Introduction
MyoD was first cloned in 1987 and termed MyoD1
(1). The protein has a basic helical
three-dimensional crystal structure containing a basic
helix-loop-helix domain that is able to bind to other proteins that
also possess this domain, including myocyte-enhancing factor 2,
myogenin and creatine kinase (CK). Its adjacent basic region is
required for it to bind to the promoters or enhancers of numerous
muscle-specific genes, including CK and myogenin (1,2). The
N-terminus of MyoD contains a histidine-cysteine domain and a
transcription activation domain, which are associated with the
transcriptional activation of MyoD target genes, and the C-terminus
contains a facultative helical (helix III) domain that may be
associated with chromatin remodeling (3–5).
As a member of the muscle transcription factor
family, MyoD has decisive roles in muscle differentiation,
including muscle conversion and the maintenance of muscle
differentiation (1,2). Recent studies have demonstrated that a
synthetic MyoD polypeptide has a high affinity for the inhibitor of
DNA-binding proteins (ID), and thus may inhibit the binding of ID
with DNA, thereby inhibiting the proliferation of cancer cells
(6). In addition, Dey et al
(7) identified that MyoD is an
important cytokine in cerebellar development and a tumor suppressor
gene in medulloblastoma. These previous studies strongly indicate
the existence of a close association between MyoD and cancer
cells.
As a major organ, skeletal muscle is rich in
lymphatic vessels with an abundant blood supply. However, few
studies have demonstrated cancer metastasis to skeletal muscle
tissue (8–12). MyoD expression may be increased
following skeletal muscle injury or its invasion by cancer cells
(13,14). The present study aimed to test the
hypothesis that MyoD may act as an endogenous cytokine to inhibit
the growth of metastatic cancer. Its expression was assessed in
breast cancer tissue and cell lines and in C2C12 skeletal muscle
cells, and the proliferation of breast cancer cells was evaluated
following co-culture with control or MyoD-silenced skeletal muscle
cells.
Materials and methods
Cell culture and co-culture
The immortalized mouse myoblast cell line C2C12 and
the mouse breast tumor cell line 4T1 (each gifted by the Xiangya
Central Experiment Laboratory, Changsha, China) were maintained at
37°C in an atmosphere of 5% CO2 in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 100 U/ml penicillin, 100 U/ml
streptomycin and 10% heat-inactivated fetal bovine serum (all
purchased from Sigma-Aldrich China, Inc., Shanghai, China).
Transwell chambers (0.4-µm pore size; Corning
Incorporated, Corning, NY, USA) were placed into 6-well plates. The
interior of the Transwell plate was designated the upper chamber,
while the space between the plates formed the lower chamber, and
the chambers were separated by a polycarbonate membrane. Due to the
permeability of the polycarbonate membranes, components in the
lower-layer medium are able to affect the growth and movement of
cells placed in the upper chamber. In order to study the impact of
cytokines secreted by skeletal muscle cells on cancer cells,
Transwell chambers were used to form a co-culture, with skeletal
muscle cells in the lower chamber and cancer cells in the upper
chamber (15). C1C12 and 4T1 cells
were firstly cultured in a culture flask to at a cell concentration
of 5×105/ml for ~48 h until they reached 70% confluence.
The C2C12 cells were subsequently transplanted onto a 6-well plate
(Corning Incorporated) for 24 h, and the 4T1 cells were cultured in
Transwell (Corning Incorporated). The cells were co-cultured for 48
h with the 4T1 cells in the upper chambers and the C1C12 cells in
the lower chambers.
Immunohistochemical analysis
Breast cancer tissues and adjacent non-cancer
tissues were obtained from 7 randomly selected patients diagnosed
with breast cancer at the Xiangya Hospital of Central South
University (Changsha, China). Breast cancer tissue was dissected
away from normal tissue, fixed with 4% paraformaldehyde, embedded
in paraffin and cut into 5-µm sections. A primary mouse monoclonal
anti-MyoD antibody (#sc-32758; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) was used to detect MyoD expression. Briefly,
endogenous peroxidase was inhibited by soaking tissue sections in
3% H2O2. After rinsing in phosphate-buffered
saline (PBS), sections were incubated with goat serum
(Sigma-Aldrich China, Inc.) to block the non-specific binding of
antibodies, and sections were then incubated overnight at 4–8°C
with the anti-MyoD primary antibody (dilution, 1:50). After washing
in PBS, the sections were incubated with biotinylated goat
anti-rabbit IgG polyclonal antibody (dilution, 1:1,000; #A6667;
Sigma-Aldrich China, Inc.) for 1 h at room temperature and washed
again. A streptavidin-biotin-peroxidase complex (#RPN1051-2ML; GE
Healthcare Life Sciences, Shanghai, China) was then incubated with
the sections for 60 min at room temperature. After washing in PBS,
the signal was detected with 3,3-diaminobenzidine. A negative
control in which the primary antibody was omitted was included for
each biopsy. Written informed consent was obtained from all
patients and ethical approval was provided by the Medical Ethics
Committee of the Basic Medical College of Central South University
(Changsha, China).
Immunofluorescence
Sections were freed from the paraffin, rehydrated,
subjected to antigen retrieval in 10 mM sodium citrate, and treated
with hydrogen peroxide. Sections were then blocked with 5% goat
serum containing 3% Triton X-100 and incubated with the mouse
monoclonal anti-MyoD antibody (dilution, 1:200) at 10 µg/ml for 1 h
at room temperature. Next, slides were incubated with ABC reagent
(from the VECTASTAIN® Elite ABC kit; Vector
Laboratories, Inc., Burlingame, CA, USA) and Alexa Fluor
568-conjugated goat anti-mouse IgG (dilution, 1:1,000; #A-11004;
Thermo Fisher Scientific, Inc., Carlsbad, CA, USA), washed, and
incubated with Tyramide Signal Amplification reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Finally, the slides were washed and mounted using fluorescence
mounting medium (Dako Omnis; Agilent Technologies, Santa Clara, CA,
USA) containing 500 µg/l 4′,6-diamidino-2-phenylindole for nuclei
staining. Nuclei were then counterstained with 2% purified methyl
green for 2 min. The slides incubated in the dark for 24 h prior to
examination with a Zeiss LSM 510 laser-scanning confocal microscope
(Zeiss GmbH, Jena, Germany).
