Introduction
Primary central nervous system lymphoma (PCNL) is a
malignant type of non-Hodgkin's lymphoma that is only observed in
the central nervous system (1). It is
a rare extranodal lymphoma accounting for 1–6% of all intracranial
tumors, and 1–2% of all malignant lymphomas worldwide (2). PCNL has a relatively high incidence rate
among patients with acquired immune deficiency syndrome, organ
transplant recipients and patients with immunodeficiency (3).
Primary dural lymphoma (PDL) refers to a rare
subtype of PCNL with epidural or subdural involvement that is
diagnosed based on imaging (4). It
accounts for <1% of all brain lymphomas and ~0.1% of all
non-Hodgkin's lymphomas globally (5).
PDL originates outside the central nervous system of
immunologically stable individuals.
Diffuse large B-cell lymphoma (DLBCL) is a subtype
of PCNL that is pathologically diagnosed (4). The presentation of DLBCL as PDL is
extremely rare, and may result in a misdiagnosis of meningioma or
other dural-based neoplasm (4). The
neuroimaging findings of DLBCL of the dura and PDL are similar to
invasive meningioma; DLBCL of the dura and PDL present with
extra-axial lesions that appear iso- or hypointense on T1-weighted
magnetic resonance imaging (MRI) and diffusely enhance with
gadolinium administration (6,7). Furthermore, a dural tail is a frequent
finding in meningiomas and PDL (8).
However, underlying vasogenic edema appears to be more common in
PDL. Thus, the differential diagnosis of PDL based on neuro-imaging
is important.
PDL or DLBCL presenting as PDL is a very rare
disease and there is no standard treatment. As extranodal diseases
limited to a single site, they respond favorably to surgery or
focal radiation. Adjuvant treatment is necessary in the majority of
cases (6). Various treatment
combinations, including complete surgical resection, systemic
chemotherapy (CHOP and rituximab) and adjuvant radiation therapy
have been attempted in cases of primary DLBCL (9).
PDL is more indolent and has a better prognosis than
parenchymal primary CNS lymphoma or systemic lymphoma with CNS
metastasis (10). In general,
patients with PDL have a favorable outcome, with a 5-year overall
survival rate of >86%. The DLBCL relapse rate among all patients
with complete remission status ranges between 7.0 and 20.0%, with a
weighted summary proportion of 13.7%. The overall survival (OS) of
patients with DLBCL is 83%. In addition, the recurrence rates of
invasive meningioma are high (11).
The present study reports a case of DLBCL of the
dura mater with skull and scalp involvement, presenting as PDL, and
includes a brief review of the literature. The diagnosis made based
on preoperative imaging was malignant meningioma. Following
surgery, the patient received systematic chemotherapy for 4 cycles,
and the prognosis subsequent to the 4-year follow-up was favorable.
The study was approved by the ethics committee of the China-Japan
Union Hospital of Jilin University (Changchun, China) and written
informed consent was obtained from the patient.
Case report
Clinical information
A 56-year-old man presented to the China-Japan Union
Hospital of Jilin University in November 2010 with a 2-week history
of intermittent headaches, dizziness and tinnitus with no clear
cause. The headaches were described as dull and primarily confined
to the parietal-occipital area, and resolved on their own. Upon
physical examination, the patient was conscious and verbally
fluent. His pupils were equal in size, round and reactive to light.
Muscle strength and tone were normal, and no pathological reflexes
were elicited. Superficial systemic lymph nodes were not found upon
palpitation, and no abnormalities were identified in the heart and
lungs. Ultrasound examination additionally revealed no
abnormalities in the superficial systemic lymph nodes. Routine
blood [red blood cell count, 462×106/l (normal range,
400–550×106/l); hemoglobin concentration, 160 g/l
(normal range, 120–160 g/l); white blood cell count,
6.5×109/l (normal range, 4–10×109/l);
neutrophils, 65% (normal range, 50–70%); lymphocytes, 35% (normal
range, 20–40%); platelet count, 180×109/l (normal range,
100–300×109/l)], urine (white blood cells, negative;
blood, negative; specific gravity, 1.015 (normal range,
1.015–1.025); urobilinogen, 5 µmol/l (normal, <16 µmol/l);
protein, negative; glucose, negative; ketones, negative], blood
lactate dehydrogenase (110 IU/l; normal range, 80–245 IU/l) and β2
microglobulin (1,182 µg/l; normal range, 604–2,282 µg/l) test
results were normal.
