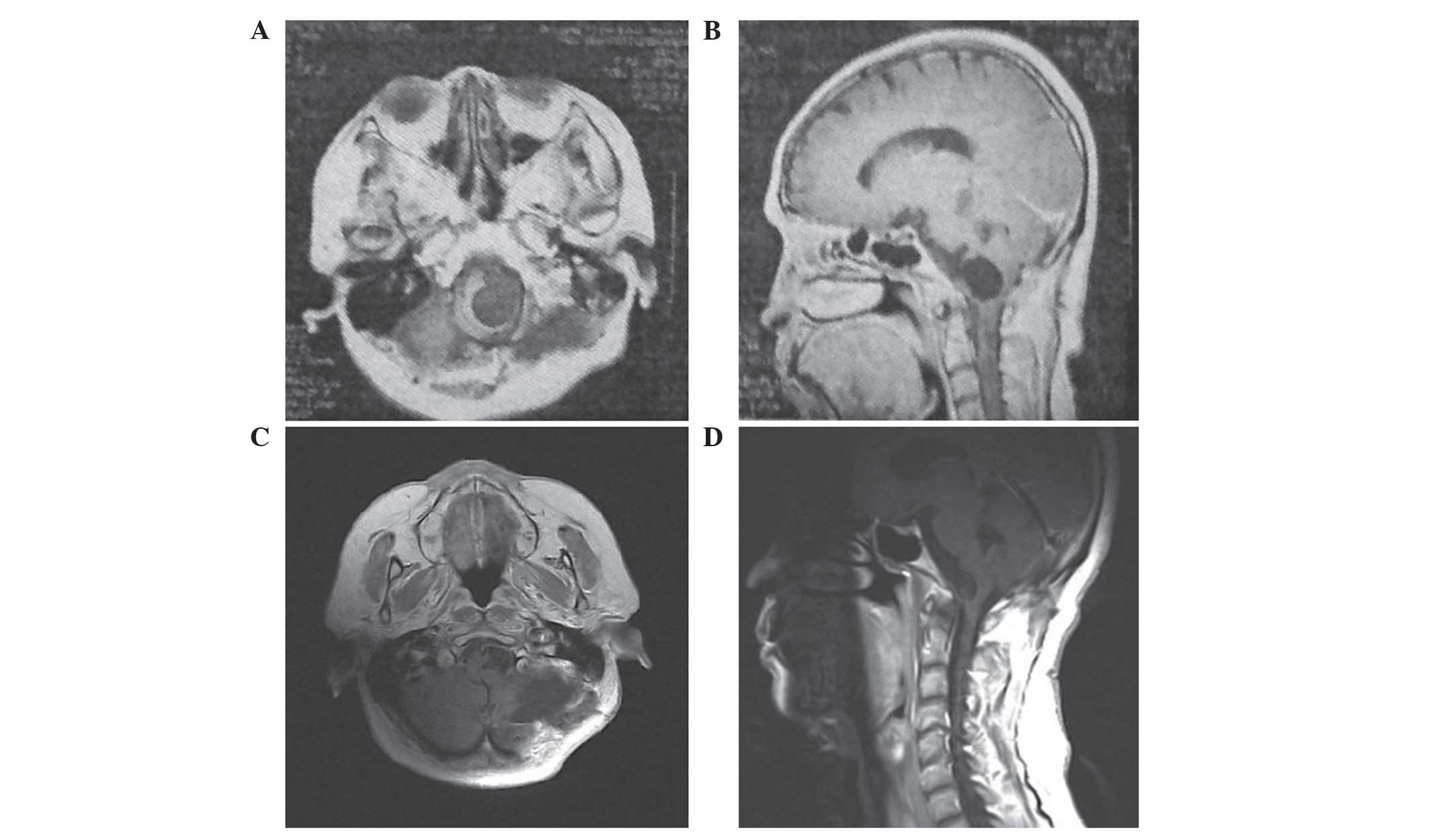
oblongata (Fig. 1). The patient
underwent a second craniotomy using the left suboccipital
retrosigmoid approach at the Beijing Tiantan Hospital. Following
the removal of the cyst contents, the cyst walls were partially
resected due to adhesion between the cyst wall and medulla
oblongata. The pathological diagnosis was of an NC. The numbness in
the right limbs and the left lower cranial nerve palsy improved
without surgical morbidity.
At 22 months after the second craniotomy, the
patient presented again with intermittent headaches and dizziness.
A radiological examination revealed supratentorial hydrocephalus. A
right ventriculo-peritoneal shunt was performed, and subsequent
improvement of the neurological symptoms was observed.
At 23 months after the second craniotomy, the
patient presented with a severe headache, projectile vomiting,
diplopia and limb fatigue. A neurological examination detected
significant palsy of the left VI and VII cranial nerves, grade IV
limb muscle strength and a positive Romberg test result, with the
patient falling to the left.
