Esophageal cancer is the eighth most common type of
cancer in the world and the sixth most common cause of mortality
from cancer, according to the results of the GLOBOCAN project,
published by the International Agency for Research on Cancer in
2012 (1). Alcohol consumption has
been demonstrated to be a major risk factor for developing
esophageal cancer, particularly esophageal squamous cell carcinoma
(ESCC) in men (2–4). Globally, prevailing alcohol intake has
been an increasingly dire health problem due to the carcinogenicity
of ethanol (5,6). Chronic ethanol ingestion leads to
nutritional deficiencies and generation of reactive oxygen species
(ROS). In addition, ethanol acts as a solvent of carcinogens
(7). Acetaldehyde, the primary
metabolite of ethanol, is highly mutagenic, due to its ability to
form exocyclic DNA adducts (8). The
diversity of carcinogenetic mechanisms may reflect the wide
interaction between ethanol and cofactors from the inner and outer
environment (9). The present study
focuses on how alcohol consumption associates with the risk of
esophageal cancer and interacts with corresponding factors, mainly
from an epidemiological aspect.
The risk of developing esophageal cancer has been
indicated to increase with an increase in alcohol intake (10–18). Fan
et al (18) demonstrated a
positive association between the total amount of ethanol intake
during lifetime and the risk of esophageal cancer. In another
study, heavy ethanol intake (>53.3 g/day) was significantly
associated with the risk of esophageal cancer, even in a relatively
short duration (≤20 years) (12).
Castellsagué et al (19)
demonstrated that, compared with individuals with a decreased daily
alcohol consumption for numerous years, those drinking large
amounts of alcohol for a shorter period of time tend to carry an
increased risk of developing esophageal cancer. Therefore, compared
with a long duration of alcohol consumption, an increased daily
amount of alcohol consumption may be a more effective risk factor
of esophageal cancer.
As the major component of alcoholic beverages,
ethanol is the determinant of the risk of esophageal cancer
(20). Acetaldehyde, though present
in trace amounts in beverages, may be another risk factor, due to
its strong carcinogenicity (21). The
most prevalent beverage in a region tends to have the greatest
relative risk (22). Baijiu (a type
of hard liquor with a high alcohol content) in China, wine in
Italy, calvados in France and spirits in South America enhance the
development of esophageal cancer, due to their high ethanol content
and great popularity in each particular region (12,19,22,23).
In contrast, wine consumption was observed to reduce the risk of
esophageal cancer in a previous cohort study (17). Despite the regional variations in
drinking habits, certain antitumor substances, including
flavonoids, that are contained in wine may explain the
inconsistencies reported across different studies (24). In previous studies, beer had a
relatively mild effect on the risk of esophageal cancer, even with
large consumption, compared with other beverages, due to its low
ethanol content (17–19,22).
There are controversies regarding the association
between the time since quitting drinking and the risk of developing
esophageal cancer. Castellsagué et al (19) and Wu et al (25) demonstrated a virtually negative
association in men. The conclusions of the study by Zambon et
al (14) were more complex.
Compared with persistent drinkers, drinking cessation was
associated with certain increased risk in the first 10 subsequent
years and with a non-significant risk reduction thereafter
(14). These differences may be due
to variations in reference and population stratification in
case-control studies.
This variant has an uncertain effect on the risk of
esophageal cancer. In the study by Castellsagué et al
(19), along with increasing daily
alcohol intake, there was a greater tendency for individuals that
started drinking at an older age to develop esophageal cancer.
However, Zambon et al (14)
showed no association between the age of drinking initiation and
the risk of developing esophageal cancer.
Different populations involved in various studies
affect the strength of the association between alcohol and
esophageal cancer. Alcohol consumption may be a great risk factor
for esophageal cancer in Caucasian populations (14,19,26);
however, the effect of alcohol consumption on the risk of
developing esophageal cancer appears to be much weaker in Asian
populations (25,27), particularly in certain regions with
high incidence of esophageal cancer, including Linxian (China),
where alcohol consumption is not a major risk factor for esophageal
cancer (28). This weak association
may be attributed to strong confounding factors, including gene
polymorphism and other carcinogens that dilute the effect of
alcohol (11,28). In studies concerning gender, the
association between alcohol consumption on the risk of developing
esophageal cancer was weaker in women compared with men, which may
be partly explained by a short history of alcohol exposure and low
alcohol prevalence among women (19,25).
A large number of studies have indicated a strong
positive association between alcohol intake and ESCC (11,12,15,17,18,29).
However, the association between alcohol intake and esophageal
adenocarcinoma (EAC) is attenuated. The two cohort studies by
Steevens et al (17,30) revealed that alcohol consumption did
not promote the risk of developing EAC or Barrett's esophagus
(precancerous lesions of EAC). Other analyses reached similar
conclusions (29,31,32). The
study by Akiyama et al (33)
showed a moderate increase in the risk of Barrett's esophagus in
Japanese male drinkers. In the meta-analysis conducted by Lubin
et al (15), odd ratios (ORs)
with drinking-years exhibited an inverse association with alcohol
consumption in <5 drinks/day consumers, and no association in
heavier consumers. The distinct outcome of EAC and ESCC may be
explained by the different pathogenesis of these two subtypes of
esophageal cancer (34).
Since numerous genes participate in the catabolism
of ethanol directly or indirectly, gene polymorphism greatly
affects the carcinogenicity of ethanol in various populations
(35). In several epidemiological
studies, even genes that are barely associated with ethanol
metabolism revealed similar effects (Table I).
Alcohol dehydrogenases (ADH) and aldehyde
dehydrogenases (ALDH) are the major enzymes that participate in the
metabolism of ethanol. Ethanol is oxidated to acetaldehyde by ADH,
and the subsequent oxidation of acetaldehyde to acetate is
catalyzed by ALDH. Polymorphism of these gene families caused by
point mutations alters enzymatic activity, resulting in potential
individual variations in acetaldehyde exposure (36–38).
The ADH1B*2 (Arg48His) allele encodes a more active
subunit of ADH1B, compared with the ADH1B*1 allele, the homodimer
of which has a ~40-fold greater maximum velocity than the
ADH1B*1/2*1 form of ADH1B (39,40). The
enzyme encoded by the ADH1C*1 (Ile350Val) allele has a 2.5-times
faster speed of acetaldehyde production than the enzyme encoded by
the ADH1C*2 allele (39,40). ADH1B*1 has been previously
demonstrated to enhance the risk of esophageal cancer in Asian
populations (41–45). By contrast, the association between
ADH1C and the risk of esophageal cancer is contradictory. Yokoyama
et al (41) and Muto et
al (46) demonstrated that
ADH1C*2 increased the risk of developing esophageal cancer;
however, the study by Wu et al (45) showed no enhanced risk. Furthermore,
ADH1C*1, but not ADH1C*2, enhanced the risk of esophageal cancer in
certain studies conducted in western countries (47,48). This
contradiction may be explained by different linkage disequilibrium
patterns among various populations (40,48). ADH7
is mainly expressed in the upper gastrointestinal tract (49), and certain studies showed that single
nucleotide polymorphisms of ADH7 were associated with esophageal
cancer in alcohol drinkers (50–52).
The ALDH2*2 (Glu487Lys) allele, which encodes an
inactive subunit of ALDH, occurs most frequently in Asian
countries, including China, Japan, Korea, Mongolia and Indochina
(53). As the major enzyme affecting
blood acetaldehyde concentration, the ALDH2*1*2 and ALDH2*2*2 forms
of ALDH produced a 6- and 19-fold increased acetaldehyde
concentrations, respectively, compared with the ALDH2*1*1 form
(54). The enhancing effect of the
ALDH2*2 allele on the risk of ESCC in Japanese men was demonstrated
in previous reports by Yokoyama et al (55,56). A
previous meta-analysis including seven case-control studies in Asia
revealed a positive association between the risk of developing
esophageal cancer and the level of alcohol consumption in subjects
carrying the ALDH2*1*2 genotype (57). The ALDH2*2 and ADH1B*1 alleles acted
in a multiplicative manner to enhance the risk of esophageal cancer
(41,42). Notably, although the ALDH2*1*2
genotype increased the risk of developing esophageal cancer, the
ALDH2*2*2 genotype reduced the risk in a previous study (42). It may be hypothesized that increased
blood levels of acetaldehyde due to the ALDH2*2 homodimer results
in the ‘alcohol flushing response’, which includes facial flushing,
nausea and tachycardia, preventing people from heavy drinking, thus
decreasing the possibility of esophageal cancer (58,59).