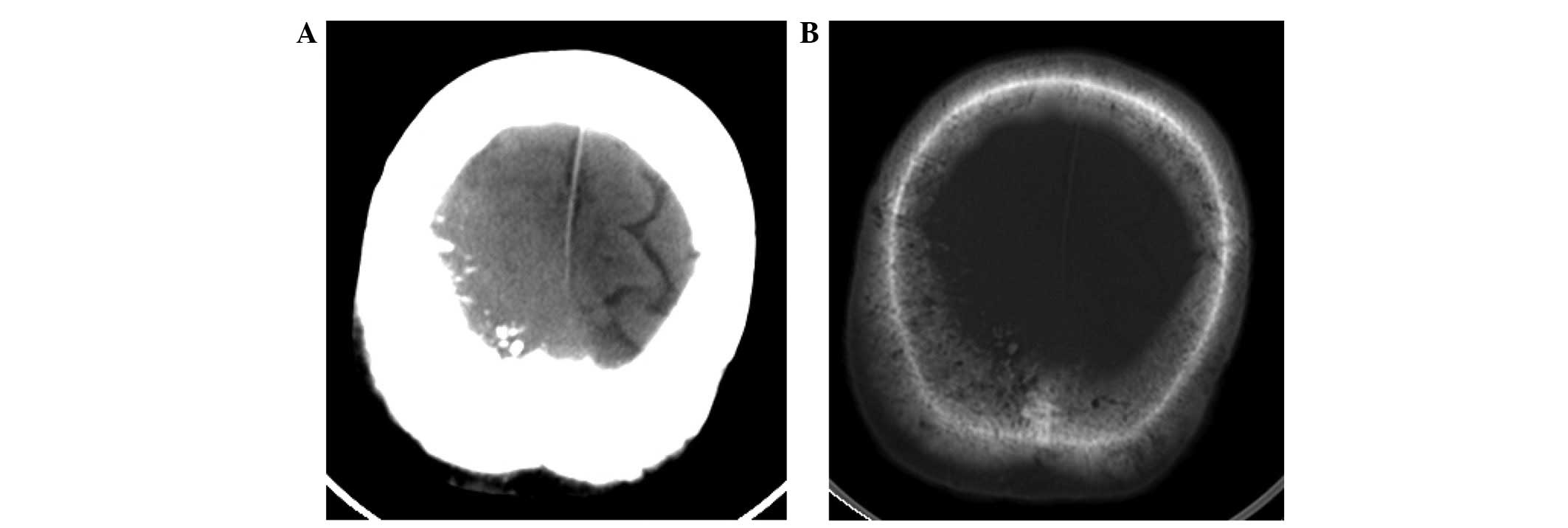
Computed tomography (CT) scanning (Lightspeed Pro16;
GE Healthcare, Chalfont, UK) of the head revealed a slightly
high-density mass beneath the right parietal skull plate, without
clear boundaries (Fig. 1A). The tumor
presented as a shadow with relatively uniform density that grew
across the cerebral falx. The bone window view revealed a rough
inner plate of the right parietal bone, and scattered high-density
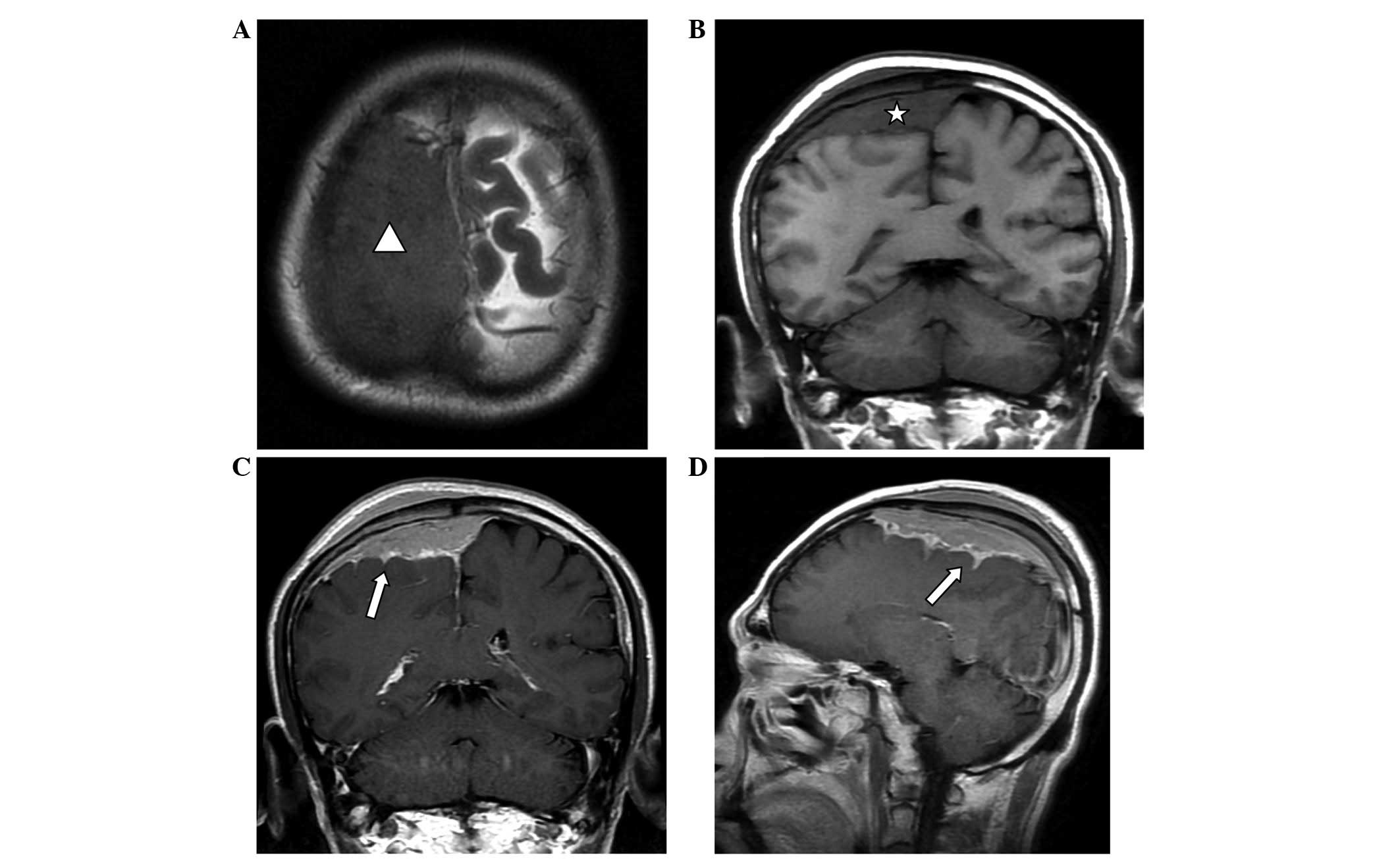
patches (Fig. 1B). MRI (Signa HDx
1.5T; GE Healthcare) revealed that a wide-base tumor attached to
the dura mater was located under the right parietal inner plate,
and was growing across the dural midline to the left (Fig. 2). Mild swelling of the soft tissues
was observed near the scalp, and the signals within the tumor were
relatively uniform, with equal-low signal on T1-weighted imaging
(WI), equal signals on T2WI and fluid-attenuated inversion recovery
and reduced tumor signals near the parietal diploë compared with
normal brain imaging. Via contrast-enhanced MRI, tumor signals were