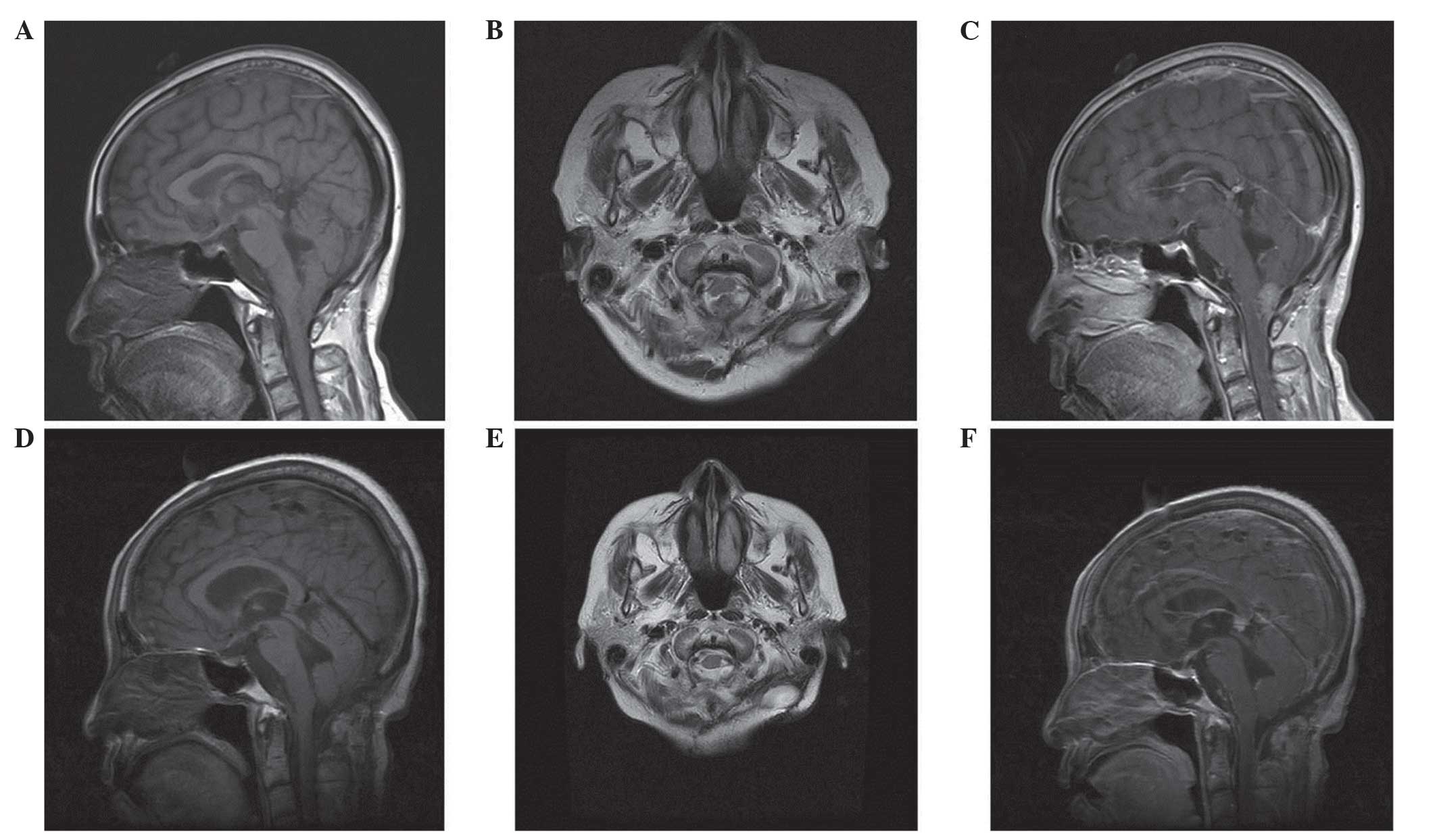
Additional MRI scans demonstrated a solid lesion
posterior to the medullar oblongata, with isointensity on the T1-
and the T2-weighted images and homogeneous enhancement following
the administration of gadolinium (Gd; Fig. 2). The lesion compressed the medullar
oblongata. No brain parenchymal edema surrounding the lesion was
noted. A total-body computed tomography scan was performed due to
the possibility of an extracranial primary neoplasm, and no
evidence of tumors in other organs or metastases was
identified.
At 25 months after the second surgery in the Beijing
Tiantan Hospital, the patient underwent a third craniotomy using a
suboccipital midline approach to relieve the brainstem compression.
Intra-operatively, the solid lesion was soft with a moderate blood
supply. A subtotal resection of the tumor and a cyst evacuation
were performed, as a total resection was not possible. Subsequent
to the resection of the lesion, the infiltration of the medulla
oblongata by the lesion was observed.
The upper limb muscle strength of the patient
improved to grade V following the surgery, and the other symptoms
were stabilized without novel neurological deficits. The patient
was discharged at 15 days post-surgery with a good cough reflex.
The follow-up revealed that after 1 month, the patient began to
experience a decreased level of consciousness and ultimately
succumbed to respiratory failure in hospital.
The specimens from the second and third surgeries
were fixed in 10% formalin and embedded in paraffin. The 4-µm thick
sections were cut and stained with hematoxylin and eosin. Selected
sections were also immunostained using the following primary
antibodies: glial fibrillary acidic protein (GFAP; monoclonal mouse
immunoglobulin (Ig) G anti-rat/chicken/human/pig; dilution, 1:100;
catalog no., MA5-12023; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), epithelial membrane antigen (EMA; monoclonal Armenian
hamster IgG anti-mouse/human; dilution, 1:50; catalog no.,
MA5-11202; Thermo Fisher Scientific, Inc.), carcinoembryonic
antigen (CEA; monoclonal mouse IgG anti-human; dilution, 1:50;
catalog no., MA5-15070 Thermo Fisher Scientific, Inc.), S-100
protein (monoclonal mouse IgG anti-human/rat/mouse/cattle;
dilution, 1:50; catalog no., MA5-12966; Thermo Fisher Scientific,
Inc.), thyroid transcription factor-1 (TTF-1; monoclonal mouse IgG
anti-human/mouse/rat; dilution, 1:100; catalog no., MA5-13961
Thermo Fisher Scientific, Inc.) and Ki-67 (MIB-1; monoclonal rabbit
IgG anti-human; dilution, 1:50; catalog no., RM9106R1; Labvision,
Fremont, CA, USA).
