Introduction
Renal cell carcinomas (RCCs) originate from the
renal cortex and constitute 90% of all primary renal neoplasms
(1). RCCs have a strong tendency to
metastasize, most commonly to the lungs, bones and liver (2). However, RCC is also renowned for
unpredictable patterns of secondary spread involving almost every
other site in the body due to the complex lymphatic drainage of the
kidneys (3). According to National
Comprehensive Cancer Network guideline, localized RCC may be
effectively treated with surgery alone, while some patients with
metastatic RCC may also benefit from it (3). Patients who develop solitary recurrence
within the lung, bone or brain may be candidates for surgical
resection of primary and metastatic tumor. Systemic therapy for
metastatic RCC can be differentiated as for cell type; first-line
therapy for patients with predominantly clear cell type include
targeted therapy with sunitinib, sorafenib, pazopanib, axitinib,
temsirolimus, everolimus, bevacizumab with interferon; treatments
for non-clear cell carcinoma include temsirolimus, everolimus,
sunitinib, sorafenib, pazopanib, axitinib, or erlotinib (3). Late recurrence is another feature of
RCC, with metastatic lesions appearing ≥10 years after surgical
treatment; however, fibrosis has rarely been associated with RCC
(3). A few studies have appeared
describing retroperitoneal and perirenal fibrosis associated with
RCC (4–6). The current study presents a case in
which the first metastatic recurrence of RCC manifested as a
fibrotic mass in the thoracic cavity 6 years after a radical