MyoD staining intensity was determined using a color
video camera (Sony DXC-950P; Sony Corporation, Tokyo, Japan)
connected to a Leica Q500 IW Imaging Workstation with MoticFluo
software v1.0 (Leica Microsystems, Cambridge, UK). A
semi-quantitative scoring method was employed by three independent
observers who were blinded to the conditions in order to record
MyoD staining expression; scores were assigned on a scale of 0–4
(0, no staining; 4, maximum staining) according to the staining
intensities.
Small interfering RNA (siRNA)
synthesis and transfection
Candidate siRNAs directed against MyoD mRNA were
designed by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Three
potential siRNAs were selected corresponding with the prediction of
single-strand domains within the mRNA secondary structure (Table I). BLAST analyses (http://blast.ncbi.nlm.nih.gov/Blast.cgi)
were performed to ensure that no additional significantly matching
mouse transcripts would be targeted by these siRNAs. MyoD and
nonsense siRNAs were synthesized by Guangzhou RiboBio Co., Ltd.,
and transfections were conducted with Invitrogen Lipofectamine 2000
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Reverse transcription (RT)-polymerase chain reaction
(PCR) and western blotting were used to detect MyoD expression
following siRNA transfection.
 | Table I.siRNA sequences and properties. |
Table I.
siRNA sequences and properties.
| siRNA | Sequences | GC% |
|---|
| 01 |
5′-GCCUGAGCAAAGUGAAUGA-dTdT-3′ | 39 |
|
|
3′-dTdT-CGGACUCGUUUCACUUACU-5′ | 39 |
| 02 |
5′-CAGCAGACGACUUCUAUGA-dTdT-3′ | 39 |
|
|
3′-dTdT-GUCGUCUGCUGAAGAUACU-5′ | 39 |
| 03 |
5′-CCAACUGCUCUGAUGGCAU-dTdT-3′ | 43 |
|
|
3′-dTdT-GGUUGACGAGACUACCGUA-5′ | 43 |
Semi-quantitative RT-PCR
The sequences of the primers for mouse MyoD were as
follows: MyoD forward, 5′-CTCCTTTGAGACAGCAGACGACTT-3′, and reverse,
5′-AAATCGCATTGGGGTTTGAGCCTG-3′; and β-actin forward,
5′-GAAACTACCTTCAACTCCATC-3′, and reverse,
5′-CGAGGCCAGGATGGAGCCGCC-3′. Primers were designed by Primer-BLAST
(www.ncbi.nlm.nih.gov/tools/primer-blast/). β-actin was
used to ascertain the presence of an equal amount of cDNA in each
reaction. A TRIzol® kit (Thermo Fisher Scientific, Inc.)
was used to extract genomic RNA. Total RNA (1 µg) purified from
siRNA-transfected cells was reverse transcribed into cDNA using AMV
reverse transcriptase (Qiagen, Venlo, Netherlands) with an RNase
inhibitor (Thermo Fisher Scientific, Inc.) and oligo(dT) primer
(Thermo Fisher Scientific, Inc.) at 40°C for 50 min, followed by
heating at 90°C for 5 min. Next, 1 µl reverse-transcriptase was
added to a 30 µl PCR mixture [Bio-Rad Laboratories (Singapore) Pte.
Ltd., Singapore] for 30 cycles. Taq polymerase was added from the
SuperScript® III One-Step RT-PCR system (Thermo Fisher
Scientific, Inc.), and each PCR cycle consisted of 93°C for 30 sec
and 54°C for 60 sec. Negative controls consisted of an equal volume
of water substituted for the volume of RNA in the RT reaction. mRNA
expression data for sample-to-sample variability in RNA input, RNA
quality and reverse transcription efficiency was normalized to
β-actin.
Western blotting
MyoD protein expression was detected by western
blotting using the aforementioned MyoD monoclonal antibody. For the
preparation of cell extracts, cells from different groups were
washed three times with ice-cold PBS and then lysed in lysis buffer
[50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM
ethylenediaminetetraacetic acid, 1% Triton X-100 and 100 µg/ml
phenylmethylsulfonyl fluoride] on ice for 20 min. Following
centrifugation at 16,000 × g for 2 min at 4°C, supernatants were
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, and then electrophoretically transferred to
nitrocellulose membranes (Invitrogen; Thermo Fisher Scientific,
Inc.). After blocking for 2 h with 5% fat-free milk at room
temperature, the membranes were incubated with the primary mouse
monoclonal anti-MyoD antibody or control mouse monoclonal
anti-β-actin secondary antibody (dilution, 1:1,000; #A1978;
Sigma-Aldrich China, Inc.) for 24 h at 4°C. The membranes were then
incubated with a secondary biotinylated goat anti rabbit IgG
polyclonal antibody (dilution, 1:1,000; #A6667; Sigma-Aldrich
China, Inc.) for 1 h at room temperature. Protein bands were
visualized using Pierce enhanced chemiluminescence reagent (Thermo
Fisher Scientific, Inc.) and Odyssey v1.2 software (LI-COR
Biosciences, Lincoln, NE, USA). The intensity of expression was
measured by comparing the target and control bands.