The histological examination of the entire specimen
from the second surgery revealed that the cyst wall was lined with
single-layer or pseudostratified columnar and non-ciliated
epithelial cells, mixed with goblet and mucin-producing cells, but
without malignant components (Fig.
3A). Single-layer ciliated epithelial cells were also observed
in certain regions (Fig. 3A and B).
Focal hyperplasia of the epithelial cells, which was similar to a
papillary structure, was observed in certain regions (Fig. 3C); however, malignant characteristics,
including nuclear pleomorphisms and frequent mitosis, were not
observed. The immunohistological staining revealed that the cells
expressed EMA and CEA, and did not express GFAP, S-100, vimentin
(Vim) or TTF-1, which confirmed the endodermal origin of the cyst.
The immunohistochemical staining for the Ki-67 proliferation marker
was not expressed in any of the cells in the specimen (Fig. 3D). Based upon these findings, the
pathological diagnosis of a typical NC without malignant
transformation was made.
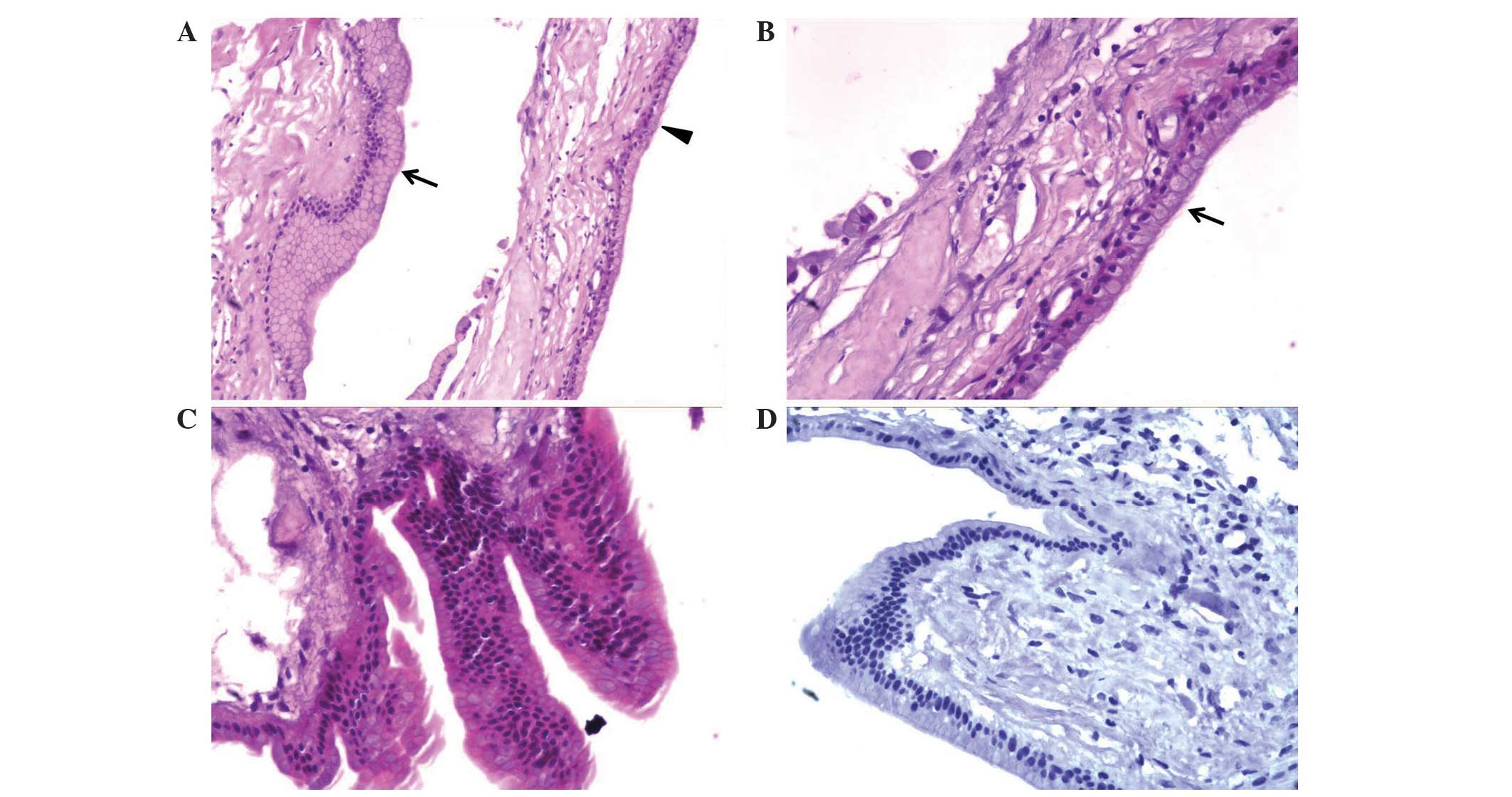 | Figure 3.Histopathological and
immunohistochemical features of the specimen obtained in the
patient's second craniotomy. (A) The cyst wall was lined with
single-layer or pseudostratified columnar and non-ciliated
epithelial cells with goblet and mucin-producing cells (arrow). In