nephrectomy. Written informed consent was obtained from the patient
for publication of this case study.
Case report
A 48-year-old man with dyspnea that had persisted
for 3 days visited the emergency department of Kangwon National
University Hospital of Chuncheon (Chuncheon, Korea) in March, 2014.
The patient was a current smoker, with a smoking history of 20
pack-years. The patient had undergone a right radical nephrectomy
for stage II clear cell carcinoma of the kidney 6 years previously.
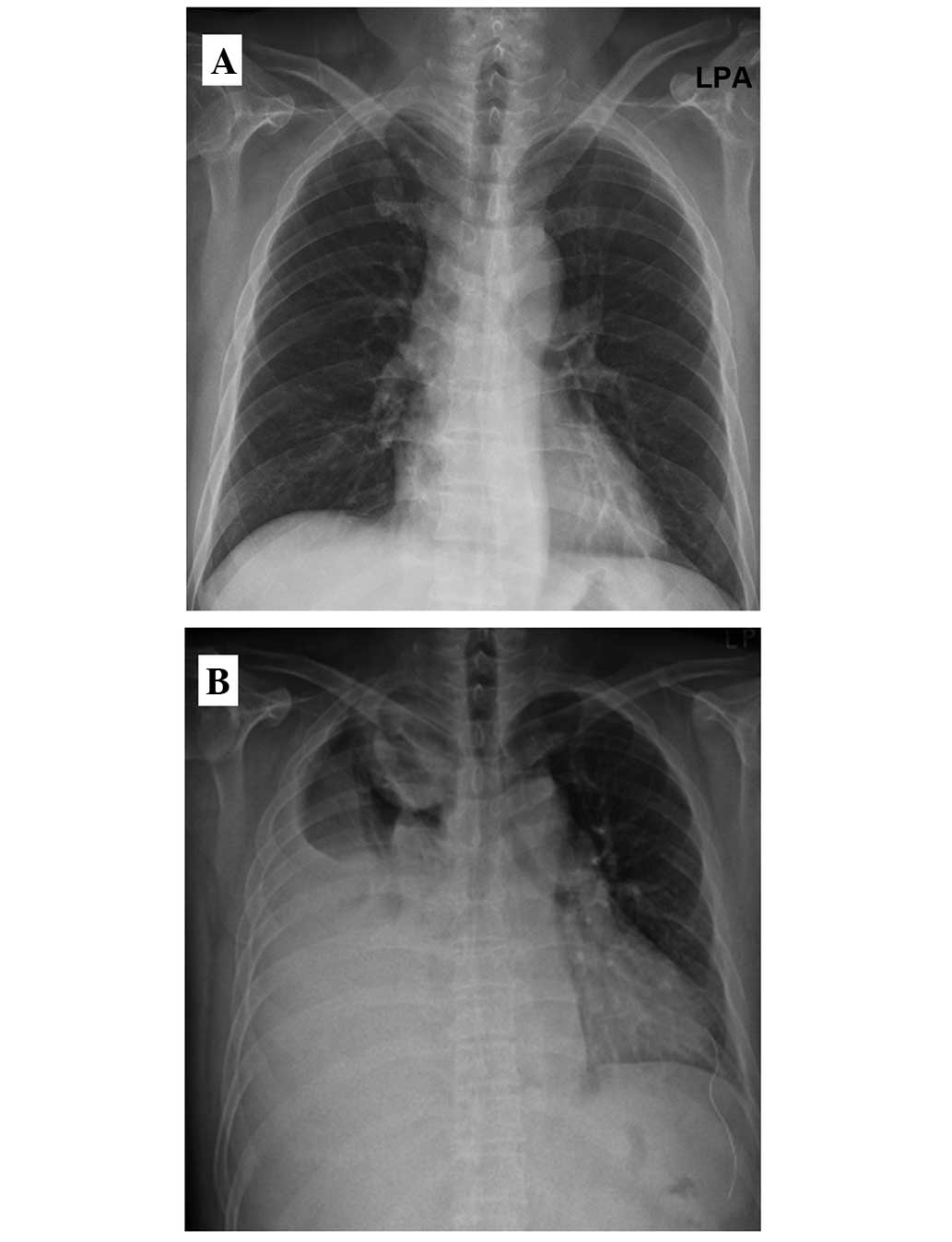
At this time, the chest radiography was unremarkable (Fig. 1A), and the patient received regular
follow-ups with no signs of metastatic disease. Upon the current
admission, chest radiography revealed pleural effusion in the right
thorax with an egg-sized mass shadow within the right upper lung
(RUL) field (Fig. 1B). Upon
examination, the patient's vital signs were within normal limits,
but breath sounds were diminished for the right lung. Laboratory
data showed the following: White blood cell count,
10,100/mm3 (normal range, 3,800–10,000/mm3);
segmented neutrophils, 70% (normal range, 40–70%); lymphocytes, 18%
(normal range, 20–50%); monocytes, 8% (normal range, 3–9%);
eosinophils, 2% (normal range, 0–5%); hemoglobin, 13.2 g/dl (normal
range, 13.3–16.5 g/dl); serum lactate dehydrogenase (LDH), 336 U/l
(normal range, <190 U/l); and total protein, 8.2 g/dl (normal
range, 5.7–8.2 g/dL). Thoracentesis was performed, and the pleural
fluid was serosanguinous in color, with a pH of 7.0. The red blood