Individuals that possessed the ALDH2*1/2*2 genotype and also
carried ADH2*1/2*1 did not exhibit flushing following drinking.
These individuals tended to be heavy drinkers and had the greatest
risk of developing esophageal cancer (58).
Cytochrome P450 2E1 (CYP2E1) is the major enzyme in
the microsomal ethanol oxidation system (60). Induced by ethanol consumption, CYP2E1
metabolically activates procarcinogens and produces noxious ROS
during ethanol oxidation (61).
Compared with CYP2E1*c1 (Pst I-/Rsa I+), the mutant CYP2E1*c2 (Pst
I+/Rsa I-) is considered to display a decreased activity (38). Individuals with the CYP2E1*c1*c1
genotype possessed a much greater risk of developing ESCC compared
with those carrying the CYP2E1*c2 allele among Chinese drinkers;
Furthermore, CYP2E1*c1*c1 exhibits a synergistic interaction with
ALDH2*1*2 and methylenetetrahydrofolate reductase (MTHFR)677
(C/T+T/T) (62,63). However, in several cases, particularly
in Caucasians, gene polymorphisms of CYP2E1 have no association
with esophageal cancer (64–67).
Polymorphisms of the enzymes involved in the folate
metabolic pathways also impact the development of esophageal
cancer. MTHFR, a key enzyme in folate metabolism, is important for
DNA methylation (68,69). Altered activity of MTHFR may cause DNA
hypomethylation, a process associated with carcinogenesis (70,71). The
most commonly studied MTHFR mutant is the change from C to T at
nucleotide 677 of the MTHFR gene, which results in an alanine to
valine substitution in the MTHFR enzyme (72). The homozygote and heterozygote of 667T
exhibit a 30 and 65% activity, respectively, compared with the 100%
activity exhibited by the 677CC genotype (73). Previous case-control studies and
meta-analyses have revealed that the MTHFR C677T allele increased
the risk of developing ESCC (74–78). In
coordination with ADLH2*2, individuals with C677T demonstrated
elevated ORs (74). The MTHFR 677T
allele collaborated with alcohol consumption to increase the risk
of esophageal cancer in former, moderate and heavy drinkers
(75). However, the interaction
between MTHFR 677T and alcohol intake is not consistent in certain
meta-analyses (77,78). A previous study conducted in Japan
reached the opposite conclusion, and reported that the MTHFR 677TT
genotype significantly decreased esophageal cancer risk in heavy
drinkers (79). This inconsistency
may be due to the regional variations in folate consumption among
different populations, which would suggest a significant
gene-nutrient interaction between folate consumption and MTHFR
genotype (78–80).
Certain gene polymorphisms with little association
with the metabolism of alcohol somewhat alter the risk of
esophageal cancer. According to the review by Hiyama et al
(81), carcinogen
metabolism-associated genes [such as cytochrome P450 family 1
subfamily A member 1 (CYP1A1), glutathione S-transferases (GSTs)
and natural antisense transcripts], DNA repair genes (such as X-ray
repair complementing defective repair in Chinese hamster cells 1
and xeroderma pigmentosum group D), cell cycle control genes (such
as tumor protein p53 and cyclin D1) and oncogenes (such as v-myc
avian myelocytomatosis viral oncogene lung carcinoma derived
homolog) showed varying degrees of association with esophageal
cancer. However, inconsistencies exist in various studies, and
certain studies showed that the aforementioned genes interacted
with alcohol consumption in the development of esophageal cancer.
The GSTM1 deletion, GSTP1 341C/T+341T/T and CYP1A1 Val/Val
genotypes were indicated to possess much greater ORs in alcohol
drinkers compared with non-drinkers in two case-control studies
(82,83).
Similarly to alcohol beverages, tobacco has spread
all over the world, with >1 billion current smokers (84). The majority of alcohol drinkers are
also tobacco smokers (85).
Epidemiological studies involving the combined effects of tobacco
and alcohol have consistently revealed the existence of a positive
synergistic effect between these two factors on the risk of
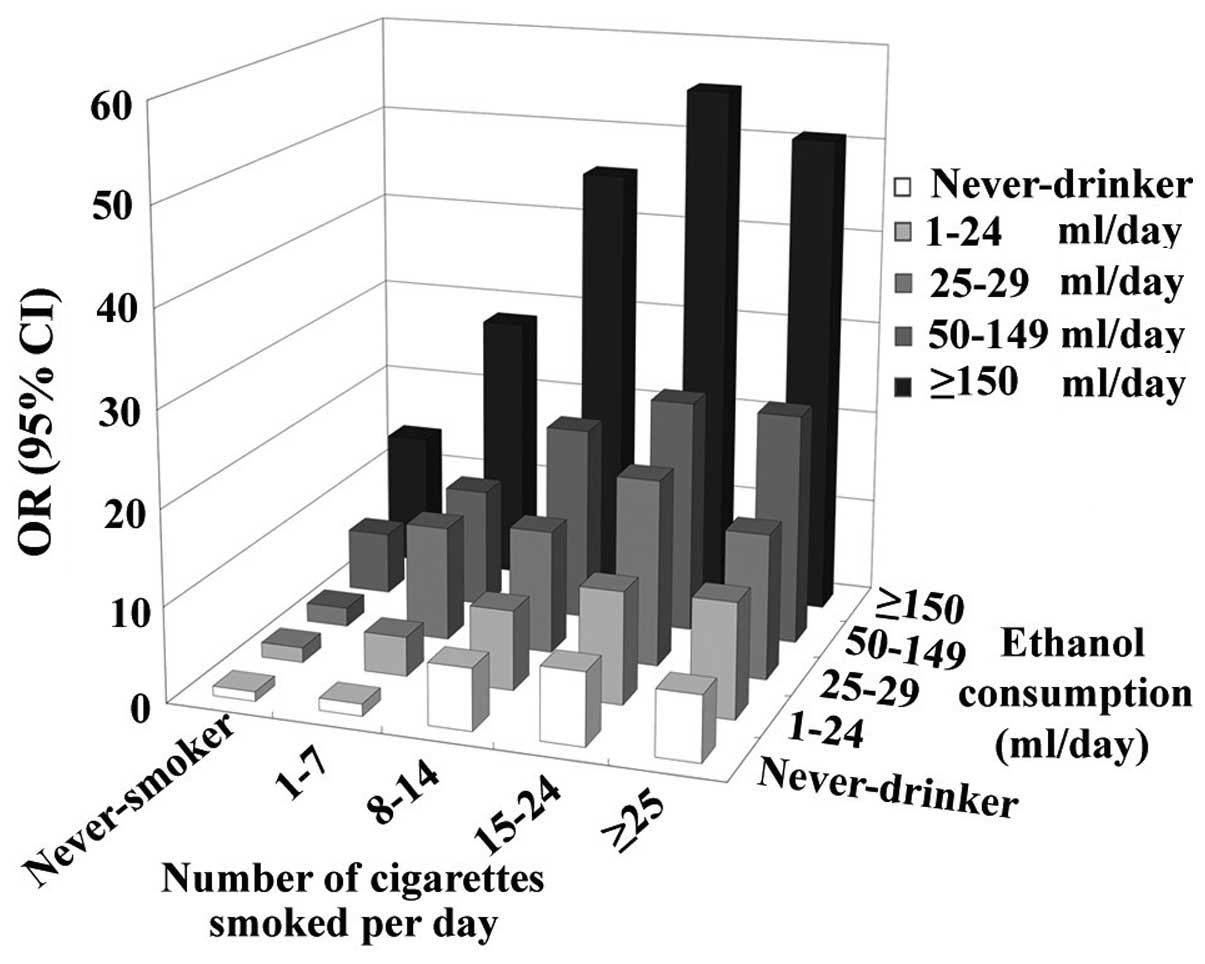
developing esophageal cancer (14,17–19,25,86–88)
(Fig. 1). The two variants usually
exhibit a mutual dose-response association (14,17,19), and a
combined OR reached a value of 130 in an Italian study (14). However, the effect of this combination
appeared to be much weaker in Asian populations, with a combined OR
of <10 at the highest alcohol and tobacco use (18,25).