Cell cycle analysis using propidium
iodide (PI) and flow cytometry
Cell cycle analysis was conducted at 72 h after
transfection. 4T1 cells (5×105) from the tested groups were
harvested by brief trypsinization, washed twice with PBS, fixed in
70% ethanol overnight and stained with PI (final concentration, 20
mg/ml)/Triton X-100 solution containing 10 mg/ml RNase (DNase-free
(Thermo Fisher Scientific, Inc.). Following incubation at 37°C for
30 min, the samples were analyzed using a FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and the
populations of cells in G1, S and G2 were quantified. The BD
FACSDiva software (BD Biosciences) can export data files in FCS 2.0
or 3.0 default formats. The ModFit LT v3.0 software package (Verity
Software House, Topsham, ME, USA) was used. The Click-iT® EdU Alexa
Fluor® 488 Imaging Kit (Thermo Fisher Scientific, Inc.) is a
superior alternative to traditional proliferation assays that is
optimized for fluorescence microscopy applications.
5-ethynyl-20-deoxyuridine (EdU)
assay
EdU is a nucleoside analog of thymidine that is
incorporated into DNA during active DNA synthesis by proliferating
cells, and may be visualized by the addition of a fluorescent
molecule. Thus, proliferating 4T1 cells were detected using a
Cell-Light™ EdU Apollo®567 in vitro Imaging Kit
(Guangzhou RiboBio Co., Ltd.) according to the manufacturer's
protocol. Briefly, cells were incubated with 500 µl of 50 µM EdU
for 3 h before fixation, permeabilization and visualization of EdU
staining. Cell nuclei were stained with 10 mg/ml Hoechst 33342
(Guangzhou RiboBio Co., Ltd) for 30 min. Quantification of the
staining intensity was determined using a color video camera
(DXC-950P; Sony, Tokyo, Japan). The camera was connected to a Leica
Imaging Workstation with MoticFluo 1.0 imaging software (Leica
Q500IW; Leica, Cambridge, UK).
Statistical analysis
All experiments were performed at least five times.
All data are presented as the mean ± standard error of the mean.
For all determinations, the differences were considered significant
when P<0.05. The unpaired t-test was used for comparing
two groups. All statistical analyses were performed using SPSS
software version 18 (SPSS, Inc., Chicago, IL, USA).
Results
Proliferation of 4T1 cells may be
suppressed by C2C12 cells
To identify whether skeletal muscle cells can
inhibit the proliferation of cancer cells, mouse breast tumor cells
(4T1 cells) and mouse myoblast cells (C2C12 cells) were co-cultured
on Transwell plates. Mouse breast tumor cells and mouse breast
tumors were co-cultured as controls. PI staining and flow cytometry
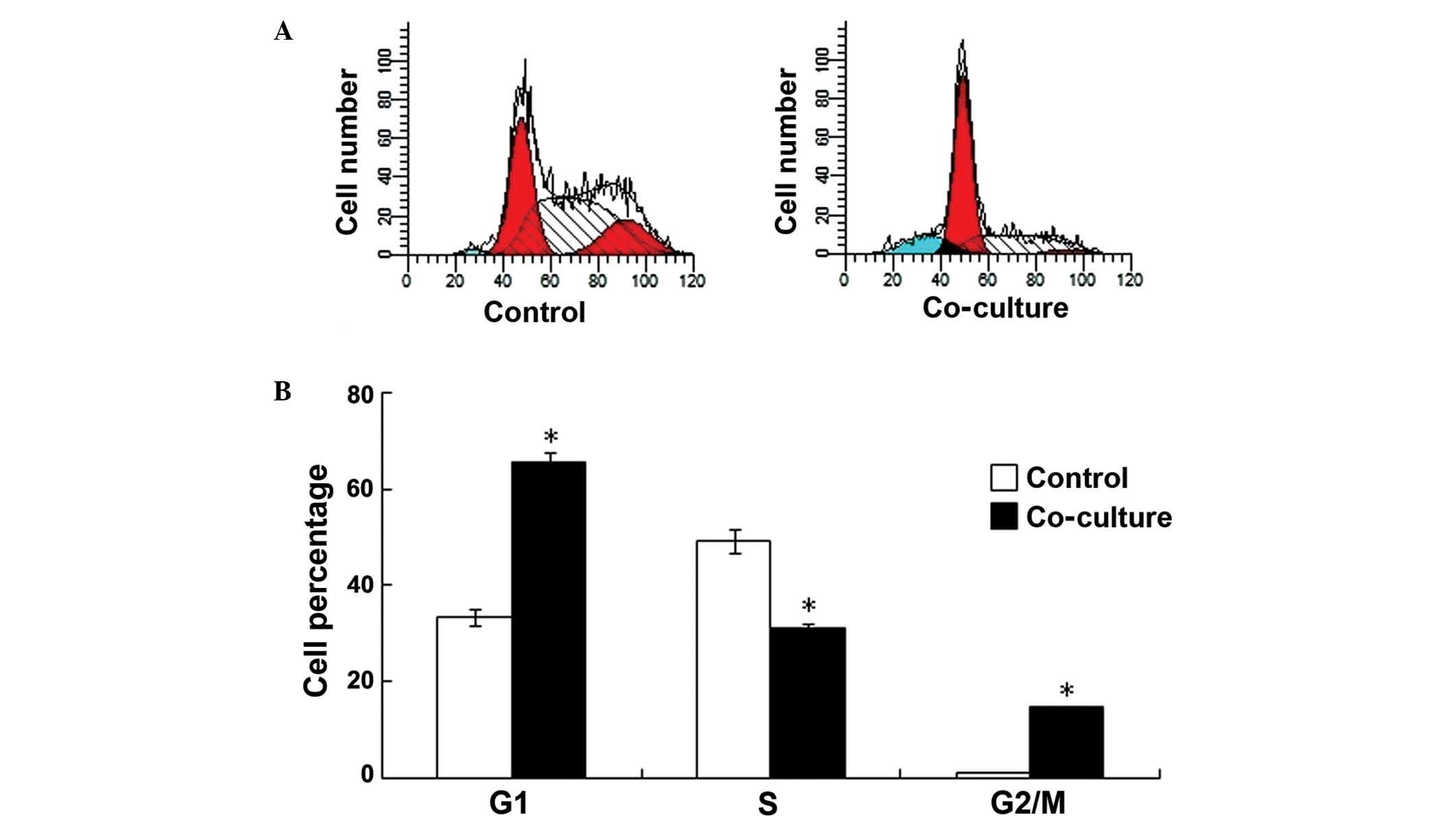
were used to detect the proliferation of the 4T1 cells. As shown in
Fig. 1, at 48 h after co-culture, 65%
of the cells were in G1 phase and 33% were in S phase in the
experimental group, compared to 31% in G1 phase and 56% that were
in S phase in the control group (G1: P=0.0376 vs. control group; S:
P=0.0396 vs. control group; G2/M: P=0.0479 vs. control group; n=6),
demonstrating that the proliferation of the 4T1 cells was inhibited
following co-culture with the C2C12 cells (Fig. 1A and B).