cell count was 134,000/mm3, the white blood cell count
was 410/mm3 (polymorphonuclear cells, 14%; and
mononuclear cells, 80%), the glucose level was 132 mg/dl, the
protein level was 0.626 g/dl and the LDH level was 523 U/l. As the
patient had been diagnosed with RCC in the past, and the results of
examination had shown unilateral monocyte-dominant exudative
pleural effusion with a mass shadow on X-ray, a computed tomography
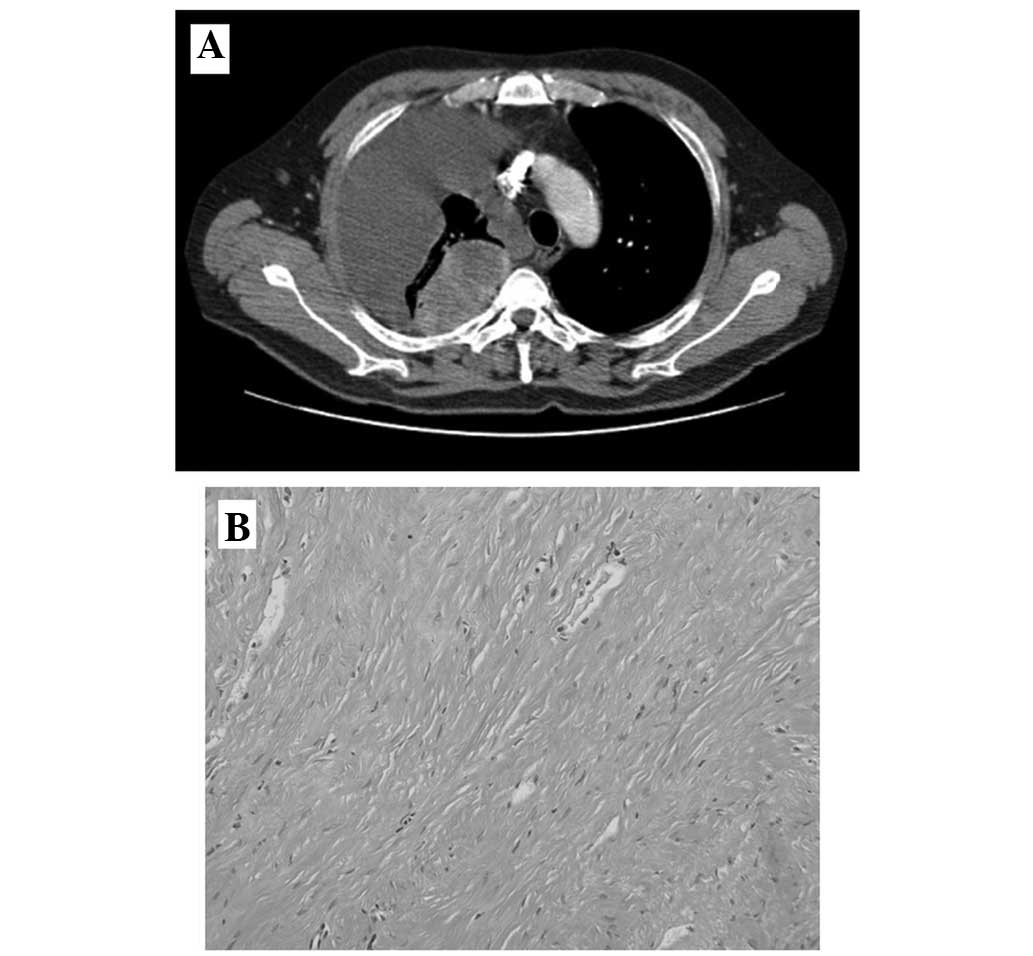
(CT) scan was now performed. CT showed a round mass, 7 cm in
diameter, on the RUL, with heterogeneous enhancement, and multiple
nodules of various sizes in the lungs, suggestive of primary lung
cancer or metastatic RCC (Fig. 2A).
To determine the correct differential diagnosis between primary
cancer of the thorax, such as lung cancer or mesothelioma, and
metastatic recurrence of RCC, a CT-guided percutaneous needle
aspiration biopsy was performed on the main mass. However,
histopathological assessment revealed a dense fibrous lesion
without malignant cells (Fig. 2B).
Briefly, biopsy tissues were fixed by 10% formaldehyde (overnight),
then paraffin embedded (PE) blocks were produced. They were cut at
4-µm thickness, mounted onto slides, and then stained using a
hematoxylin and eosin stain (Dako, Glostrup, Denmark) protocol. For
immunohistochemical staining, slides were incubated overnight at 4
°C with primary antibodies, including monoclonal mouse Wilms tumor
protein (cat. no. 6F-H2; ready to use; Cell Marque™; Sigma-Aldrich,
St. Louis, MO, USA), monoclonal rabbit anti-mouse calretinin (cat.