Similarly, women had a less combined OR compared with men (19,25). The
variation between populations may be attributed to regional and
gender differences in terms of tobacco prevalence (25,89).
Reportedly, ~66.9% of men but only 4.2% of women are tobacco
smokers in China, whereas the prevalence of smoking among men and
women was estimated to be 35 and 22%, respectively, in developed
countries, and 50 and 9%, respectively, in developing countries
(25,89).
Tobacco smoke contains >60 carcinogens, including
tobacco-specific nitrosamines such as
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and
N'-nitrosonornicotine, and polycyclic aromatic hydrocarbons (PAHs)
such as benzo[a]pyrene and acetaldehyde (84,90,91).
Tobacco smoke interacts with ethanol in the initiation of cancer in
several ways: i) Ethanol activates the CYP members that metabolize
tobacco procarcinogens to carcinogens (91); ii) ethanol increases cellular membrane
permeability and acts as a solvent to facilitate the penetration of
molecules like PAHs into the intracellular domain of mucosal
epithelial cells (91); and iii)
tobacco smoke, as a direct source of acetaldehyde or as a regulator
of the population of oral bacteria, cooperatively elevates
acetaldehyde exposure in a direct or indirect way by 7-fold,
compared with alcohol drinking alone (92).
Epidemiological evidence of the associations between
salivary acetaldehyde and esophageal cancer remains limited, but
several experiments conducted in animals may aid to elucidate the
local carcinogenic effects of acetaldehyde on the mucosa of the
upper digestive tract. Previous studies suggested that long-term
alcohol consumption may induce increased cell proliferation in the
oral and esophageal mucosa of rats (93,94). As a
more direct model for reflecting the effect of salivary
acetaldehyde, rats that drank water with an increased concentration
of acetaldehyde showed hyperplasia and hyperproliferation in the
epithelia of their upper gastrointestinal tract (95).
Oral microbes and prolonged ethanol use are two
major factors in the generation of salivary acetaldehyde. This
hypothesis has been previously demonstrated in vivo by
Homann et al (96). In this
study, moderate ethanol ingestion resulted in carcinogenic amounts
of acetaldehyde in the saliva; whereas using an antiseptic
mouthwash with chlorhexidine prior to ethanol exposure, in
vivo acetaldehyde production decreased by >50%, with a
parallel evident decrease in bacterial counts. Yokoyama et
al (97) showed that following 3
weeks of abstinence, the microorganism count and salivary
acetaldehyde production decreased in alcoholics. These results
indicate a certain mutual effect between ethanol and oral
microorganisms. Chronic alcohol consumption may increase bacterial
concentrations through affecting salivary gland morphology and
decreasing salivary flow (98,99).
As another promoter of microbial acetaldehyde
production, tobacco smoking may exhibit a strong association with
increased salivary acetaldehyde during alcohol drinking. Smokers
that smoke while drinking have 7-fold increased salivary
acetaldehyde levels compared with non-smokers (100). With the exception of the direct
contribution of acetaldehyde by tobacco smoke, the alteration of
oral microorganisms by smoking is also a major source of the
increased concentration of salivary acetaldehyde (99). As previously reported, increased yeast
infections and conversions from Gram-negative to Gram-positive
bacteria has been demonstrated in smokers (99,101,102).
However, oral bacteria may activate the nitrosamines from tobacco
smoking to carcinogenic adducts by forming hydroxylated products
(103–105).
In previous studies, people ingesting a greater
quantity and variety of fresh vegetable and fruits were less likely
to develop esophageal cancer (112–115).
Among numerous anticarcinogenic nutrients contained in plant foods,
folate intake has been widely demonstrated to be closely associated
with cancer of the brain, lung, esophagus, pancreas, colorectum,
breast, cervix and breast in previous epidemiological studies
(116). Three meta-analyses
involving worldwide case-control studies conducted between 1988 and
2011 reached a consensus that folate intake may effectively protect
individuals from ESCC and EAC, with a pooled OR and relative risk
(RR) between 0.50 and 0.66 (78,117,118).
In a large cohort study, a decreased intake of folate compared with
the daily median intake (405 µg) demonstrated an inverse
association with the risk of developing ESCC, but increased intake
showed no association; furthermore, there were no significant
associations between dietary folate and risk of EAC (119). One hypothesis proposed that folate
intake was not linearly associated with cancer risk, with a
protective effect only in moderate folate intake but no protection
or even tumor promotion in low or excessive ingestion (120). In that study, the cohort had a
relatively high median of folate consumption, which may explain
this inconsistency with other studies.
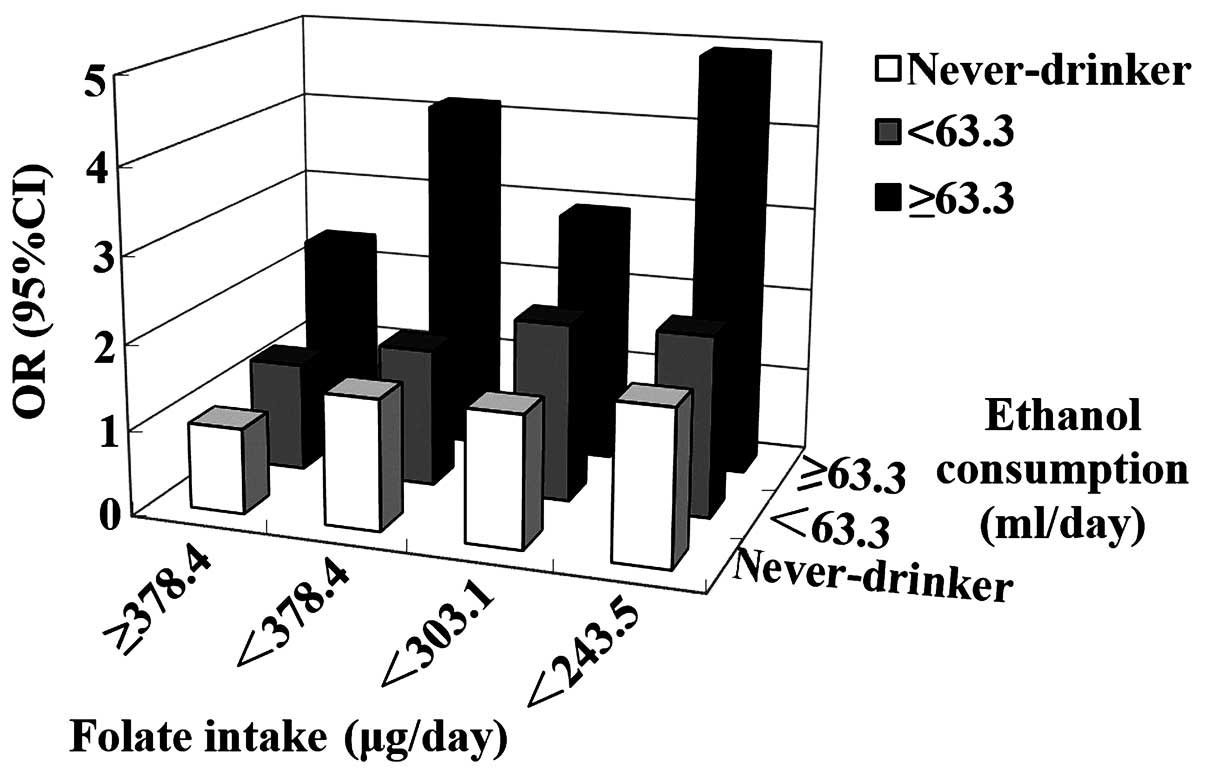
A significant interaction has been observed between
folate and alcohol intake in upper digestive tract cancer. In the
case-control study by Ibiebele et al (121), individuals with increased alcohol
and decreased folate intake demonstrated a 3-fold OR of ESCC
compared with individuals with decreased alcohol and increased
folate intake. Matsuo et al (122) reported a similar association in oral
and pharyngeal cancer (Fig. 2).