observed to be markedly enhanced, demonstrating a relatively long
dural tail sign at the edge. The lesion was observed to invade into
the pia mater, with enhancements of a jagged shape in the right
parietal sulci, indicating leptomeningeal involvement, as well as
mild homogeneous enhancement in the diploë and soft tissues under
the scalp, near the parietal bone. The diagnosis made based on
preoperative imaging was invasive meningioma.
Surgery and pathology
Under general anesthesia, the patient underwent
craniotomy and tumor resection. During the surgery, it was observed
that the tumor was located in the dura mater, and was pale gray,
firm and adjacent to the pia mater, with skull and skin
involvement. Resected tissues were fixed in 10% formalin (Jinan
Baibo Biotechnology Co., Ltd., Jinan, China) and paraffin (Shanghai
Hualing Recovery Equipment Factory, Shanghai, China)-embedded, cut
into 1–3 µm sections, stained with hematoxylin and eosin
[Huayueyang Biotechnology (Beijing) Co., Ltd., Beijing, China] and
observed under a microscope (CX31; Olympus Corporation, Tokyo,
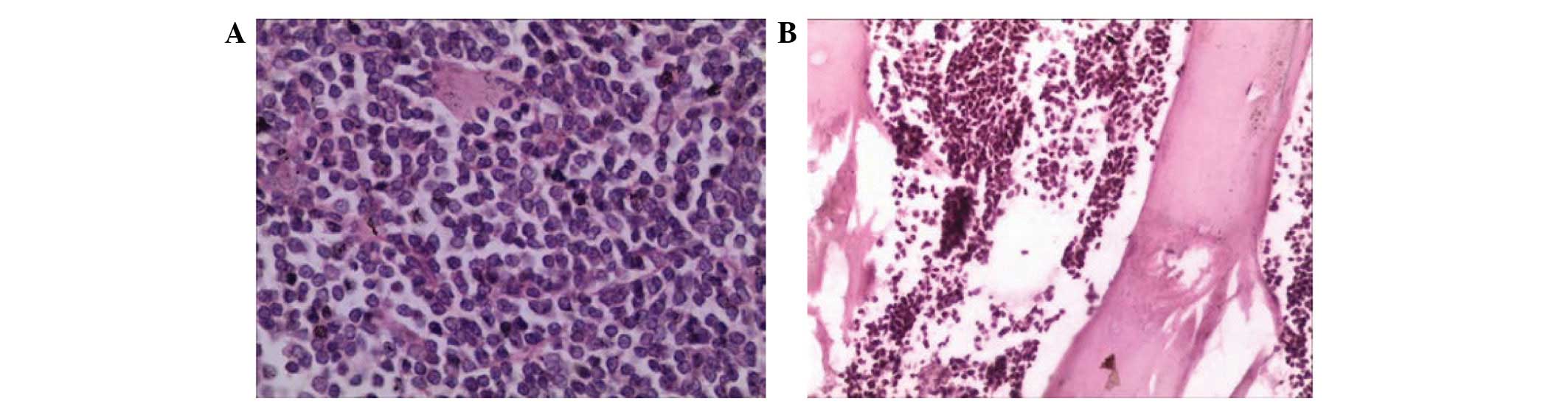
Japan). The postoperative pathological diagnosis was DLBCL. Tumors
were additionally observed inside the skull and subcutaneous
tissues, as revealed by hematoxylin and eosin staining (Fig. 3). These tumors were resected, and
cranioplasty using titanium mesh was performed.
Immunohistochemistry was performed mouse monoclonal antibodies
against cluster of differentiation (CD)21 (cat no. EP3093), CD23
(cat no. SP23), CD79 (cat no. SP18), B-cell lymphoma-2 (cat. no.
MAB-0014) and Ki-67 (cat no. MX006) (Fuzhou Maixin Biotech. Co.,
Ltd., Fuzhou, China), all at dilutions of 1:100. The results
revealed elevated expression of CD21, CD20, B-cell lymphoma 2,
CD23, CD79 and <30% Ki-67, indicating a low cell proliferation
index (Fig. 4).
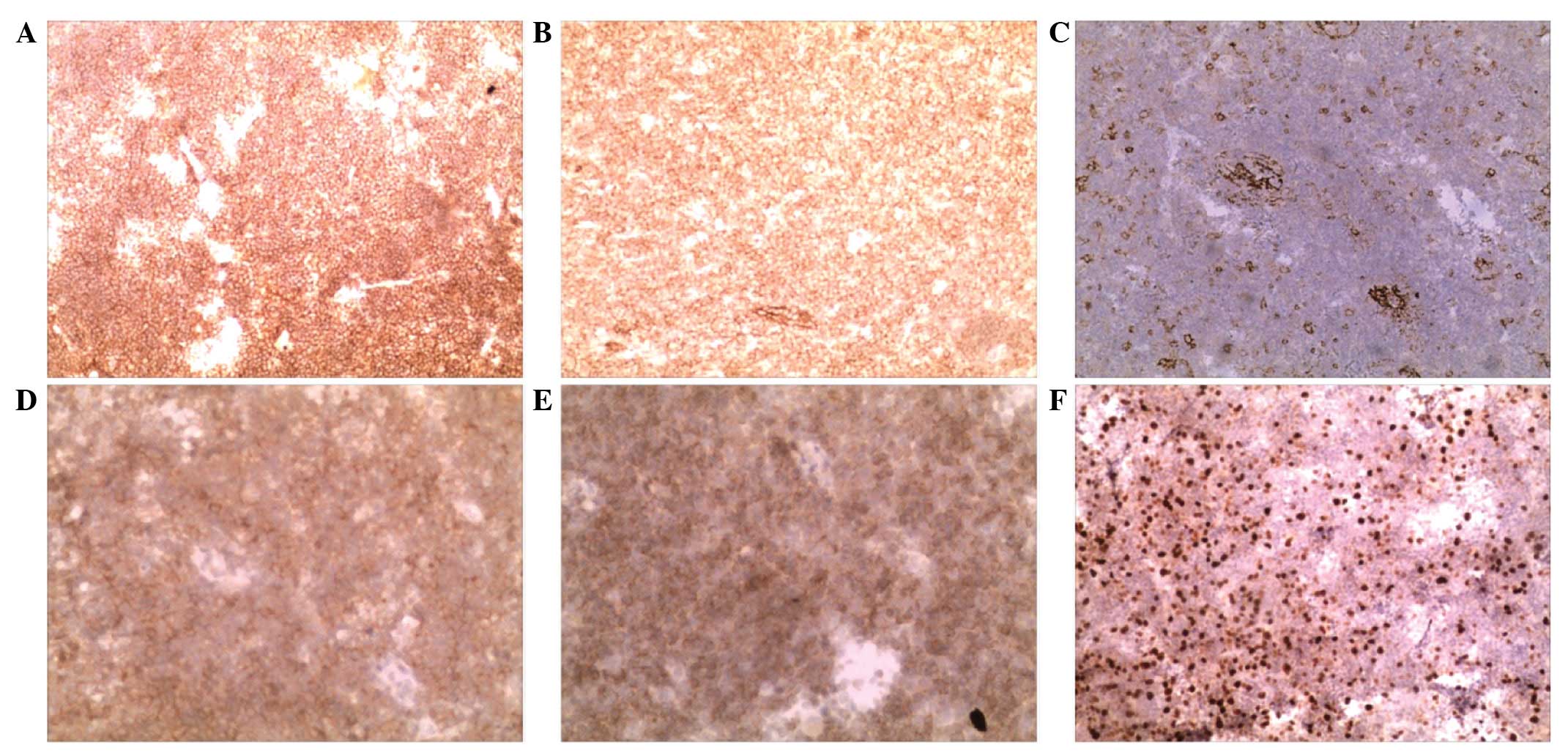 | Figure 4.Immunohistochemistry results. (A) CD20
diffusely positive expression (magnification, ×10), (B) CD21
diffusely positive expression (magnification, ×10), (C) CD23