certain regions, a single layer of ciliated epithelial cells was
observed (arrowhead). Original magnification, ×100. (B)
Single-layer ciliated epithelial cells without a malignant
component were observed. Original magnification, ×200. (C) Focal
hyperplasia of the epithelial cells, which is similar to the
papillary structure, was observed in certain regions, and malignant
characteristics, including nuclear pleomorphisms and frequent
mitosis, were not detected. Original magnification, ×200. (D)
Immunohistochemical staining for the Ki-67 proliferation marker did
not reveal any positively stained cells in the specimen. Original
magnification, ×200. |
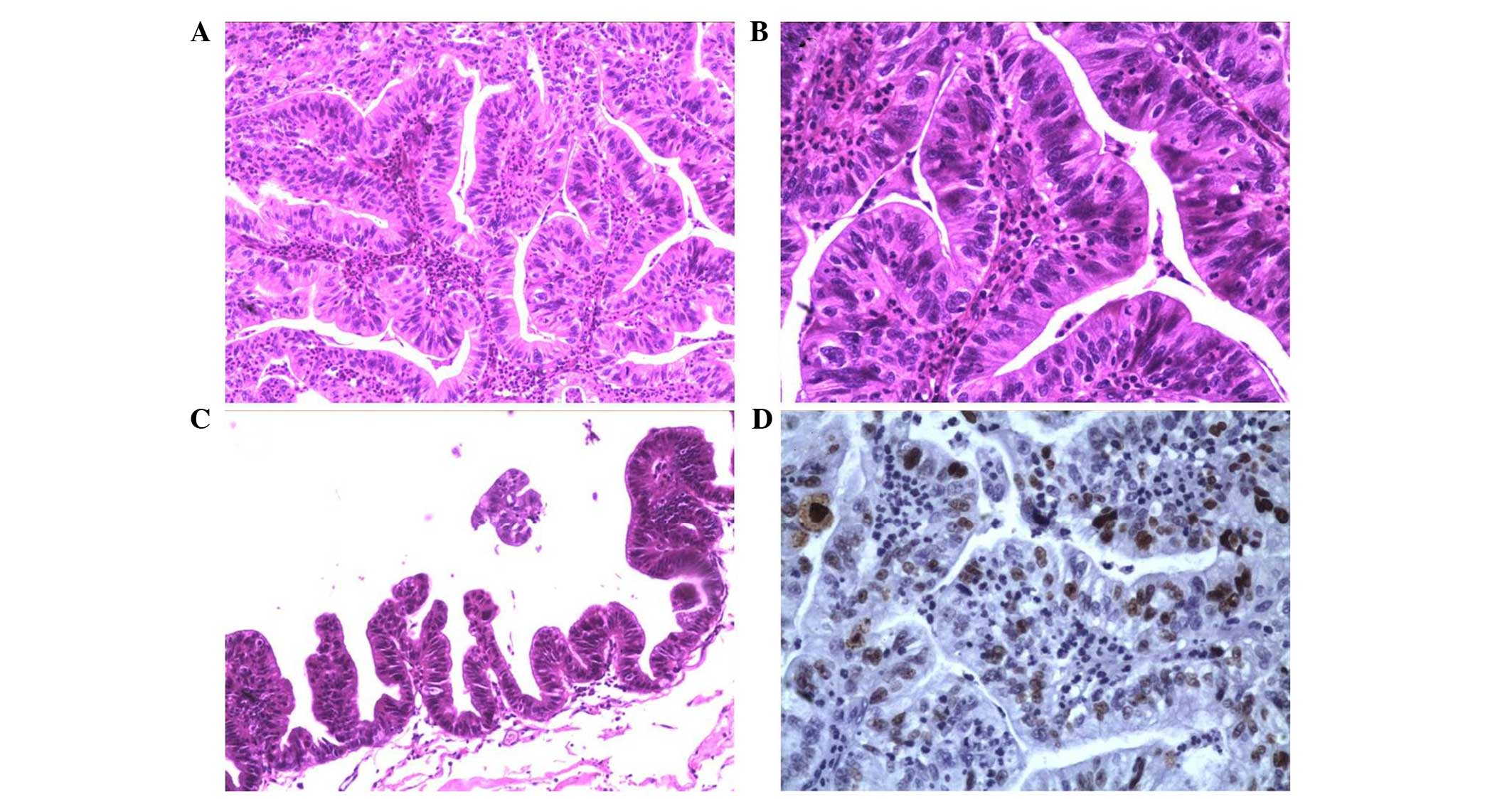
A histological examination of the tissue that was
removed during the final craniotomy of the patient revealed
well-formed papillae with prominent fibrovascular cores that were
lined by malignant epithelial cells containing enlarged
hyperchromatic nuclei with high nuclear-to-cytoplasmic ratios and
nuclear abnormalities. Mitotic figures were easily observed
(Fig. 4A and B). Frequent irregular
folds of the dysplastic epithelial cells that showed a tendency to
create well-formed papillae structures were observed in certain
regions (Fig. 4C). The EMA and CEA
immunohistochemical staining was more prominent in this final
specimen compared with the prior surgical specimen.