Another large prospective cohort study additionally confirmed the
antagonistic interaction between alcohol and folate intake in oral
cancer (123).
Folate is important in DNA metabolism, since it
mediates the synthesis of S-adenosylmethionine, a methyl donor used
in biological methylation reactions and de novo deoxynucleoside
triphosphate synthesis (116).
Folate depletion may be oncogenic through altered DNA/RNA
methylation, disruption of DNA integrity and disruption of DNA
repair (116). Alcohol ingestion is
a primary cause of folate deficiency (124,125).
In addition to dietary inadequacy, alcohol may decrease internal
folate levels through intestinal malabsorption, decreased hepatic
storage and increased renal excretion (124,125).
Folate homeostasis depends on transporter proteins, including
reduced folate carrier, proton-coupled folate transporter,
folate-binding protein, mitochondrial folate transporter and
enzymes such as folylpolyglutamate synthetase (126–129).
Chronic ethanol exposure may downregulate gene expression, thus
impairing the transportation of folate across membranes (126–129).
With the exception of causing folate deficiency, ethanol intake
interferes widely in folate-dependent intermediary metabolism by
inhibiting enzymes in the one-carbon metabolism, particularly
methionine synthase and its associated products of metabolism, thus
disturbing the synthesis of nucleotides (130). By contrast, folate deficiency
facilitates the adverse effects of alcohol in methionine metabolism
and promotes alcohol-induced oxidative cell injury (124).
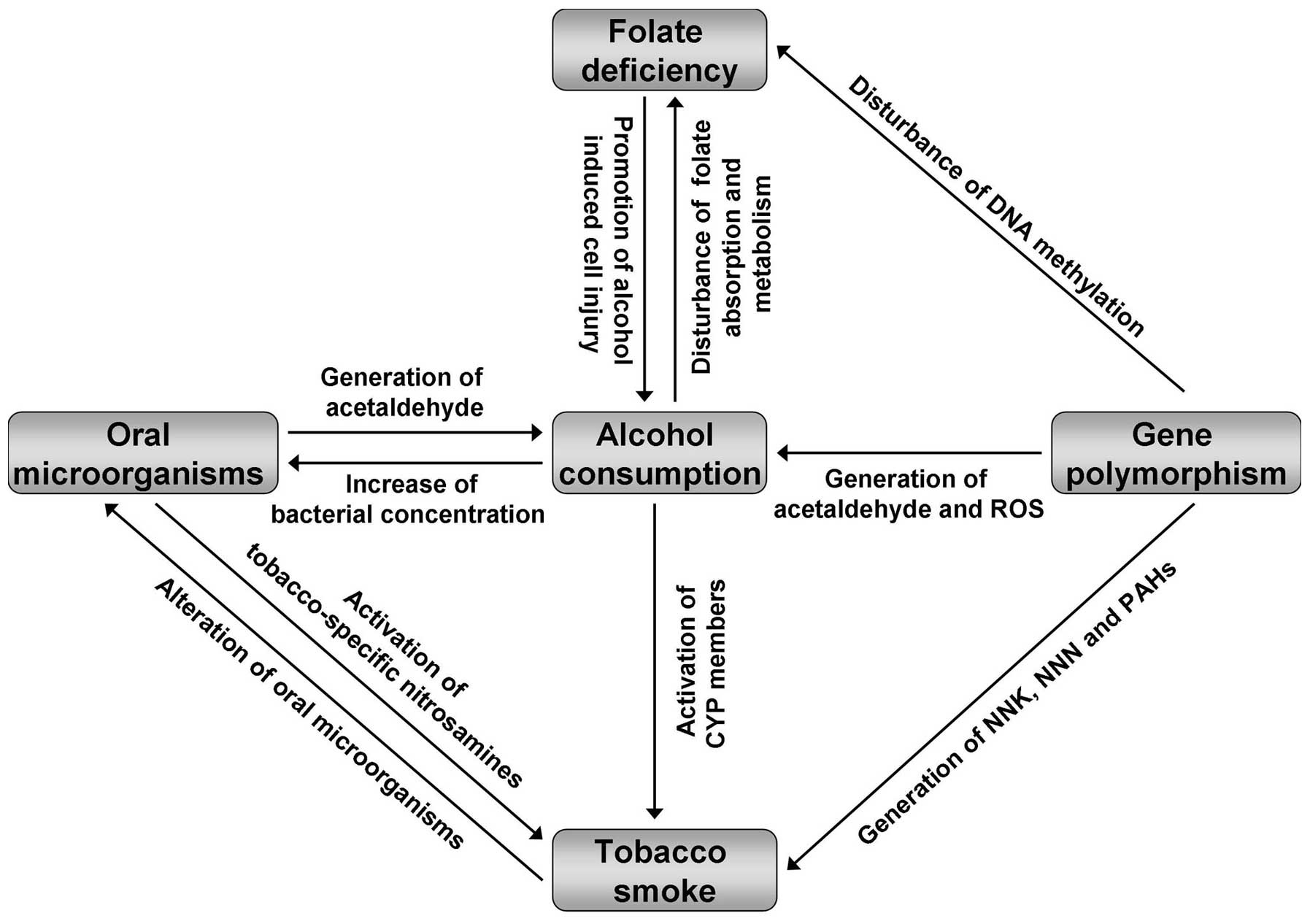
Alcohol consumption significantly increases the risk
of esophageal cancer, exhibiting a dose-response association with
daily intake and an altered efficiency in various beverage types,
populations and cancer subtypes. Gene polymorphisms, tobacco
smoking, oral microorganisms and folate deficiency act as
collaborators with concurrent alcohol use (Fig. 3). Current evidence suggests that,
rather than mere alcohol consumption, the synergy of alcohol
consumption and corresponding factors is important for the
development of esophageal cancer. Therefore, quitting alcohol
drinking and tobacco smoking, maintaining an adequate oral hygiene
and ingesting adequate levels of plant foods may effectively
protect high-risk individuals from developing esophageal
cancer.
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danaei G, Vander Hoorn S, Lopez AD, Murray
CJ and Ezzati M: Comparative Risk Assessment collaborating group
(Cancers): Causes of cancer in the world: Comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang JB, Fan JH, Liang H, Li J, Xiao HJ,
Wei WQ, Dawsey SM, Qiao YL and Boffetta P: Attributable causes of
esophageal cancer incidence and mortality in China. PLoS One.
7:e422812012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engel LS, Chow WH, Vaughan TL, Gammon MD,
Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam
H, et al: Population attributable risks of esophageal and gastric
cancers. J Natl Cancer Inst. 95:1404–1413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones L, Bates G, McCoy E and Bellis MA:
Relationship between alcohol-attributable disease and socioeconomic
status, and the role of alcohol cons-umption in this relationship:
A systematic review and meta-analysis. BMC Public Health.
15:4002015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim SS, Vos T, Flaxman AD, Danaei G,
Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee
M, Atkinson C, et al: A comparative risk assessment of burden of
disease and injury attributable to 67 risk factors and risk factor
clusters in 21 regions, 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2224–2260. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pöschl G and Seitz HK: Alcohol and cancer.