positive expression (magnification, ×10), (D) CD79 positive
expression (magnification, ×20), (E) B-cell lymphoma 2 positive
expression (magnification, ×20), (F) Ki-67 positive expression
(magnification, ×10). Diffusely positive expression is indicated by
>75% positive cells; positive expression is indicated by 25–75%
positive cells. CD, cluster of differentiation. |
Postoperative follow-up
Following surgery, the patient underwent adjuvant
cyclophosphamide, hydroxydaunorubicin, Oncovin®,
prednisone (CHOP) chemotherapy every 3 weeks for 4 cycles (750
mg/m2 cyclophosphamide intravenously on day 1; 50
mg/m2 adriamycin intravenously on day 1; 1.4
mg/m2 vincristine intravenously on day 1; and 60 mg
prednisone orally on days 1–5). The patient remained in a good
condition 26 months subsequent to the surgery, with no neurological
abnormalities. Subsequently the patient underwent artificial
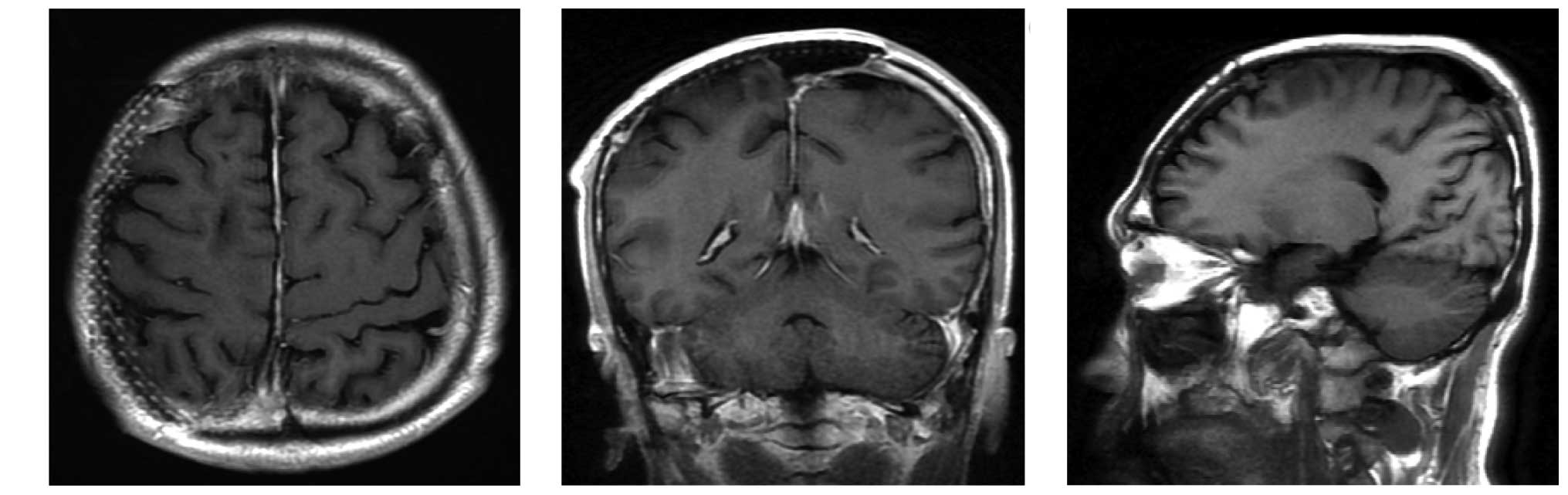
cranial plate implantation. A total of 50 months subsequent to
resection of the tumor, no signs of tumor recurrence were detected
by MRI (Fig. 5).