Silencing efficiency of MyoD
siRNAs
RNA interference was used to generate a C2C12 cell
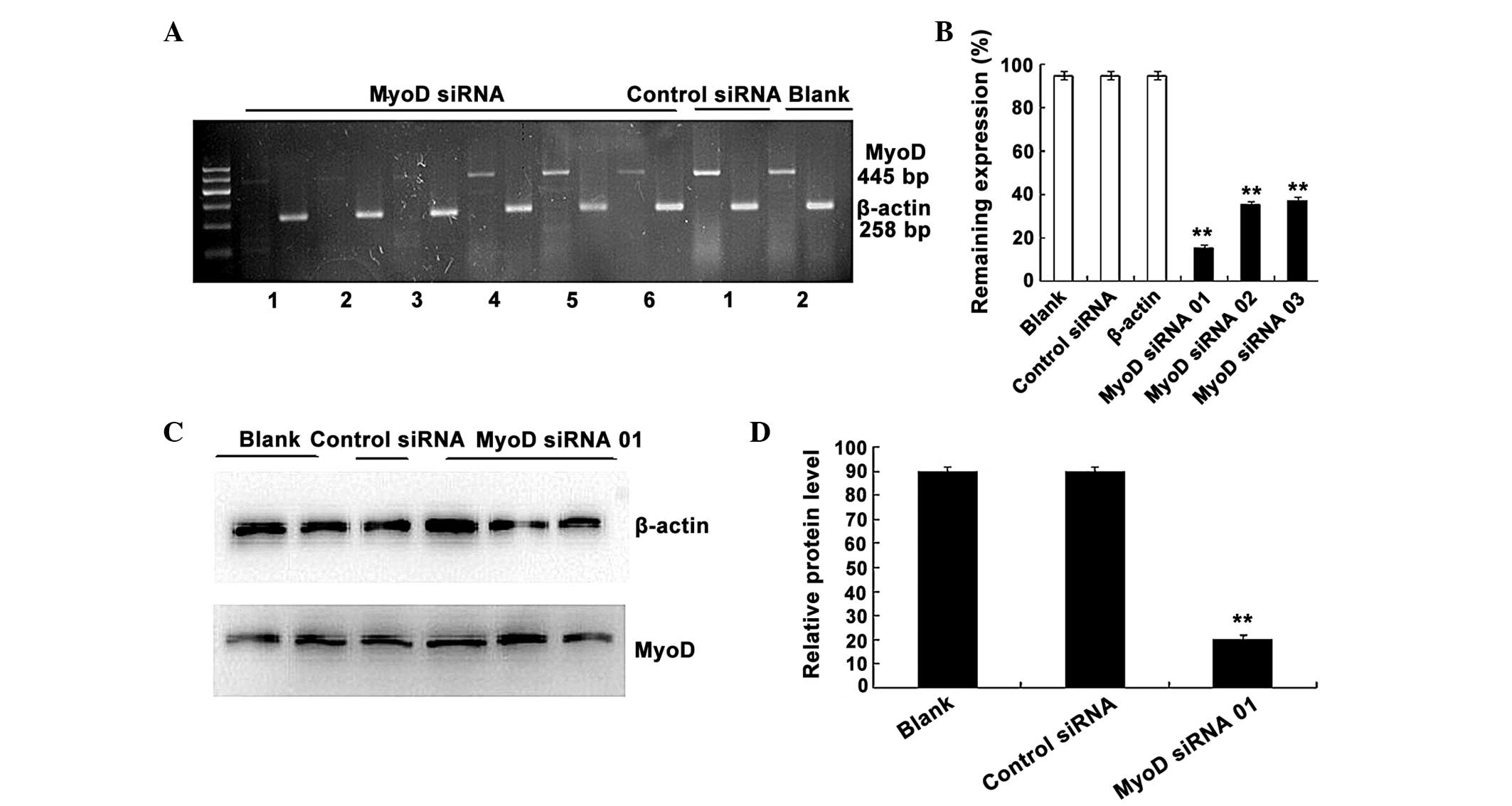
line with targeted silencing of MyoD. To verify the silencing
efficiency of MyoD siRNA, three candidate siRNAs were designed and
numbered 01, 02 and 03, respectively. The expression of MyoD mRNA
in C2C12 cells was detected using RT-PCR following siRNA
transfection at the recommended concentration of 100 nM. As shown
in Fig. 2A and B, all three siRNAs
effectively downregulated MyoD expression in the C2C12 cells
(P=0.00134 vs. control siRNA; n=3), and the silencing efficiency of
siRNA 01 reached ~70%. Therefore siRNA 01 was selected for use in
subsequent experiments.
siRNA 01 was transfected into C2C12 cells using
Lipofectamine 2000 and western blotting was performed to
investigate MyoD protein expression at 72 h after transfection
(Fig. 2C and D). The results
demonstrated that the expression of MyoD was markedly reduced
compared with the control group, which consisted of untransfected
cells. Gray value analysis indicated that MyoD expression in the
experimental group was decreased by ~70% compared with the control
group (P=0.00149 vs. control siRNA; n=3), which was consistent with
the RT-PCR results, and the nonsense siRNA had no effect on MyoD
expression.