Alcohol Alcohol. 39:155–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brooks PJ and Theruvathu JA: DNA adducts
from acetaldehyde: Implications for alcohol-related carcinogenesis.
Alcohol. 35:187–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seitz HK and Stickel F: Molecular
mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer.
7:599–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oze I, Matsuo K, Wakai K, Nagata C, Mizoue
T, Tanaka K, Tsuji I, Sasazuki S, Inoue M and Tsugane S: Research
Group for the Development and Evaluation of Cancer Prevention
Strategies in Japan: Alcohol drinking and esophageal cancer risk:
An evaluation based on a systematic review of epidemiologic
evidence among the Japanese population. Jpn J Clin Oncol.
41:677–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Islami F, Fedirko V, Tramacere I, Bagnardi
V, Jenab M, Scotti L, Rota M, Corrao G, Garavello W, Schüz J, et
al: Alcohol drinking and esophageal squamous cell carcinoma with
focus on light-drinkers and never-smokers: A systematic review and
meta-analysis. Int J Cancer. 129:2473–2484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumagai N, Wakai T, Akazawa K, Ling Y,
Wang S, Shan B, Okuhara Y, Hatakeyama Y and Kataoka H: Heavy
alcohol intake is a risk factor for esophageal squamous cell
carcinoma among middle-aged men: A case-control and simulation
study. Mol Clin Oncol. 1:811–816. 2013.PubMed/NCBI
|
|
13
|
Gao YT, McLaughlin JK, Blot WJ, Ji BT,
Benichou J, Dai Q and Fraumeni JF Jr: Risk factors for esophageal
cancer in Shanghai, China. I. Role of cigarette smoking and alcohol
drinking. Int J Cancer. 58:192–196. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zambon P, Talamini R, La Vecchia C, Dal
Maso L, Negri E, Tognazzo S, Simonato L and Franceschi S: Smoking,
type of alcoholic beverage and squamous-cell oesophageal cancer in
northern Italy. Int J Cancer. 86:144–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lubin JH, Cook MB, Pandeya N, Vaughan TL,
Abnet CC, Giffen C, Webb PM, Murray LJ, Casson AG, Risch HA, et al:
The importance of exposure rate on odds ratios by cigarette smoking
and alcohol consumption for esophageal adenocarcinoma and squamous
cell carcinoma in the Barrett's Esophagus and Esophageal
Adenocarcinoma Consortium. Cancer Epidemiol. 36:306–316. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polesel J, Dal Maso L, Bagnardi V,
Zucchetto A, Zambon A, Levi F, La Vecchia C and Franceschi S:
Estimating dose-response relationship between ethanol and risk of
cancer using regression spline models. Int J Cancer. 114:836–841.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steevens J, Schouten LJ, Goldbohm RA and
van den Brandt PA: Alcohol consumption, cigarette smoking and risk
of subtypes of oesophageal and gastric cancer: A prospective cohort
study. Gut. 59:39–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan Y, Yuan JM, Wang R, Gao YT and Yu MC:
Alcohol, tobacco, and diet in relation to esophageal cancer: The
Shanghai Cohort Study. Nutr Cancer. 60:354–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castellsagué X, Muñoz N, De Stefani E,
Victora CG, Castelletto R, Rolón PA and Quintana MJ: Independent
and joint effects of tobacco smoking and alcohol drinking on the
risk of esophageal cancer in men and women. Int J Cancer.
82:657–664. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Linderborg K, Joly JP, Visapää JP and
Salaspuro M: Potential mechanism for Calvados-related oesophageal
cancer. Food Chem Toxicol. 46:476–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bosetti C, La Vecchia C, Negri E and
Franceschi S: Wine and other types of alcoholic beverages and the
risk of esophageal cancer. Eur J Clin Nutr. 54:918–920. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Launoy G, Milan C, Day NE, Faivre J,
Pienkowski P and Gignoux M: Oesophageal cancer in France: Potential
importance of hot alcoholic drinks. Int J Cancer. 71:917–923. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neuhouser ML: Dietary flavonoids and
cancer risk: Evidence from human population studies. Nutr Cancer.
50:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu M, Zhao JK, Zhang ZF, Han RQ, Yang J,
Zhou JY, Wang XS, Zhang XF, Liu AM, van't Veer P, et al: Smoking
and alcohol drinking increased the risk of esophageal cancer among
Chinese men but not women in a high-risk population. Cancer Causes
Control. 22:649–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vioque J, Barber X, Bolumar F, Porta M,
Santibáñez M, de la Hera MG and Moreno-Osset E: PANESOES Study
Group: Esophageal cancer risk by type of alcohol drinking and
smoking: A case-control study in Spain. BMC Cancer. 8:2212008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Tang L, Sun G, Tang Y, Xie Y, Wang
S, Hu X, Gao W, Cox SB and Wang JS: Etiological study of esophageal
squamous cell carcinoma in an endemic region: A population-based
case control study in Huaian, China. BMC Cancer. 6:2872006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey
SM, Dong ZW, Mark SD, Qiao YL and Taylor PR: Prospective study of
risk factors for esophageal and gastric cancers in the Linxian
general population trial cohort in China. Int J Cancer.
113:456–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Freedman ND, Murray LJ, Kamangar F, Abnet
CC, Cook MB, Nyrén O, Ye W, Wu AH, Bernstein L, Brown LM, et al:
Alcohol intake and risk of oesophageal adenocarcinoma: A pooled
analysis from the BEACON Consortium. Gut. 60:1029–1037. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steevens J, Schouten LJ, Driessen AL,
Huysentruyt CJ, Keulemans YC, Goldbohm RA and van den Brandt PA: A
prospective cohort study on overweight, smoking, alcohol
consumption, and risk of Barrett's esophagus. Cancer Epidemiol
Biomarkers Prev. 20:345–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tramacere I, Pelucchi C, Bagnardi V, Rota
M, Scotti L, Islami F, Corrao G, Boffetta P, La Vecchia C and Negri
E: A meta-analysis on alcohol drinking and esophageal and gastric
cardia adenocarcinoma risk. Ann Oncol. 23:287–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thrift AP, Kramer JR, Richardson PA and
El-Serag HB: No significant effects of smoking or alcohol
consumption on risk of Barrett's esophagus. Dig Dis Sci.
59:108–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akiyama T, Inamori M, Iida H, Mawatari H,
Endo H, Hosono K, Yoneda K, Fujita K, Yoneda M, Takahashi H, et al:
Alcohol consumption is associated with an increased risk of erosive
esophagitis and Barrett's epithelium in Japanese men. BMC
Gastroenterol. 8:582008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siewert JR and Ott K: Are squamous and
adenocarcinomas of the esophagus the same disease? Semin Radiat
Oncol. 17:38–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Druesne-Pecollo N, Tehard B, Mallet Y,
Gerber M, Norat T, Hercberg S and Latino-Martel P: Alcohol and
genetic polymorphisms: Effect on risk of alcohol-related cancer.
Lancet Oncol. 10:173–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Edenberg HJ: The genetics of alcohol
metabolism: Role of alcohol dehydrogenase and aldehyde
dehydrogenase variants. Alcohol Res Health. 30:5–13.
2007.PubMed/NCBI
|
|
37
|
Brennan P, Lewis S, Hashibe M, Bell DA,
Boffetta P, Bouchardy C, Caporaso N, Chen C, Coutelle C, Diehl SR,
et al: Pooled analysis of alcohol dehydrogenase genotypes and head
and neck cancer: A HuGE review. Am J Epidemiol. 159:1–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boffetta P and Hashibe M: Alcohol and
cancer. Lancet Oncol. 7:149–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bosron WF and Li TK: Genetic polymorphism
of human liver alcohol and aldehyde dehydrogenases, and their
relationship to alcohol metabolism and alcoholism. Hepatology.
6:502–510. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Osier M, Pakstis AJ, Kidd JR, Lee JF, Yin
SJ, Ko HC, Edenberg HJ, Lu RB and Kidd KK: Linkage disequilibrium
at the ADH2 and ADH3 loci and risk of alcoholism. Am J Hum Genet.