Immunohistochemical staining revealed that GFAP, Vim and TTF-1 were
not expressed. The Ki-67 proliferative index had increased to
20–40% (Fig. 4D). The pathological
diagnosis was of a well-differentiated papillary adenocarcinoma
that originated from an initially benign NC.
Discussion
NCs, also called enterogenous (13,15),
enterogenic (17), endodermal
(14) or archenteric (18) cysts, are congenital lesions of
presumed ectodermal origin that rarely appear in the cranio-spinal
axis. The precise pathogenesis of NCs remains unclear. The most
popular hypothesis is that NCs develop from the failing separation
between the foregut or the respiratory buds and the notochord
during alimentary canal formation (1). A total surgical resection of the lesion
is the most effective therapeutic method, as the residual cyst
walls, particularly walls that are large enough to overlap, may
cause recurrence (1,6,8). Although
recurrence and dissemination are not uncommon in NCs (19–21), the
malignant transformation of NC is extremely rare.
Hamlat et al (22) and Bejjani et al (23) defined the criteria for the malignant
transformation of intracranial epidermoid and dermoid cysts;
however, no strict criteria for the malignant transformation of NCs
have been previously developed. In order to determine the behavior
of these lesions, previously published reports regarding
intracranial and intraspinal malignant-transformed NCs were
reviewed, following strict criteria for the selection of cases: The
tumor must be restricted to the intracranial or intraspinal
intradural compartment; the malignant transformation of a primary
NC must have a benign NC wall component; and the malignant
transformation in a recurrent NC must have preexisting
pathologically confirmed NC. Metastasized carcinomas were excluded.
Following these criteria, it was found that 8 cases (9–16) of
malignantly transformed NCs have been described to date (Table I). In addition, malignant
transformations were also identified in NCs outside of the
cranio-spinal axis. Nakayama et al (24) reported a mucinous cyst adenocarcinoma
in the sigmoid colon that was considered to originate from a
NC.
 | Table I.Clinical data from the published
literature regarding intracranial neurenteric cysts with malignant
transformations. |
Table I.
Clinical data from the published
literature regarding intracranial neurenteric cysts with malignant
transformations.
| First author,
year | Age,
yearsa/gender | P or R | PFT, months/T | Primary site | Cyst location | MT phenotype | Expressed in
IHC | Not expressed in
IHC | Therapy | FU, months | Outcome | Ref. |
|---|
| Present study | 50/F | R | 22/GTR | Left CPA | Posterior to
MO | Papillary
adenocarcinoma | CEA, EMA,
Ki-67=20–40%, | TTF-1, Vim,
GFAP | STR | 1 | Succumbed |
|
| Okabe et al,
2014 | Late 50s/F | R | 24/PR | Right cerebral
hemisphere | In situ | Adenocarcinoma | CK5, MUC5AC, p63
Ki-67>80% | CDX-2, CK20, MUC2,
MUC6, TTF-1, p16, p53 | GTR, PR | 3 | Stable | (9) |
| Surash et
al, 2009 | 46/M | R | 168/GTR | Right CPA | Left CPA | Adenocarcinoma | Ki-67>20% | – | PR, RT (50.4
Gy) | 10 | Stable | (10) |
| Dunham et
al, 2009 | 58/F | P | – | – | Right parietal
lobe | Invasive mucinous
papillary cystadenocarcinoma | EMA, CAM 5.2, CEA,
CK19(f), CK7(f), CK20(f) | AFP, S-100, GFAP,
TTF-1 | GTR | 38 | Stable | (11) |
| Wang et al,
2009 | 26/F | P | – | – | Left CPA | Columnar epithelium
with atypical nuclei | EMA, CK, CA19–9,
CEA, Ki-67>50% | GFAP, S-100 | GTR, RT (50.0
Gy) | 6 | Dissemination | (12) |
| Gessi et al,
2008 | 25/M | P, no R | 4/GTR | Right CPA, also
extended contralaterally | Right CPA,
cerebellum | In situ
papillary adenocarcinoma | CK, EMA, CEA | GFAP, Vim, S100,
p53, synaptophysin | GTR, PR | >4 | Recurred 4 months
later, succumbed | (13) |
| Monaco et
al, 2003 | 36/M | P | – | – | Cisterna magna, 4th
ventricle | Carcinoma in
situ | AE1/AE3 CK, CEA(f),
S100(f) Ki-67>80% | Vim, GFAP | GTR | 24 | Stable | (14) |
| Sahara et
al, 2001 | 53/M | R | 42/GTR | Anteriolateral to
MO | In situ | Adenocarcinoma with
papillary proliferation | EMA, CEA, S-100, CA
19–9, CK7, CK20, Ki-67=6.7%, p53=8% | GFAP, CA-125,
chromogranin A | PT, RT (50 Gy),
CT# | 12 | Recurred 6 months
later, succumbed | (16) |
| Ho et al,
1998 | 45/F | P, no R | 16/GTR | Right parietal
region | Right
hemisphere | Papillary
adenocarcinoma | CK, EMA, CEA, Ki-67
(rare) | GFAP, p53 | GTR | 21 | No malignant
recurrence | (15) |
Notably, all 9 studies, including the present study,
were intracranial lesions, even though intraspinal NCs are more
common compared with intracranial NCs. Intracranial NCs have been
reported to comprise ~10% of all NCs in the central nervous system
(3,8).