Discussion
PCNL has a relatively high incidence among patients
with acquired immune deficiency syndrome, organ transplant
recipients and patients with immunodeficiency (3). PDL is a rare type of PCNL and accounts
for 0.6% of all intracranial tumors (12). PDL originates in the dura mater, and
differs from other central nervous system lymphomas in its
biological mechanism (13). PDL is
typically a low-level marginal zone BCL, whereas other types of
central nervous system lymphomas are high-level diffuse large BCLs
(14). The majority of patients with
PDL do not exhibit immune system abnormalities, unlike patients
with primary malignant lymphoma of the brain, who are known to
experience immune dysfunction (8).
Patients with PDL frequently present with headaches, scalp
swelling, meningeal irritations, epilepsy and symptoms of cranial
nerve involvement (15).
Murray et al (16) conducted a retrospective analysis of
693 cases with brain lymphoma, and reported that the mean age at
diagnosis was 52 years (range, 30–65 years), with a male to female
ratio of 1.5:1, values similar to those for systemic lymphoma
(17). By contrast, Shoko et
al (18) examined 25 cases of
dural malignant BCL, and reported the mean age to be 48.7 years
(range, 28–66 years), a value that is similar to that of primary
brain lymphoma; however, the male to female ratio was 2:23,
markedly different from that of systemic lymphomas. The patient in
the present study was diagnosed with primary DLBCL, based on the
findings of histology and immunohistochemistry. Pathological
examination revealed scalp and skull involvement, which is rare.
Due to the lack of lymphatic tissue in the dura, the pathogenesis
of PDL remains unclear, but several hypotheses have been proposed,
including chronic inflammation, chronic infection, autoimmune
diseases and meningeal epithelial components (8,19,20).
As PDL lacks typical tumor imaging manifestations,
its diagnosis is considerably challenging (21). Non-enhanced CT scans typically reveal
a mass of equal or slightly higher density compared with normal
brain imaging and, in cases of skull involvement, CT scans with the
bone window setting show a rough cranial plate or clear bone damage
(21). Non-enhanced MRI scans
typically reveal equal or slightly higher T1WI signals, and
diffusion-weighted imaging frequently shows a relatively high
signal (21). In cases of brain
parenchyma involvement, varying degrees of edema may be observed
upon T1- and T2-weighted imaging (8);
skull involvement is manifested by a reduced signal in the diploë.
In the present study, enhanced MRI revealed the tumor to be
markedly enhanced, exhibiting a relatively long dural tail sign in
the periphery. The diffusion may be restricted, reflecting compact
cellularity (21). Leptomeningeal
involvement may be identified by enhancement of a jagged shape in
adjacent sulci upon MRI. PDL tends to be located in areas that are
rich in meningeal cells (22), and
frequently forms a focal lump or plaque-like thickening of the dura
mater, thereby producing imaging manifestations similar to those of
other meningeal lesions (23). The
differential diagnosis of PDL often includes epidural hematoma,
anaplastic meningioma, hemangiopericytoma, meningeal metastasis and
meningeal sarcoma (8,24). Menniti et al (25) retrospectively reviewed 14 cases of PDL
previously reported in the literature. Out of these 14 cases, a
single case was initially diagnosed as a subdural hematoma, while
the remaining cases were diagnosed as meningiomas. The body of PDL
is typically relatively flat, with a fairly long dural tail sign
(24). Intratumoral calcification is
rarely observed, and hyperplasia or hardening of the adjacent skull
bone is almost never exhibited; thus, PDL can be differentiated
from meningiomas (21). A history of
trauma may assist with the differentiation between epidural
hematoma and PDL; however, Iaccarino et al (26) reported a rare clinical case of PDL,
with clinical symptoms and imaging results similar to those of
chronic epidural hematoma. They additionally reviewed 4 cases of
PDL that were misdiagnosed as chronic subdural hematomas, in 2
cases of which chronic subdural hematoma and PDL coexisted, making
the diagnosis challenging (26). When
imaging techniques are unable to provide a clear diagnosis,
pathological examination, following craniotomy or directed biopsy,
is the only approach to confirm the diagnosis (27).