Proliferation of 4T1 cells is
inhibited by MyoD
To explore the effects of MyoD and other cytokines
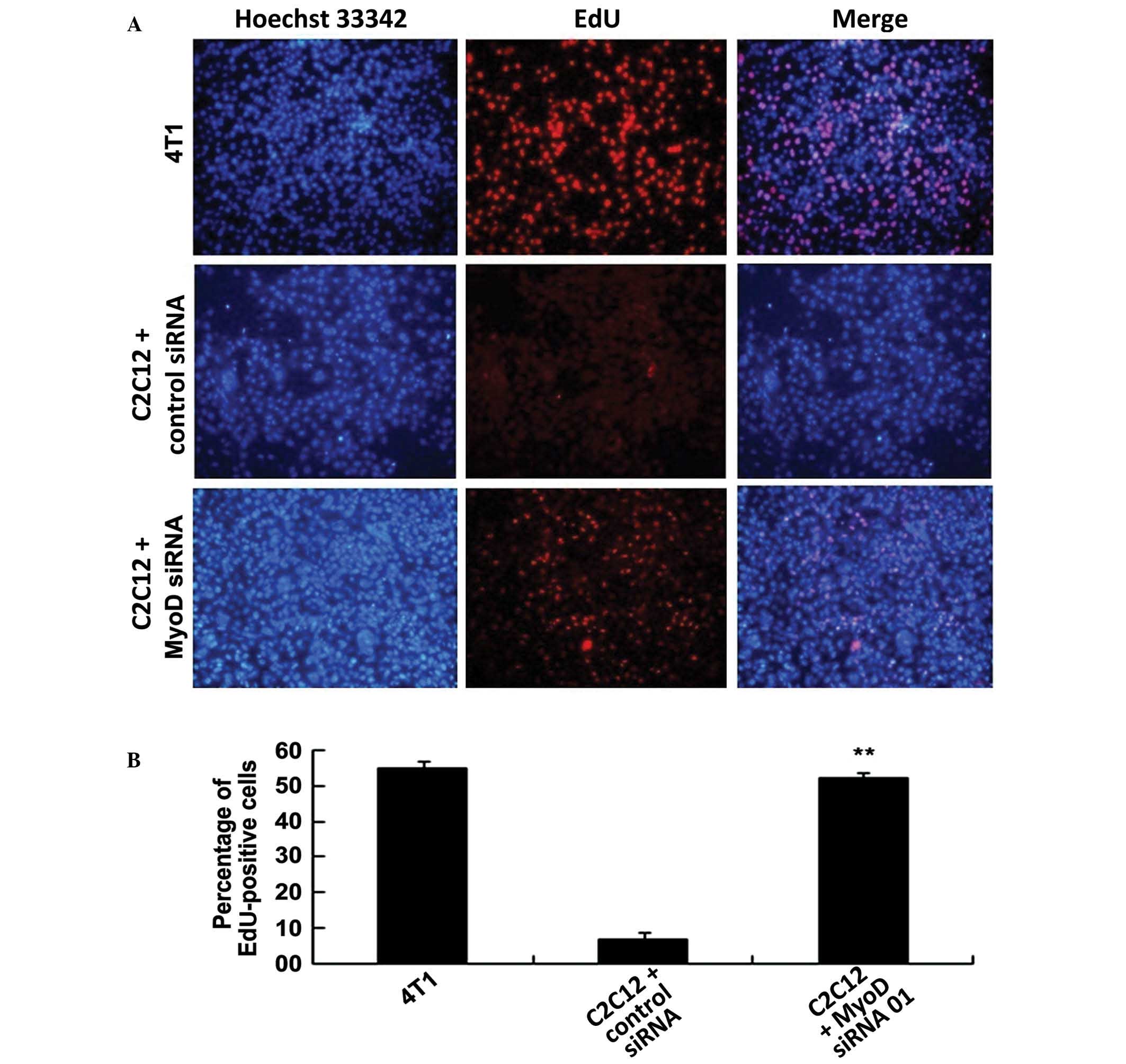
from skeletal muscle cells on cancer cells, MyoD-silenced C2C12
cells were co-cultured with 4T1 cells in Transwell plates and EdU
assays were performed to detect cancer cell proliferation following
48 h of co-culture (Fig. 3A and B).
Fluorescence microscopy clearly showed that the 4T1 cells alone
actively proliferated and exhibited extremely strong EdU
fluorescent labeling (~50% of cell population; n=3). The EdU
fluorescence of the 4T1 cells co-cultured with the untransfected
C2C12 cells was extremely sparse and was detected in ~10% of all
cells (untransfected C2C12 vs. 4T1, P=0.00648; n=3). However, the
EdU fluorescence of the 4T1 cells co-cultured with the C2C12 cells
that had been transfected with MyoD siRNA was moderately bright,
accounting for ~40% of all cells with gray signals (C2C12+MyoD
siRNA vs. 4T1, P=0.00130; n=3). The proliferation of the 4T1 cells
co-cultured with the C2C12 cells transfected with nonsense siRNA
was similar to that of the 4T1 cells co-cultured with the
untransfected C2C12 cells (C2C12+control siRNA vs. 4T1, P=0.00539;
n=3). These results indicate that MyoD is able to markedly inhibit
the proliferation of the 4T1 cells.