Other reports have indicated that NCs are ~3 times more common in
the spine compared with the brain (4); therefore, the lack of studies reporting
an intraspinal NC with a malignant transformation is notable. In
addition, in 3/9 studies, the NC was located in the supratentorial
region (9,11,15), and 2
of those cases reported intramedullary lesions located in the
cerebral hemisphere (9,11). Intracranial NCs that developed within
the posterior fossa accounted for 70–90% of all intracranial NC
cases (1). Intramedullary
supratentorial NCs appear to be much more rare. Despite the limited
number of studies reviewed in the present study, the location
distribution of NCs with malignant transformation did not coincide
with the location distribution of all NCs. In addition, the time
span of the 9 studies is hypothesized to have weakened this
limitation.
The precise pathogenesis of the transformation and
the original NCs remains unclear. Mittal et al (2) proposed that NCs are caused by anomalous
endodermal cell migration that occurs dorsally through the
primitive neurenteric canal into the ectoderm. This theory
plausibly explains the progressively decreasing incidence of
intraspinal, posterior fossa and supratentorial NCs. Based on this
hypothesis, the ectopic endodermal cells that travelled the
furthest distance may have been more likely to develop into
dysplasia or a malignant transformation. This hypothesis may
explain the variation in the location distribution between NCs with
a malignant transformation and all NCs, acting as an internal
cause. Other studies have suggested that chronic inflammation, most
likely due to repeated cyst rupture or subtotal resection of the
cyst walls, may predispose tissue to malignantly transform from
benign epidermoid and dermoid cysts to squamous cell carcinomas
(22,25). A similar principle may apply to the
development of malignantly transformed NCs and act as an external
cause. These hypotheses are based on the existing limited cases and
require additional studies in the future.
The male-to-female ratio of all 9 cases was 4:5,
which did not indicate a significant difference between genders.
The age of the 9 patients with NC and malignant transformation
ranged between 25–60 years (6,9–14). To the best of our knowledge, no
patients have been reported with malignantly transformed NC during
childhood.
In 5 of the 9 patients (11–15), the
malignant transformation occurred in the primary NC, including in
the 2 youngest patients (12,13). The other 4 patients, including the
patient from the present study, possessed benign primary NCs that
transformed into malignant NCs upon recurrence (9,10,12,16). The
progression-free time between the benign primary NC and the
malignant-transformed recurrent NC ranged from 22–168 months.
Intracranial NCs often appear with hyperintensity or
isointensity on T1-weighted images and typical hyperintensity on
T2-weighted images, without edema on MRI scans, and occasionally
with rim enhancement or partial rim enhancement on Gd-enhanced MRI
scans (1,4). In the 9 studies reviewed in the present
study, enhanced T1-weighted images were provided in 8 cases; of
which, 6 cases, including the present case, showed enhancement
(9,10,11,15).
Unlike the typical rim enhancement observed in benign NCs, the
enhancement of NCs with malignant transformations was focal and
heterogeneous. No direct evidence proves that these atypical
enhanced regions are the transformed malignant component. Only 1
patient, who did not possess enhancement in the primary malignantly
transformed NC, possessed enhancement that was confirmed as tumor
dissemination (12). In addition, 4
patients possessed multiloculated cysts on the MRI scans (9,13,14,16), and 2
patients possessed part-cystic, part-solid structures on the MRI
scans (11,15). The lesion of the patient in the
present study appeared as an enhanced solid mass that compressed
the medulla oblongata. These radiological characteristics varied
from those of the benign NCs, which appeared as a smooth cyst on
the MRI scans. Additionally, surrounding edema, which is common
with intracranial metastatic tumors, was identified in only 1
patient (16). For this patient, who
possessed an extramedullary malignantly transformed NC, the
surrounding edema was local, which differs from the extensive edema
of metastatic tumors. The exact radiological spectrum was not
calculated due to the limited number of studies that were reviewed.
However, adult primary or recurrent NCs with atypical radiological
features, including multiloculated cysts, part-cystic and
part-solid structures, or focal heterogeneous enhancement, are
recommended to be assessed with the possibility of NC with
malignant transformation in mind, and a relatively aggressive
surgical strategy may be considered, as opposed to continued
observation.
In the present literature review, the most common
malignant pattern was adenocarcinoma on histological examination,
which was identified in 6 patients, including the patient of the
present case (9,10,13,15,16).
Papillary adenocarcinoma, characterized by distinct papillary
architecture, was diagnosed in 4 patients, including the patient of
the present case (13,15,16). The
other malignant patterns were papillary cystadenocarcinoma
(11) and intraepithelial carcinoma
(14). One intramedullary malignantly
transformed NC was consistent with a papillary cyst adenocarcinoma
with carcinomatous invasion of the brain parenchyma (11). Malignant epithelia accompanied with
regional benign NC epithelia were present in 6 patients (9,11–15). The other 3 patients, including the
patient of the present case, did not possess benign epithelia in
the transformed lesion (10,16). Two cases were of carcinoma in
situ (14,16). Okabe et al (9) reported a mucinous adenocarcinoma with
bronchopulmonary differentiation arising from the NC, which was
almost the same as the pulmonary type I congenital adenomatoid
malformation (CCAM); however, the K-Ras mutation and p-16
expression that frequently accompany CCAM were not detected.