64:1147–1157. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yokoyama A, Kato H, Yokoyama T, Tsujinaka
T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M, et
al: Genetic polymorphisms of alcohol and aldehyde dehydrogenases
and glutathione S-transferase M1 and drinking, smoking, and diet in
Japanese men with esophageal squamous cell carcinoma.
Carcinogenesis. 23:1851–1859. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang GH, Mai RQ and Huang B:
Meta-analysis of ADH1B and ALDH2 polymorphisms and esophageal
cancer risk in China. World J Gastroenterol. 16:6020–6025.
2010.PubMed/NCBI
|
|
43
|
Lee CH, Lee JM, Wu DC, Goan YG, Chou SH,
Wu IC, Kao EL, Chan TF, Huang MC, Chen PS, et al: Carcinogenetic
impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of
the esophagus with regard to the consumption of alcohol, tobacco
and betel quid. Int J Cancer. 122:1347–1356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Y, Ji R, Wei X, Gu L, Chen L, Rong Y,
Wang R, Zhang Z, Liu B and Xia S: Esophageal squamous cell
carcinoma and ALDH2 and ADH1B polymorphisms in Chinese females.
Asian Pac J Cancer Prev. 12:2065–2068. 2011.PubMed/NCBI
|
|
45
|
Wu M, Chang SC, Kampman E, Yang J, Wang
XS, Gu XP, Han RQ, Liu AM, Wallar G, Zhou JY, et al: Single
nucleotide polymorphisms of ADH1B, ADH1C and ALDH2 genes and
esophageal cancer: A population-based case-control study in China.
Int J Cancer. 132:1868–1877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Muto M, Nakane M, Hitomi Y, Yoshida S,
Sasaki S, Ohtsu A, Yoshida S, Ebihara S and Esumi H: Association
between aldehyde dehydrogenase gene polymorphisms and the
phenomenon of field cancerization in patients with head and neck
cancer. Carcinogenesis. 23:1759–1765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Terry MB, Gammon MD, Zhang FF, Vaughan TL,
Chow WH, Risch HA, Schoenberg JB, Mayne ST, Stanford JL, West AB,
et al: Alcohol dehydrogenase 3 and risk of esophageal and gastric
adenocarcinomas. Cancer Causes Control. 18:1039–1046. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hashibe M, Boffetta P, Zaridze D, Shangina
O, Szeszenia-Dabrowska N, Mates D, Janout V, Fabiánová E, Bencko V,
Moullan N, et al: Evidence for an important role of alcohol- and
aldehyde-metabolizing genes in cancers of the upper aerodigestive
tract. Cancer Epidemiol Biomarkers Prev. 15:696–703. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jairam S and Edenberg HJ: An
enhancer-blocking element regulates the cell-specific expression of
alcohol dehydrogenase 7. Gene. 547:239–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang J, Wei J, Xu X, Pan W, Ge Y, Zhou C,
Liu C, Gao J, Yang M and Mao W: Replication study of ESCC
susceptibility genetic polymorphisms locating in the
ADH1B-ADH1C-ADH7 cluster identified by GWAS. PLoS One.
9:e940962014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hashibe M, McKay JD, Curado MP, Oliveira
JC, Koifman S, Koifman R, Zaridze D, Shangina O, Wünsch-Filho V,
Eluf-Neto J, et al: Multiple ADH genes are associated with upper
aerodigestive cancers. Nat Genet. 40:707–709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oze I, Matsuo K, Suzuki T, Kawase T,
Watanabe M, Hiraki A, Ito H, Hosono S, Ozawa T, Hatooka S, et al:
Impact of multiple alcohol dehydrogenase gene polymorphisms on risk
of upper aerodigestive tract cancers in a Japanese population.
Cancer Epidemiol Biomarkers Prev. 18:3097–3102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li H, Borinskaya S, Yoshimura K, Kal'ina
N, Marusin A, Stepanov VA, Qin Z, Khaliq S, Lee MY, Yang Y, et al:
Refined geographic distribution of the oriental ALDH2*504Lys (nee
487Lys) variant. Ann Hum Genet. 73:335–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mizoi Y, Yamamoto K, Ueno Y, Fukunaga T
and Harada S: Involvement of genetic polymorphism of alcohol and
aldehyde dehydrogenases in individual variation of alcohol
metabolism. Alcohol Alcohol. 29:707–710. 1994.PubMed/NCBI
|
|
55
|
Yokoyama A, Muramatsu T, Ohmori T,
Makuuchi H, Higuchi S, Matsushita S, Yoshino K, Maruyama K, Nakano
M and Ishii H: Multiple primary esophageal and concurrent upper
aerodigestive tract cancer and the aldehyde dehydrogenase-2
genotype of Japanese alcoholics. Cancer. 77:1986–1990. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yokoyama A, Muramatsu T, Ohmori T,
Yokoyama T, Okuyama K, Takahashi H, Hasegawa Y, Higuchi S, Maruyama
K, Shirakura K and Ishii H: Alcohol-related cancers and aldehyde
dehydrogenase-2 in Japanese alcoholics. Carcinogenesis.
19:1383–1387. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lewis SJ and Smith GD: Alcohol, ALDH2, and
esophageal cancer: A meta-analysis which illustrates the potentials
and limitations of a Mendelian randomization approach. Cancer
Epidemiol Biomarkers Prev. 14:1967–1971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yokoyama T, Yokoyama A, Kato H, Tsujinaka
T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M, et
al: Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes,
and risk for esophageal squamous cell carcinoma in Japanese men.
Cancer Epidemiol Biomarkers Prev. 12:1227–1233. 2003.PubMed/NCBI
|
|
59
|
Brooks PJ, Enoch MA, Goldman D, Li TK and
Yokoyama A: The alcohol flushing response: An unrecognized risk
factor for esophageal cancer from alcohol consumption. PLoS Med.
6:e502009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lewis DF, Bird MG and Parke DV: Molecular
modelling of CYP2E1 enzymes from rat, mouse and man: An explanation
for species differences in butadiene metabolism and potential
carcinogenicity, and rationalization of CYP2E substrate
specificity. Toxicology. 118:93–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gonzalez FJ: Role of cytochromes P450 in
chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat
Res. 569:101–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo YM, Wang Q, Liu YZ, Chen HM, Qi Z and
Guo QH: Genetic polymorphisms in cytochrome P4502E1, alcohol and
aldehyde dehydrogenases and the risk of esophageal squamous cell
carcinoma in Gansu Chinese males. World J Gastroenterol.
14:1444–1449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qin JM, Yang L, Chen B, Wang XM, Li F,
Liao PH and He L: Interaction of methylenetetrahydrofolate
reductase C677T, cytochrome P4502E1 polymorphism and environment
factors in esophageal cancer in Kazakh population. World J
Gastroenterol. 14:6986–6992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Morita S, Yano M, Shiozaki H, Tsujinaka T,
Ebisui C, Morimoto T, Kishibuti M, Fujita J, Ogawa A, Taniguchi M,
et al: CYP1A1, CYP2E1 and GSTM1 polymorphisms are not associated
with susceptibility to squamous-cell carcinoma of the esophagus.
Int J Cancer. 71:192–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lucas D, Ménez C, Floch F, Gourlaouen Y,
Sparfel O, Joannet I, Bodénez P, Jezequel J, Gouérou H, Berthou F,
et al: Cytochromes P4502E1 and P4501A1 genotypes and susceptibility
to cirrhosis or upper aerodigestive tract cancer in alcoholic
caucasians. Alcohol Clin Exp Res. 20:1033–1037. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rossini A, Rapozo DC, Soares Lima SC,
Guimarães DP, Ferreira MA, Teixeira R, Kruel CD, Barros SG,
Andreollo NA, Acatauassú R, et al: Polymorphisms of GSTP1 and
GSTT1, but not of CYP2A6, CYP2E1 or GSTM1, modify the risk for
esophageal cancer in a western population. Carcinogenesis.