MyoD is weakly expressed in 4T1 and
human breast cancer cells
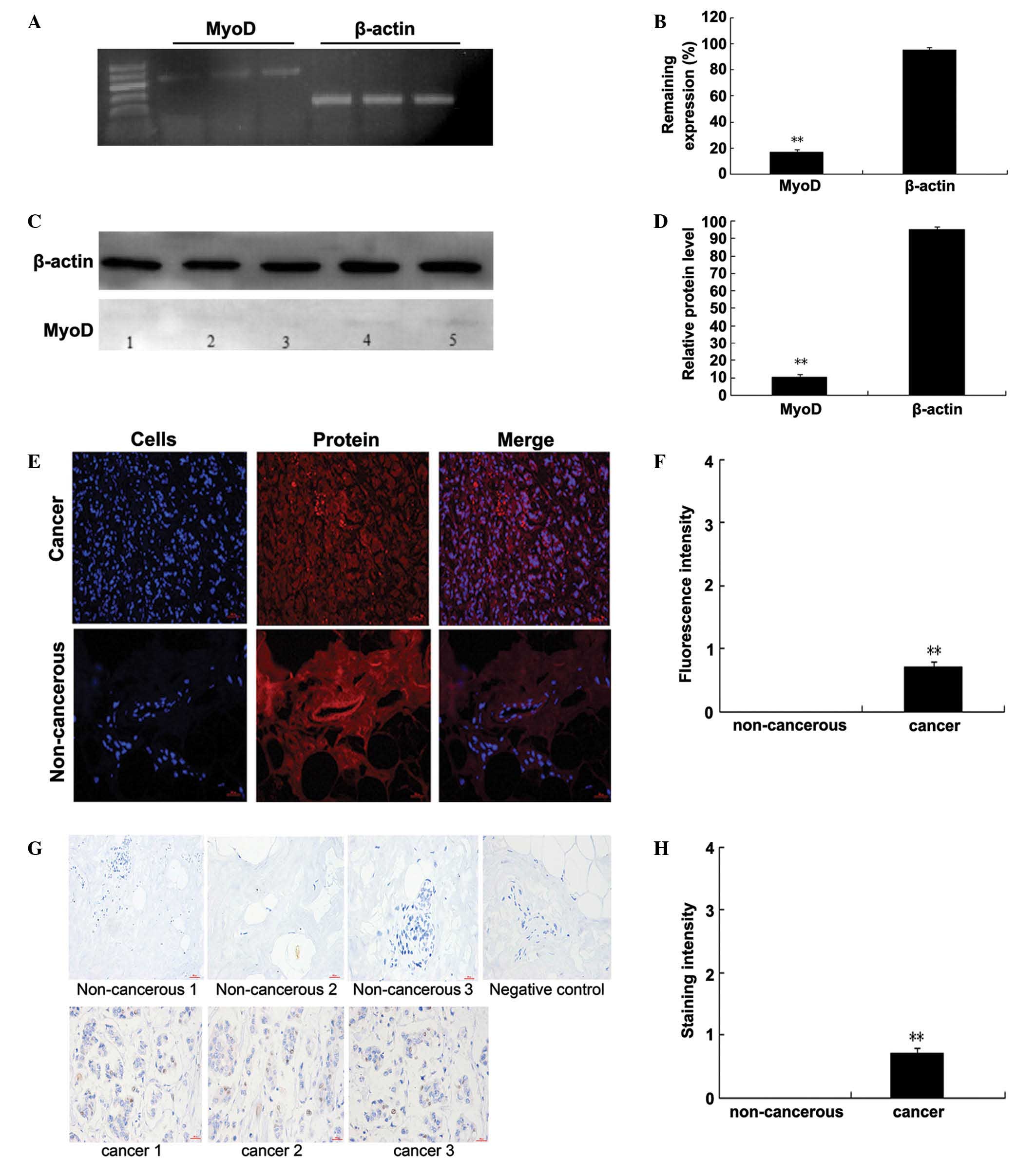
To detect whether cancer cells express MyoD, TRIzol
was used to extract genomic RNA and total protein from 4T1 cells
after 48 h of culturing in 6-well plates and then performed RT-PCR
and western blotting. Only faint bands were observed by RT-PCR
(P=0.00278 vs. β-actin; n=3) and western blotting (P=0.00324 vs.
β-actin; n=3), indicating weak MyoD expression in the 4T1 cells
(Fig. 4A–D).
To further characterize MyoD expression in breast
cancer tissue, breast cancer and normal tissue samples were
obtained from 7 randomly selected breast cancer patients and
assessed its expression using immunofluorescence and
immunohistochemistry. The two assays revealed weak MyoD expression
in the breast cancer and control human breast tissues
(immunofluorescence: 0.97±0.01, P=0.00448 vs. control, n=7,
unpaired t-test; immunohistochemistry: 0.95±0.01, P=0.00377
vs. control, n=7, unpaired t-test) (Fig. 4E–H). In addition, Fig. 4 shows evident differences between the
cancer and control tissues. The present study aimed to test the
expression of MyoD in the tissues. The other differences between
the control tissues and the cancer may be due to the atypia of
cancer cells. To the best of our knowledge, this is the first
report of low levels of MyoD expression in breast cancer
tissue.