Genetic analyses were not provided in the previous
studies. This aspect of malignantly transformed NCs requires
additional study, as comparing the gene expression in primary
benign and recurrent malignantly transformed lesions may uncover
the pathogenesis of malignantly transformed NCs. At present, no
cases of the malignant transformation of NCs into squamous cell
carcinoma have been observed. In the present study, the focal
hyperplasia of epithelial cells prior to malignant transformation
were identified. In addition, the frequent irregular folds of the
dysplastic epithelium cells that showed a tendency to create a
well-formed papillae structure were observed. These phenomena make
up an intact process of malignant transformation, which supports
our recommendation that NC with epithelial cells and focal
hyperplasia requires careful examination and follow-up.
The Ki-67 labeling index of the malignantly
transformed regions in the immunohistochemical examination ranged
from rare to 80% expression (9,10,12,14–16).
Sahara et al (16) indicated
that the staining of CEA and cancer antigen 19–9 in the recurrent
malignantly transformed specimen was stronger compared with that in
the initial benign specimen, and that the Ki-67 labeling index
increased from 0% in the first surgical specimen to 6.7% in the
second. In total, 3 patients possessed Ki-67 labeling index scores
of >50% (9,12,14), 1 of
whom remained stable in the 2 years of follow-up. Wang et al
(12) proposed that an increased
Ki-67 labeling index score and the invasion of the tumor cells led
to short recurrence and dissemination periods.
Surgery remains the major treatment for malignantly
transformed and benign NCs. A total resection of the cyst content
and cyst walls is recommended for a good prognosis (12). Since certain malignantly transformed
NCs occur in recurrent NCs, adhesion between the NC and surrounding
structures may result in an incomplete resection (10,16). In
addition, primary malignant NCs may be adhered to surrounding
tissues. Gessi et al (13)
reported severe adhesion between a primary malignant NC and the
surrounding structures, with mucinous cystic fluid. Monaco et
al (14) described a primary
malignantly transformed NC with clear, colorless and watery cystic
fluid; however, adhesion between the cyst walls and surrounding
tissues was not reported in the study. One primary intramedullary
malignantly transformed NC with pathologically confirmed brain
invasion was separated from the adjacent parenchymal white matter
(11). These variable characteristics
indicate that a recurrent malignantly transformed NC with mucinous
fluid may adhere more easily to adjacent tissues. A previous study
that reported the case of a benign intracranial NC indicated that
the greater the size and the thinner the wall of the lesion, the
stronger the adhesion to the surrounding tissue was (7). Due to the limited number of cases
reviewed in the present study, this phenomenon cannot be confirmed
for malignantly transformed NCs. The 2 of the 9 studies that
reported the largest lesions did not mention severe adhesion
between the lesion and surrounding tissues (9,11).
Malignant characteristics often indicate that adjuvant treatments,
including radiotherapy (11,16) and chemotherapy (16), may be used. In a study regarding the
malignant transformation of epidermoid and dermoid cysts, radiation
therapy appeared to be beneficial, and the majority of patients
that received radiation therapy survived for >1 year (22). Sahara et al (16) reported a case of malignantly
transformed NC that was treated with a partial resection, 50 Gy
radiotherapy and chemotherapy, including carboplatin and etoposide.
The lesion recurred 6 months after surgery and eventually resulted
in the mortality of the patient. Adjuvant treatment with
radiotherapy was used in 2 other studies; 1 patient remained stable
for 10 months after partial resection surgery (10), while in the other patient, the tumor
disseminated 6 months after a total excision of the cyst (12).
In total, 4 patients from the 9 reviewed studies
remained stable without disease progression or recurrence for the
duration of the follow-up, which ranged from 3–38 months (9,11,14,16). As
the follow-up times were fairly short, additional follow-up
examinations will be required for these 4 patients. Notably, 1
patient relapsed in ~15 months; however, the specimen from the
second surgery showed fragments of the cyst wall that were
identical to the benign component of the first specimen, without a
malignant component or brain invasion (15). The exclusion of a malignant
transformation of a recurrent lesion is challenging as the
fenestration of the cyst wall of the second surgery may omit the
malignant component. The remaining patients appeared to have poor
prognoses. For 1 patient the tumor disseminated in 6 months
(12), and 2 patients relapsed within
6 months and eventually succumbed to the disease (13,16). The
causes of the mortalities were associated with severe brainstem
compression (13) and the extension
of the tumor (16). In the present
study, the patient's respiratory failure may have been due to
brainstem compression and the infiltration of the dorsal medulla
oblongata.
Overall, the malignant transformation of NCs in the
central nervous system is extremely rare. Based on the findings of
the present case and the review of the previous literature, strict
criteria for malignant-transformed NCs can be defined. The location
distribution of NCs with a malignant transformation does not
coincide with the location distribution of all NCs. In general, the
malignant transformation of NCs occurred in adult patients and
demonstrated atypical radiological features. Therefore, an adult
primary or recurrent NC with atypical radiological features may be
more likely to become malignant. Surgery is the optimal treatment
for malignantly transformed NCs, and close follow-up is required.
The therapeutic effectiveness of adjuvant chemotherapy and
radiotherapy requires additional study.