Due to the paucity of cases in individual series, no
standard treatment has been established for intracranial primary
DLBCL. Various treatment combinations, including complete surgical
resection, systemic chemotherapy (CHOP and rituximab) and adjuvant
radiation therapy have been attempted in cases of primary DLBCL
(6,9,28,29). Despite the success of surgical
resection of the tumor in the present case, postoperative adjuvant
chemotherapy was also employed. Radiotherapy was not offered as
part of the treatment to avoid neurotoxicity, considering that this
patient may potentially achieve long-term survival. The reasons for
the selection of systemic chemotherapy as a treatment option were
the involvement of the skull and scalp, and the fact that the
chemotherapy drugs had free access to the PDL and did not need to
pass through the blood-brain barrier. There is no definitive answer
to whether radiotherapy should have been administered to the
present patient. It was considered that the neurotoxicity caused by
radiotherapy was too great a risk for a healthy patient, and should
only be used as salvage treatment in case of relapse (30).
The prognosis of malignant B-cell-type dural
lymphoma is relatively good. Surgical excision is considered
adequate for the treatment of the disease, regardless of whether
postoperative local radiotherapy is performed (31). Shoko et al (18) reviewed 21 cases of malignant
B-cell-type dural lymphoma, and observed that the average survival
time of patients, including 19 patients who remained alive when the
paper was published, was 29.3 months. By contrast, the survival
time of non-Hodgkin's central nervous system tumor patients was
observed to be 12–18 months (17),
with only 8% of patients surviving for >3 years (32). Thus, the favorable outcome of the
present case is similar to that of previous case reports.
In conclusion, the present study described a rare
case of DLBCL presenting as PDL with skull and scalp involvement,
which was diagnosed based on preoperative imaging and pathology.
Follow-up non-enhanced CT scans, as well as non-enhanced and
enhanced MRI scans, were required. Early diagnosis and treatment is
crucial for patients with DLBCL presenting as PDL; therefore, upon
the detection of a diffuse infiltrative dural lesion, additional
examinations should be performed to confirm or rule out PDL.
Despite its rarity, primary malignant lymphoma should be considered
in the differential diagnosis of scalp masses. Treatment should be
individualized and should involve a multidisciplinary team
approach, including neurosurgery, radiation and oncology
professionals.
Acknowledgements
The present study was financially supported by the
International Cooperation Projects of the Science and Technology
Agency of Jilin Province (grant no. 20130413027GH) and the Natural
Science Foundation of Jilin Province (grant no. 20160101023JC)
References
|
1
|
Ervin T and Canellos GP: Successful
treatment of recurrent primary central nervous system lymphoma with
high-dose methotrexate. Cancer. 45:1556–1557. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abrey LE, Yahalom J and DeAngelis LM:
Treatment for primary CNS lymphoma: The next step. J Clin Oncol.
18:3144–3150. 2000.PubMed/NCBI
|
|
3
|
Ferreri AJ, Abrey LE, Blay JY, Borisch B,
Hochman J, Neuwelt EA, Yahalom J, Zucca E, Cavalli F, Armitage J
and Batchelor T: Summary statement on primary central nervous
system lymphomas from the Eighth international conference on
Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J
Clin Oncol. 21:2407–2414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plotkin S and Batchelor T: Primary
nervous-system lymphoma. Lancet Oncol. 2:354–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Said R, Rizk S and Dai Q: Clinical
challenges of primary diffuse large B-cell Lymphoma of the dura:
Case report and literature review. ISRN Hematol. 2011:9452122011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brito ABC, Reis F, de Souza CA, Vassallo J
and Lima CSP: Intracranial primary dural diffuse large B-cell
lymphoma successfully treated with chemotherapy. Int J Clin Exp
Med. 7:456–460. 2014.PubMed/NCBI
|
|
8
|
Iwamoto FM and Abrey LE: Primary dural
lymphomas: A review. Neurosurg Focus. 21:E52006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reis F, Schwingel R, Queiroz Lde S and de
Zanardi VA: Primary dural lymphoma: A rare subtype of primary
central nervous system lymphoma (PCNSL). Arq Neuropsiquiatr.
69:264–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thieblemont C, Berger F, Dumontet C,
Moullet I, Bouafia F, Felman P, Salles G and Coiffier B:
Mucosa-associated lymphoidtissue lymphoma is a disseminated disease
in one third of 158 patients analyzed. Blood. 95:802–806.