Discussion
Skeletal muscle is widely distributed and is an
infrequent site of cancer metastasis (9). The current study was conducted to test
the hypothesis that an endogenous tumor suppressor factor may be
associated with the low incidence of cancer metastasis in skeletal
muscle. MyoD secretion increases when skeletal muscle is injured or
invaded by cancer cells, suggesting that there is an association
between MyoD and tissue wound repair (1). We hypothesized that MyoD may be an
endogenous tumor suppressor factor that is also associated with the
low occurrence of cancer in skeletal muscle (16).
MyoD is a DNA-binding protein that also has a
significant role in skeletal muscle differentiation due to its
importance in muscle conversion (6).
Recently, Dey et al (7)
demonstrated that MyoD is an important cytokine during cerebellar
development and is a tumor suppressor gene in medulloblastoma. In
fact, MyoD may regulate gene expression as a DNA-binding protein.
Chen et al (6) used synthetic
peptide fragments of MyoD to block the binding of DNA with ID,
which is an important regulator of cell proliferation. After ID
binding is blocked, cancer cell proliferation decreases (17), indicating a possible pathway by which
MyoD may inhibit the proliferation of these cells. However, MyoD is
a large protein, and its ability to enter the cell and affect DNA
duplication require further verification (18,19).
As it is difficult to monitor the biological
activity of MyoD in vitro, a MyoD-silenced model of mouse
myoblast C2C12 cells was constructed in the present study. The
C2C12 cells were co-cultured with 4T1 mouse breast cancer cells in
Transwell chambers to explore the effects of MyoD on the
proliferation of cancer cells. PI and EdU assays were used to
assess cancer cell proliferation and the results revealed that the
proliferation of the 4T1 cells was markedly inhibited by the C2C12
cells. PI staining results revealed that the population of cancer
cells in S phase was 20% lower following co-culture with skeletal
muscle cells, and the population in G1 phase was 35% higher than
that of the control group. These results indicate that skeletal
muscle cells may inhibit cancer cell proliferation by regulating
the cell cycle.
To further assess the effects of MyoD on cancer cell
proliferation, mouse breast tumor cells were co-cultured with
MyoD-silenced mouse myoblast cells. Proliferation of the 4T1 cancer
cells was significantly inhibited in the group that was co-cultured
with the control (untransfected) C2C12 cells. However, in the group
that was co-cultured with the MyoD-silenced C2C12 cells, the 4T1
cells exhibited no change in proliferative activity compared with
the 4T1 cells cultured in the absence of C2C12 cells. These results
suggest that MyoD was responsible for inhibiting the proliferation
of the 4T1 cells, indicating that this protein may act as an
endogenous factor to inhibit cancer cell proliferation (20).
The current study also evaluated MyoD expression in
4T1 cells by RT-PCR and western blotting. MyoD protein was found to
be expressed at low levels in the 4T1 cells. In addition, its
expression was assessed in the control human breast tissue and
breast cancer tissue samples obtained from randomly selected breast
cancer patients; no obvious MyoD expression was observed in the
non-cancerous tissues, whereas this protein was expressed in the
breast cancer tissue. Similarly to other tumor suppressor genes
that are expressed only in cancer cells, such as α-fetoprotein
(21), MyoD was expressed in the
cancer cells at levels insufficient to inhibit their proliferation.
A recent study performed by Dey et al (7) has shown that MyoD is a tumor suppressor
gene in medulloblastoma. The current results indicated that MyoD
may act as a tumor suppressor gene in 4T1 cells; however, this
conclusion requires further verification. In addition, the low
incidence of skeletal muscle metastasis is not limited to one type
of cancer cell, suggesting that MyoD may be a suppressor of
multiple types of cancer (8,9).
MyoD also serves important roles in muscle
transformation in the skeletal muscle microenvironment. Several
previous studies have reported the successful transformation of fat
cells into skeletal muscle cells by MyoD transfection in
vitro (22), and this technique
has been widely applied in chicken, pork and beef production
(23–25). Skeletal muscle spends a long period of
time undergoing tissue differentiation, maturation and repair
(26,27). Cancer cells are a class of cells with
high proliferative abilities (28–30). We
suspect that it may also be possible to transform cancer cells into
normal muscle tissue by taking advantage of the muscle conversion
function of MyoD, the accomplishment of which would represent
progress in cancer research.
In conclusion, the current study demonstrates for
the first time that MyoD plays a critical role in cancer
development by inhibiting the proliferation of cancer cells.
Furthermore, it may act as a tumor suppressor gene in multiple
types of cancer cells. These results will aid in the elucidation of
the mechanisms underlying the low incidence of cancer metastasis in
skeletal muscle.
Acknowledgements
The authors would like to thank the Department of
Pathology of Xiangya Hospital for their support in obtaining the
specimens. In addition, this work was supported by NSFC (grant nos.
81170016, 81270065, 81370116 and 81570026), Hunan Natural Science
Foundation and National Basic Research Program of China (973
Program) (grant nos. 2013JJ4030, 2015JJ2147 and 2012CB518104),
Youth Support Program of China Science Communication (grant no.
2015[45]) and National Innovative Entrepreneurship Training Program
funded projects to University Students of Central South University
(grant no. 201410533096).
References
|
1
|
Weintraub H, Davis R, Tapscott S, Thayer
M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R and
Hollenberg S: The MyoD gene family: Nodal point during
specification of the muscle cell lineage. Science. 251:761–766.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirai H, Tani T and Kikyo N: Structure and
functions of powerful transactivators: VP16, MyoD and FoxA. Int J
Dev Biol. 54:1589–1596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asakura A, Lyons GE and Tapscott SJ: The
regulation of MyoD gene expression: Conserved elements mediate
expression in embryonic axial muscle. Dev Biol. 171:386–398. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishibashi J, Perry RL, Asakura A and
Rudnicki MA: MyoD induces myogenic differentiation through
cooperation of its NH2-and COOH-terminal regions. J Cell Biol.