28:2537–2542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dura P, Berkers T, van Veen EM, Salomon J,
te Morsche RH, Roelofs HM, Kristinsson JO, Wobbes T, Witteman BJ,
Tan AC, et al: Polymorphisms in alcohol-metabolizing enzymes and
esophageal carcinoma susceptibility: A Dutch Caucasian case-control
study. J Hum Genet. 58:742–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Trimmer EE: Methylenetetrahydrofolate
reductase: Biochemical characterization and medical significance.
Curr Pharm Des. 19:2574–2593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ly A, Hoyt L, Crowell J and Kim YI: Folate
and DNA methylation. Antioxid Redox Sign. 17:302–326. 2012.
View Article : Google Scholar
|
|
70
|
Frosst P, Blom HJ, Milos R, Goyette P,
Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA and
van den Heuvel LP: A candidate genetic risk factor for vascular
disease: A common mutation in methylenetetrahydrofolate reductase.
Nat Genet. 10:111–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stern LL, Mason JB, Selhub J and Choi SW:
Genomic DNA hypomethylation, a characteristic of most cancers, is
present in peripheral leukocytes of individuals who are homozygous
for the C677T polymorphism in the methylenetetrahydrofolate
reductase gene. Cancer Epidemiol Biomarkers Prev. 9:849–853.
2000.PubMed/NCBI
|
|
72
|
Goyette P, Sumner JS, Milos R, Duncan AM,
Rosenblatt DS, Matthews RG and Rozen R: Human
methylenetetrahydrofolate reductase: Isolation of cDNA, mapping and
mutation identification. Nat Genet. 7:195–200. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bailey LB and Gregory JF III:
Polymorphisms of methylenetetrahydrofolate reductase and other
enzymes: Metabolic significance, risks and impact on folate
requirement. J Nutr. 129:919–922. 1999.PubMed/NCBI
|
|
74
|
Li QD, Li H, Wang MS, Diao TY, Zhou ZY,
Fang QX, Yang FY and Li QH: Multi-susceptibility genes associated
with the risk of the development stages of esophageal squamous cell
cancer in Feicheng County. BMC Gastroenterol. 11:742011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao P, Lin F, Li Z, Lin B, Lin J and Luo
R: Folate intake, methylenetetrahydrofolate reductase
polymorphisms, and risk of esophageal cancer. Asian Pac J Cancer
Prev. 12:2019–2023. 2011.PubMed/NCBI
|
|
76
|
Li D, Diao Y and Li H, Fang X and Li H:
Association of the polymorphisms of MTHFR C677T, VDR C352T and MPO
G463A with risk for esophageal squamous cell dysplasia and
carcinoma. Arch Med Res. 39:594–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Langevin SM, Lin D, Matsuo K, Gao CM,
Takezaki T, Stolzenberg-Solomon RZ, Vasavi M, Hasan Q and Taioli E:
Review and pooled analysis of studies on MTHFR C677T polymorphism
and esophageal cancer. Toxicol Lett. 184:73–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu YX, Wang B, Wan MH, Tang WF, Huang FK
and Li C: Meta-analysis of the relationship between the
metholenetetrahydrofolate reductase C677T genetic polymorphism,
folate intake and esophageal cancer. Asian Pac J Cancer Prev.
12:247–252. 2011.PubMed/NCBI
|
|
79
|
Yang CX, Matsuo K, Ito H, Shinoda M,
Hatooka S, Hirose K, Wakai K, Saito T, Suzuki T, Maeda T and Tajima
K: Gene-environment interactions between alcohol drinking and the
MTHFR C677T polymorphism impact on esophageal cancer risk: Results
of a case-control study in Japan. Carcinogenesis. 26:1285–1290.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Song C, Xing D, Tan W, Wei Q and Lin D:
Methylene-tetrahydrofolate reductase polymorphisms increase risk of
esophageal squamous cell carcinoma in a Chinese population. Cancer
Res. 61:3272–3275. 2001.PubMed/NCBI
|
|
81
|
Hiyama T, Yoshihara M, Tanaka S and
Chayama K: Genetic polymorphisms and esophageal cancer risk. Int J
Cancer. 121:1643–1658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang AH, Sun CS, Li LS, Huang JY, Chen QS
and Xu DZ: Genetic susceptibility and environmental factors of
esophageal cancer in Xi'an. World J Gastroenterol. 10:940–944.
2004.PubMed/NCBI
|
|
83
|
Li D, Dandara C and Parker MI: The 341C/T
polymorphism in the GSTP1 gene is associated with increased risk of
oesophageal cancer. BMC Genet. 11:472010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Secretan B, Straif K, Baan R, Grosse Y, El
Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L and Cogliano V: WHO International Agency for Research on
Cancer Monograph Working Group: A review of human carcinogens -
Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish.
Lancet Oncol. 10:1033–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Littleton J, Barron S, Prendergast M and
Nixon SJ: Smoking kills (alcoholics)! Shouldn't we do something
about it? Alcohol Alcohol. 42:167–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Prabhu A, Obi KO and Rubenstein JH: The
synergistic effects of alcohol and tobacco consumption on the risk
of esophageal squamous cell carcinoma: A meta-analysis. Am J
Gastroenterol. 109:822–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yaegashi Y, Onoda T, Morioka S, Hashimoto
T, Takeshita T, Sakata K and Tamakoshi A: Joint effects of smoking
and alcohol drinking on esophageal cancer mortality in Japanese
men: Findings from the Japan collaborative cohort study. Asian Pac
J Cancer Prev. 15:1023–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen J, Zhang N, Wakai T, Wei L, He Y,
Kumagai N, Kitsu K, Wang S and Akazawa K: Effect of the interaction
between the amount and duration of alcohol consumption and tobacco
smoking on the risk of esophageal cancer: A case-control study. Exp
Ther Med. 1:991–997. 2010.PubMed/NCBI
|
|
89
|
Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet
JM, Taylor CE, Becker K and Xu J: Smoking in China: Findings of the
1996 National Prevalence Survey. JAMA. 282:1247–1253. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S,
Egashira A, Saeki H, Kakeji Y, Morita M, Sakaguchi Y, et al:
Alcohol drinking, cigarette smoking, and the development of
squamous cell carcinoma of the esophagus: Molecular mechanisms of
carcinogenesis. Int J Clin Oncol. 15:135–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lopes CF, de Angelis BB, Prudente HM, de
Souza BV, Cardoso SV and de Azambuja Ribeiro RI: Concomitant
consumption of marijuana, alcohol and tobacco in oral squamous cell
carcinoma development and progression: Recent advances and
challenges. Arch Oral Biol. 57:1026–1033. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Salaspuro M: Interactions of alcohol and
tobacco in gastrointestinal cancer. J Gastroenterol Hepatol.
28:12522013.
|
|
93
|
Simanowski UA, Suter P, Stickel F, Maier
H, Waldherr R, Smith D, Russell RM and Seitz HK: Esophageal
epithelial hyperproliferation following long-term alcohol
consumption in rats: Effects of age and salivary gland function. J
Natl Cancer Inst. 85:2030–2033. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Maier H, Weidauer H, Zöller J, Seitz HK,
Flentje M, Mall G and Born IA: Effect of chronic alcohol
consumption on the morphology of the oral mucosa. Alcohol Clin Exp
Res. 18:387–391. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Homann N, Kärkkäinen P, Koivisto T, Nosova
T, Jokelainen K and Salaspuro M: Effects of acetaldehyde on cell
regeneration and differentiation of the upper gastrointestinal
tract mucosa. J Natl Cancer Inst. 89:1692–1697. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Homann N, Jousimies-Somer H, Jokelainen K,
Heine R and Salaspuro M: High acetaldehyde levels in saliva after
ethanol consumption: Methodological aspects and pathogenetic
implications. Carcinogenesis. 18:1739–1743. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yokoyama A, Tsutsumi E, Imazeki H, Suwa Y,
Nakamura C and Yokoyama T: Contribution of the alcohol
dehydrogenase-1B genotype and oral microorganisms to high salivary
acetaldehyde concentrations in Japanese alcoholic men. Int J
Cancer. 121:1047–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Maier H, Born IA, Veith S, Adler D and
Seitz HK: The effect of chronic ethanol consumption on salivary
gland morphology and function in the rat. Alcohol Clin Exp Res.