Glossary
Abbreviations
Abbreviations:
|
NC
|
neurenteric cyst
|
|
MRI
|
magnetic resonance imaging
|
|
EMA
|
epithelial membrane antigen
|
|
CEA
|
carcinoembryonic antigen
|
|
TTF-1
|
thyroid transcription factor 1
|
|
GFAP
|
glial fibrillary acidic protein
|
|
Vim
|
vimentin
|
References
|
1
|
Gauden AJ, Khurana VG, Tsui AE and Kaye
AH: Intracranial neuroenteric cysts: A concise review including an
illustrative patient. J Clin Neurosci. 19:352–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mittal S, Petrecca K, Sabbagh AJ, Rayes M,
Melançon D, Guiot MC and Olivier A: Supratentorial neurenteric
cysts - A fascinating entity of uncertain embryopathogenesis. Clin
Neurol Neurosurg. 112:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brooks BS, Duvall ER, el Gammal T, Garcia
JH, Gupta KL and Kapila A: Neuroimaging features of neurenteric
cysts: Analysis of nine cases and review of the literature. AJNR Am
J Neuroradiol. 14:735–746. 1993.PubMed/NCBI
|
|
4
|
Preece MT, Osborn AG, Chin SS and
Smirniotopoulos JG: Intracranial neurenteric cysts: Imaging and
pathology spectrum. AJNR Am J Neuroradiol. 27:1211–1216.
2006.PubMed/NCBI
|
|
5
|
Simon JA, Olan WJ and Santi M:
Intracranial neurenteric cysts: A differential diagnosis and
review. Radiographics. 17:1587–1593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Zhang J, Wu Z, Jia G, Zhang L, Hao
S and Geng S: Diagnosis and management of adult intracranial
neurenteric cysts. Neurosurgery. 68:44–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Oliveira RS, Cinalli G, Roujeau T,
Sainte-Rose C, Pierre-Kahn A and Zerah M: Neurenteric cysts in
children: 16 consecutive cases and review of the literature. J
Neurosurg. 103(Suppl 6): S512–S523. 2005.
|
|
8
|
Al-Ahmed IH, Boughamoura M, Dirks P,
Kulkarni AV, Rutka JT and Drake JM: Neurosurgical management of
neurenteric cysts in children. J Neurosurg Pediatr. 11:511–517.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okabe H, Katsura K, Yamano T, Tenjin H,
Nakahara Y, Ishida M and Kato T: Mucinous adenocarcinoma arising
from supratentorial intramedullary neuroenteric cyst with
broncho-pulmonary differentiation. Neuropathology. 34:420–424.
2014.PubMed/NCBI
|
|
10
|
Surash S, Ismail A, Loughrey C and van
Hille P: Malignant transformation of a neurenteric cyst in the
posterior fossa following complete excision. Br J Neurosurg.
23:458–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dunham CP, Curry B and Hamilton M:
Malignant transformation of an intraaxial-supratentorial
neurenteric cyst - case report and review of the literature. Clin
Neuropathol. 28:460–466. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W, Piao YS, Gui QP, Zhang XH and Lu
DH: Cerebellopontine angle neurenteric cyst with focal malignant
features. Neuropathology. 29:91–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gessi M, Legnani FG, Maderna E, Casali C,
Solero CL, Pollo B and DiMeco F: Mucinous low-grade adenocarcinoma
arising in an intracranial enterogenous cyst: Case report.
Neurosurgery. 62:E972–E973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monaco R, Boscaino A, Di Blasi A,
D'Antonio A, Profeta G, De Falco R and Nappi O: Intraepithelial
carcinoma arising in an endodermal cyst of the posterior fossa.
Neuropathology. 23:219–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho LC, Olivi A, Cho CH, Burger PC, Simeone
F and Tihan T: Well-differentiated papillary adenocarcinoma arising
in a supratentorial enterogenous cyst: Case report. Neurosurgery.
43:1474–1477. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahara Y, Nagasaka T, Takayasu M, Takagi
T, Hata N and Yoshida J: Recurrence of a neurenteric cyst with
malignant transformation in the foramen magnum after total
resection. Case report. J Neurosurg. 95:341–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lara M, Pascual D, Aparicio MA, Ruiz L,
Miranda D, Gomez-Moreta JA and Hernandez Vicente J: Giant and
recurrent enterogenous cyst of the frontal lobe: Case report.
Childs Nerv Syst. 27:1333–1339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hutchison J and Thomson JD: Congenital
archenteric cysts. Br J Surg. 41:15–20. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimura H, Nagatomi A, Ochi M and Kurisu K:
Intracranial neurenteric cyst with recurrence and extensive
craniospinal dissemination. Acta Neurochir (Wien). 148:347–352.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaynes P, Bousquet P, Sol JC, Delisle MB,
Richaud J and Lagarrigue J: Recurrent intracranial neurenteric
cysts. Acta Neurochir (Wien). 140:905–911. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perry A, Scheithauer BW, Zaias BW and
Minassian HV: Aggressive enterogenous cyst with extensive
craniospinal spread: Case report. Neurosurgery. 44:401–404,
404–405. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamlat A, Hua ZF, Saikali S, Laurent JF,
Gedouin D, Ben-Hassel M and Guegan Y: Malignant transformation of
intra-cranial epithelial cysts: Systematic article review. J
Neurooncol. 74:187–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bejjani GK, Wright DC, Schessel D and
Sekhar LN: Endodermal cysts of the posterior fossa. Report of three
cases and review of the literature. J Neurosurg. 89:326–335. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakayama H, Akikusa B, Kondo Y, Saito N,
Sarashina H and Okui K: Mucinous cystadenocarcinoma of the colon.
Report of a case. Dis Colon Rectum. 32:243–246. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abramson RC, Morawetz RB and Schlitt M:
Multiple complications from an intracranial epidermoid cyst: Case
report and literature review. Neurosurgery. 24:574–578. 1989.
View Article : Google Scholar : PubMed/NCBI
|