171:471–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
L'honoré A, Commère PH, Ouimette JF,
Montarras D, Drouin J and Buckingham M: Redox regulation by Pitx2
and Pitx3 is critical for fetal myogenesis. Dev Cell. 29:392–405.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CH, Kuo SC, Huang LJ, Hsu MH and Lung
FD: Affinity of synthetic peptide fragments of MyoD for Id1 protein
and their biological effects in several cancer cells. J Pept Sci.
16:231–241. 2010.PubMed/NCBI
|
|
7
|
Dey J, Dubuc AM, Pedro KD, Thirstrup D,
Mecham B, Northcott PA, Wu X, Shih D, Tapscott SJ, LeBlanc M, et
al: MyoD is a tumor suppressor gene in medulloblastoma. Cancer Res.
73:6828–6837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herrera L: Skeletal muscle metastases. Dis
Colon Rectum. 31:5791988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Surov A: Skeletal muscle metastases. Jpn J
Radiol. 32:308–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waning DL and Guise TA: Molecular
mechanisms of bone metastasis and associated muscle weakness. Clin
Cancer Res. 20:3071–3077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carey K, Bestic J, Attia S, Cortese C and
Jain M: Diffuse skeletal muscle metastases from sacral chordoma.
Skeletal Radiol. 43:985–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta P, Gill M, Gupta S, Sachdeva B,
Sansanwal P and Sen R: Metastatic Carcinoma in Skeletal Muscle-A
Rare Presentation. Int J Health Sci Res (IJHSR). 4:347–351.
2014.
|
|
13
|
Hatade T, Takeuchi K, Fujita N, Arakawa T
and Miki A: Effect of heat stress soon after muscle injury on the
expression of MyoD and myogenin during regeneration process. J
Musculoskelet Neuronal Interact. 14:325–333. 2014.PubMed/NCBI
|
|
14
|
Szuhai K, de Jong D, Leung WY, Fletcher CD
and Hogendoorn PC: Transactivating mutation of the MYOD1 gene is a
frequent event in adult spindle cell rhabdomyosarcoma. J Pathol.
232:300–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Demircan PC, Sariboyaci AE, Unal ZS, Gacar
G, Subasi C and Karaoz E: Immunoregulatory effects of human dental
pulp-derived stem cells on T cells: Comparison of transwell
co-culture and mixed lymphocyte reaction systems. Cytotherapy.
13:1205–1220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Battistelli C, Busanello A and Maione R:
Functional interplay between MyoD and CTCF in regulating long-range
chromatin interactions during differentiation. J Cell Sci.
127:3757–3767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lyden D, Young AZ, Zagzag D, Yan W, Gerald
W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K and Benezra
R: Id1 and Id3 are required for neurogenesis, angiogenesis and
vascularization of tumour xenografts. Nature. 401:670–677. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berks BC, Lea SM and Stansfeld PJ:
Structural biology of Tat protein transport. Curr Opin Struct Biol.
27:32–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sorrentino V, Pepperkok R, Davis RL,
Ansorge W and Philipson L: Cell proliferation inhibited by MyoD1
independently of myogenic differentiation. Nature. 345:813–815.
1990. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rajabi HN, Takahashi C and Ewen ME:
Retinoblastoma protein and MyoD function together to effect the
repression of Fra-1 and in turn cyclin D1 during terminal cell
cycle arrest associated with myogenesis. J Biol Chem.
289:23417–23427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gillespie JR and Uversky VN: Structure and
function of alpha-fetoprotein: A biophysical overview. Biochim
Biophys Acta. 1480:41–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uezumi A, Fukada S, Yamamoto N, Takeda S
and Tsuchida K: Mesenchymal progenitors distinct from satellite
cells contribute to ectopic fat cell formation in skeletal muscle.
Nat Cell Biol. 12:143–152. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cazzato D, Assi E, Moscheni C, Brunelli S,
De Palma C, Cervia D, Perrotta C and Clementi E: Nitric oxide
drives embryonic myogenesis in chicken through the upregulation of
myogenic differentiation factors. Exp Cell Res. 320:269–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pena RN, Quintanilla R, Manunza A,
Gallardo D, Casellas J and Amills M: Application of the microarray
technology to the transcriptional analysis of muscle phenotypes in
pigs. Anim Genet. 45:311–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Q, Lee HG, Kang SK, Baik M and Choi
YJ: Heat-shock protein beta 1 regulates androgen-mediated bovine
myogenesis. Biotechnol Lett. 36:1225–1231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li VC and Kirschner MW: Molecular ties
between the cell cycle and differentiation in embryonic stem cells.
Proc Natl Acad Sci USA. 111:9503–9508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Polesskaya A, Cuvellier S, Naguibneva I,
Duquet A, Moss EG and Harel-Bellan A: Lin-28 binds IGF-2 mRNA and
participates in skeletal myogenesis by increasing translation
efficiency. Genes Dev. 21:1125–1138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lasorella A, Benezra R and Iavarone A: The
ID proteins: Master regulators of cancer stem cells and tumour
aggressiveness. Nat Rev Cancer. 14:77–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emlet DR, Gupta P, Holgado-Madruga M, Del
Vecchio CA, Mitra SS, Han SY, Li G, Jensen KC, Vogel H, Xu LW, et
al: Targeting a glioblastoma cancer stem-cell population defined by
EGF receptor variant III. Cancer Res. 74:1238–1249. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Mendoza FH and Rodriguez Alanya E:
Cancer Stem Cells in Brain Tumors. Stem Cells in Cancer: Should We
Believe or Not? Grande E and Antón Aparicio L: Springer.
(Netherlands). 229–243. 2014.
|