10:425–427. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Salaspuro MP: Acetaldehyde, microbes, and
cancer of the digestive tract. Crit Rev Clin Lab Sci. 40:183–208.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Salaspuro V and Salaspuro M: Synergistic
effect of alcohol drinking and smoking on in vivo acetaldehyde
concentration in saliva. Int J Cancer. 111:480–483. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Holmstrup P and Bessermann M: Clinical,
therapeutic, and pathogenic aspects of chronic oral multifocal
candidiasis. Oral Surg Oral Med Oral Pathol. 56:388–395. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Colman G, Beighton D, Chalk AJ and Wake S:
Cigarette smoking and the microbial flora of the mouth. Aust Dent
J. 21:111–118. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Verna L, Whysner J and Williams GM:
N-nitrosodiethylamine mechanistic data and risk assessment:
Bioactivation, DNA-adduct formation, mutagenicity, and tumor
initiation. Pharmacol Ther. 71:57–81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shapiro KB, Hotchkiss JH and Roe DA:
Quantitative relationship between oral nitrate-reducing activity
and the endogenous formation of N-nitrosoamino acids in humans.
Food Chem Toxicol. 29:751–755. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ahn J, Chen CY and Hayes RB: Oral
microbiome and oral and gastrointestinal cancer risk. Cancer Causes
Control. 23:399–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pavlova SI, Jin L, Gasparovich SR and Tao
L: Multiple alcohol dehydrogenases but no functional acetaldehyde
dehydrogenase causing excessive acetaldehyde production from
ethanol by oral streptococci. Microbiology. 159:1437–1446. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Muto M, Hitomi Y, Ohtsu A, Shimada H,
Kashiwase Y, Sasaki H, Yoshida S and Esumi H: Acetaldehyde
production by non-pathogenic Neisseria in human oral microflora:
Implications for carcinogenesis in upper aerodigestive tract. Int J
Cancer. 88:342–350. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Väkeväinen S, Tillonen J, Blom M,
Jousimies-Somer H and Salaspuro M: Acetaldehyde production and
other ADH-related characteristics of aerobic bacteria isolated from
hypochlorhydric human stomach. Alcohol Clin Exp Res. 25:421–426.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Homann N, Tillonen J, Meurman JH,
Rintamäki H, Lindqvist C, Rautio M, Jousimies-Somer H and Salaspuro
M: Increased salivary acetaldehyde levels in heavy drinkers and
smokers: A microbiological approach to oral cavity cancer.
Carcinogenesis. 21:663–668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Homann N, Tillonen J, Rintamäki H,
Salaspuro M, Lindqvist C and Meurman JH: Poor dental status
increases acetaldehyde production from ethanol in saliva: A
possible link to increased oral cancer risk among heavy drinkers.
Oral Oncol. 37:153–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tillonen J, Homann N, Rautio M,
Jousimies-Somer H and Salaspuro M: Role of yeasts in the salivary
acetaldehyde production from ethanol among risk groups for
ethanol-associated oral cavity cancer. Alcohol Clin Exp Res.
23:1409–1415. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tang L, Lee AH, Xu F, Zhang T, Lei J and
Binns CW: Fruit and vegetable consumption and risk of esophageal
cancer: A case-control study in north-west China. Dis Esophagus.
27:777–782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jeurnink SM, Büchner FL, Bueno-de-Mesquita
HB, Siersema PD, Boshuizen HC, Numans ME, Dahm CC, Overvad K,
Tjønneland A, Roswall N, et al: Variety in vegetable and fruit
consumption and the risk of gastric and esophageal cancer in the
European Prospective Investigation into Cancer and Nutrition. Int J
Cancer. 131:E963–E973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lucenteforte E, Garavello W, Bosetti C,
Talamini R, Zambon P, Franceschi S, Negri E and La Vecchia C: Diet
diversity and the risk of squamous cell esophageal cancer. Int J
Cancer. 123:2397–2400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Freedman ND, Park Y, Subar AF, Hollenbeck
AR, Leitzmann MF, Schatzkin A and Abnet CC: Fruit and vegetable
intake and esophageal cancer in a large prospective cohort study.
Int J Cancer. 121:2753–2760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Choi SW and Mason JB: Folate and
carcinogenesis: An integrated scheme. J Nutr. 130:129–132.
2000.PubMed/NCBI
|
|
117
|
Larsson SC, Giovannucci E and Wolk A:
Folate intake, MTHFR polymorphisms, and risk of esophageal,
gastric, and pancreatic cancer: A meta-analysis. Gastroenterology.
131:1271–1283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tio M, Andrici J, Cox MR and Eslick GD:
Folate intake and the risk of upper gastrointestinal cancers: A
systematic review and meta-analysis. J Gastroenterol Hepatol.
29:250–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Xiao Q, Freedman ND, Ren J, Hollenbeck AR,
Abnet CC and Park Y: Intakes of folate, methionine, vitamin B6, and
vitamin B12 with risk of esophageal and gastric cancer in a large
cohort study. Br J Cancer. 110:1328–1333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ulrich CM: Folate and cancer prevention: A
closer look at a complex picture. Am J Clin Nutr. 86:271–273.
2007.PubMed/NCBI
|
|
121
|
Ibiebele TI, Hughes MC, Pandeya N, Zhao Z,
Montgomery G, Hayward N, Green AC, Whiteman DC and Webb PM: Study
of Digestive Health; Australian Cancer Study: High intake of folate
from food sources is associated with reduced risk of esophageal
cancer in an Australian population. J Nutr. 141:274–283. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Matsuo K, Rossi M, Negri E, Oze I, Hosono
S, Ito H, Watanabe M, Yatabe Y, Hasegawa Y, Tanaka H, et al:
Folate, alcohol, and aldehyde dehydrogenase 2 polymorphism and the
risk of oral and pharyngeal cancer in Japanese. Eur J Cancer Prev.
21:193–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Shanmugham JR, Zavras AI, Rosner BA and
Giovannucci EL: Alcohol-folate interactions in the risk of oral
cancer in women: A prospective cohort study. Cancer Epidemiol
Biomarkers Prev. 19:2516–2524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Halsted CH, Villanueva JA, Devlin AM and
Chandler CJ: Metabolic interactions of alcohol and folate. J Nutr.
132(Suppl 8): 2367S–2372S. 2002.PubMed/NCBI
|
|
125
|
Hamid A, Wani NA and Kaur J: New
perspectives on folate transport in relation to alcoholism-induced
folate malabsorption - association with epigenome stability and
cancer development. FEBS J. 276:2175–2191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Biswas A, Senthilkumar SR and Said HM:
Effect of chronic alcohol exposure on folate uptake by liver
mitochondria. Am J Physiol Cell Physiol. 302:C203–C209. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wani NA, Hamid A and Kaur J:
Alcohol-associated folate disturbances result in altered
methylation of folate-regulating genes. Mol Cell Biochem.
363:157–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Said HM, Mee L, Sekar VT, Ashokkumar B and
Pandol SJ: Mechanism and regulation of folate uptake by pancreatic
acinar cells: Effect of chronic alcohol consumption. Am J Physiol
Gastrointest Liver Physiol. 298:G985–G993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wani NA, Nada R, Khanduja KL and Kaur J:
Decreased activity of folate transporters in lipid rafts resulted
in reduced hepatic folate uptake in chronic alcoholism in rats.
Genes Nutr. 8:209–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mason JB and Choi SW: Effects of alcohol
on folate metabolism: Implications for carcinogenesis. Alcohol.
35:235–241. 2005. View Article : Google Scholar : PubMed/NCBI
|