2000.PubMed/NCBI
|
|
11
|
Adams HJ, Nievelstein RA and Kwee TC:
Prognostic value of complete remission status at end-of-treatment
FDG-PET in R-CHOP-treated diffuse large B-cell lymphoma: Systematic
review and meta-analysis. Br J Haematol. 170:185–191. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamada SM, Ikawa N, Toyonaga S,
Nakabayashi H, Chang Park K and Shimizu K: Primary malignant
B-cell-type dural lymphoma: Case report. Surg Neurol. 66:539–543.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benouaich A, Delord JP, Danjou M, Richaud
J, Urocoste E, Soum F, Aziza R and Roche H: Primary dural lymphoma:
A report of two cases with review of the literature. Rev Neurol
(Paris). 159:652–658. 2003.(In French). PubMed/NCBI
|
|
14
|
Venkataraman G, Rizzo KA, Chavez JJ,
Streubel B, Raffeld M, Jaffe ES and Pittaluga S: Marginal zone
lymphomas involving meningeal dura: Possible link to IgG4-related
disease. Mod Pathol. 24:355–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galarza M, Gazzeri R, Elfeky HA and
Johnson RR II: Primary diffuse large B-cell lymphoma of the dura
mater and cranial vault. Case report and literature review.
Neurosurg Focus. 21:E102006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murray K, Kun L and Cox J: Primary
malignant lymphoma of the central nervous system. Results of
treatment of 11 cases and review of the literature. J Neurosurg.
65:600–607. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parekh HC, Sharma RR, Keogh AJ and Prabhu
SS: Primary malignant non-Hodgkin's lymphoma of cranial vault: A
case report. Surg Neurol. 39:286–289. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shoko M, Ikawa N, Toyonaga S, Nakabayashi
H, Chang Park K and Shimizu K: Primary malignant B-cell-type dural
lymphoma: Case report. Surg Neurol. 66:539–543, Discussion 543.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar S, Kumar D, Kaldjian EP, Bauserman
S, Raffeld M and Jaffe ES: Primary low-grade B-cell lymphoma of the
dura: A mucosa associated lymphoid tissue-type lymphoma. Am J Surg
Pathol. 21:81–87. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Isaacson PG and Du MQ: MALT lymphoma: From
morphology to molecules. Nat Rev Cancer. 4:644–653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jacobs A, Kracht LW, Grossman A, Ruger MA,
Thomas AV, Thiel A and Herholz K: Imaging in neurooncology.
NeuroRx. 2:333–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goetz P, Lafuente J, Revesz T, Galloway M,
Dogan A and Kitchen N: Primary low-grade B-cell lymphoma of
mucosa-associated lymphoid tissue of the dura mimicking the
presentation of an acute subdural hematoma. Case report and review
of the literature. J Neurosurg. 96:611–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson MD, Powell SZ, Boyer PJ, Weil RJ
and Moots PL: Dural lesions mimicking meningiomas. Hum Pathol.
33:1211–1226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sotoudeh H and Yazdi HR: A review on dural
tail sign. World J Radiol. 2:188–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Menniti A, Moschettoni L, Liccardo G and
Lunardi P: Low-grade primary meningeal lymphoma: Case report and
review of the literature. Neurosurg Rev. 28:229–233. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iaccarino C, Schiavi P, Crafa P, Bronzoni
C, Ramponi V, Mantenuto G, Cavanna L and Servadei F: Primary dural
lymphoma mimicking a chronic epidural hematoma. Differential
diagnosis of two rare conditions. Clin Neurol Neurosurg.
115:1510–1513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimoto T, Yuki K, Sasaki T, Imada Y,
Murakami T and Kodama Y: A case of subcutaneous malignantlymphoma
with dura mater lesion. No Shinkei Geka. 31:43–47. 2003.(In
Japanese). PubMed/NCBI
|
|
28
|
DeAngelis LM, Seiferheld W, Schold SC,
Fisher B and Schultz CJ: Radiation Therapy Oncology Group Study:
Combination chemotherapy and radiotherapy for primary central
nervous system lymphoma: Radiation therapy oncology group study
93-10. J Clin Oncol. 20:4643–4648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giordano A, Perrone T, Guarini A,
Ciappetta P, Rubini G, Ricco R, Palma M, Specchia G and Liso V:
Primary intracranial dural B cell small lymphocytic lymphoma. Leuk
Lymphoma. 48:1437–1443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanjeevi A, Krishnan J, Bailey PR and
Catlett J: Extranodal marginal zone B-cell lymphoma of malt type
involving the cavernous sinus. Leuk Lymphoma. 42:1133–1137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horning SJ, Weller E, Kim K, Earle JD,
O'Connell MJ, Habermann TM and Glick JH: Chemotherapy with or
without radiotherapy in limited-stage diffuse aggressive
non-Hodgkin's lymphoma: Eastern cooperative oncology group study
1484. J Clin Oncol. 22:3032–3038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Freudenstein D, Bornemann A, Ernemann U,
Boldt R and Duffner F: Intracranial malignant B-cell lymphoma of
the dura. Clin Neuropathol. 19:34–37. 2000.PubMed/NCBI
|