no. RM-9113-S0; 1:100; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), monoclonal mouse anti-human cytokeratin 5/6 (cat. no. M7237;
1:50; Dako) and monoclonal mouse anti-human thyroid transcription
factor-1 (cat. no. PA0364; ready to use; Bond™; Leica Biosystems,
Wetzlar, Germany). Immunohistochemical staining was performed using
an auto stainer (XT System Benchmark, Ventana Medical System,
Tucson, AZ, USA) according to the manufacturer's instruction. The
pleural fluid was sent for cytological examination twice, and
demonstrated no evidence of malignancy. However, due to a strong
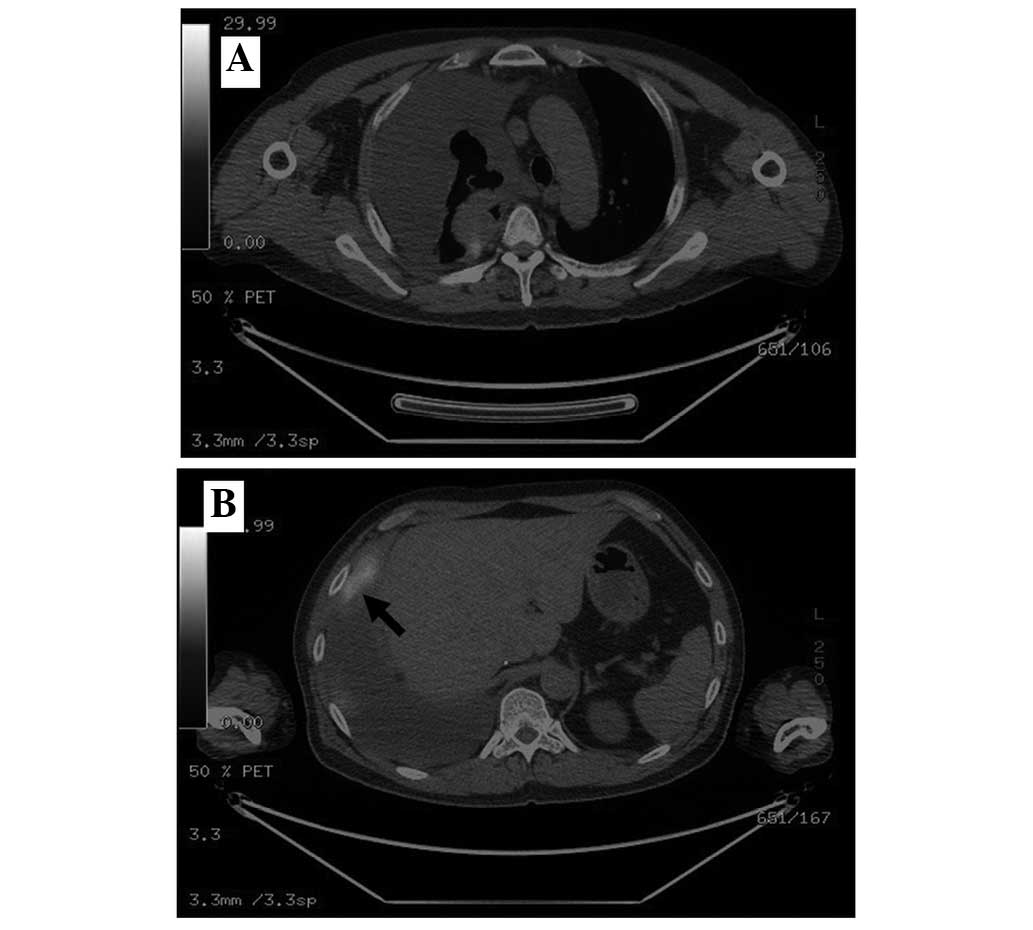
clinical suspicion of malignancy, positron emission tomography-CT
(PET-CT) was performed, which demonstrated an irregular
hypermetabolic RUL mass, with nodular thickening along the right
pleura, with a standardized uptake value (SUV) of 5.0, and small
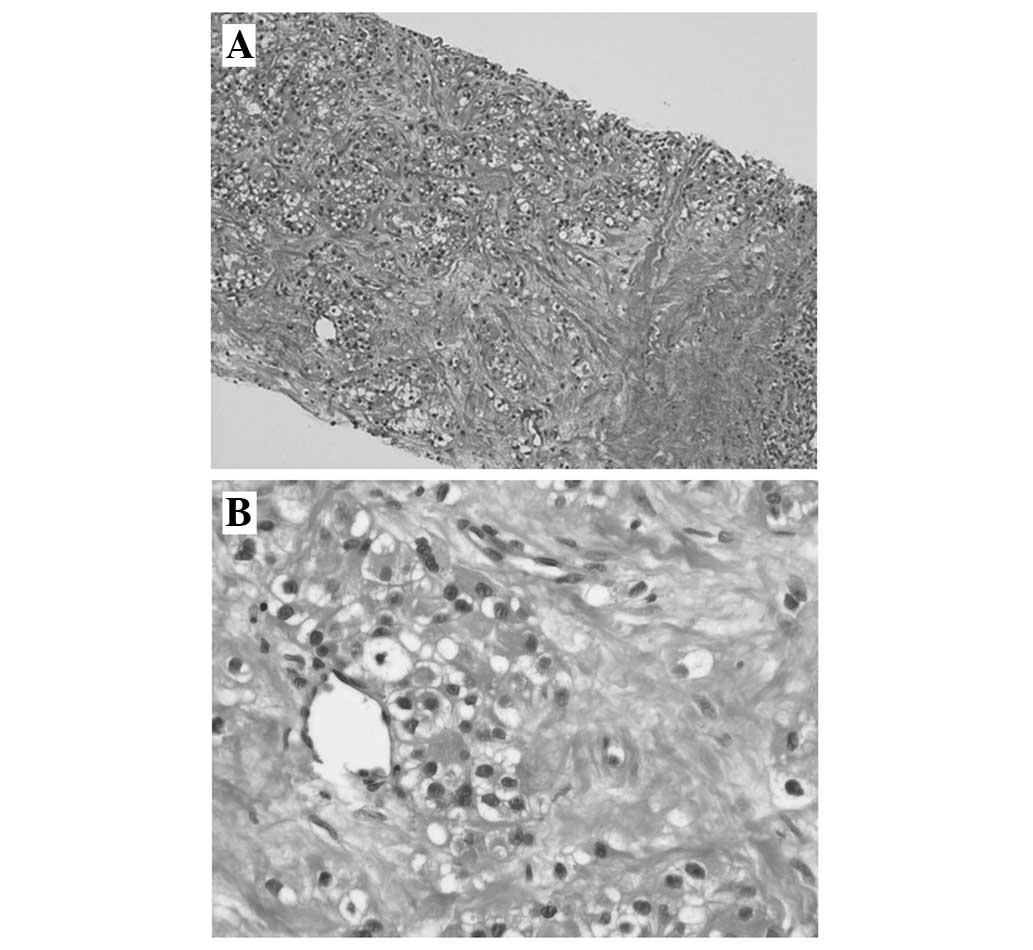
pulmonary nodules with an SUV of 2.0 (Fig. 3A and B). Ultrasound-guided biopsy of a
hypermetabolic pleural nodule visualized on PET-CT was attempted,
and histological assessment found the lesion to consist of tumor
cells with abundant clear cytoplasm and small round nuclei with
atypia, consistent with a diagnosis of metastatic clear cell
carcinoma (Fig. 4).
Immunohistochemical analyses were negative for Wilms tumor protein,
calretinin, cytokeratin 5/6 and thyroid transcription factor-1,
which excluded mesothelioma or primary lung cancer from the final
diagnosis. The patient was diagnosed with recurrent RCC and
treatment was commenced with 50 mg oral sunitinib daily. Although
there was a significant decrease in the amount of pleural effusion
after 1 month of treatment with sunitinib, the fibrotic mass did
not respond to this medication. Everolimus was administered 2
months later, as the patient developed progressive disease. At
present, the patient has exhibited stable disease for 9 months with
everolimus treatment.
Discussion
Renal cell carcinoma accounts for 90–95% of
malignant neoplasms originating from the kidney. The tumors have
been reported to be resistant to cytotoxic agents, infrequently
responsive to biological response modifiers, such as interleukin-2,
and to produce a variable clinical course for patients with
metastatic disease (7). Fibrosis is
not a usual finding associated with metastatic RCC, and only a few
studies have reported retroperitoneal or perirenal fibrosis
(4–6).
A previous case report detailed a 53-year-old male
who underwent radical nephrectomy for sarcomatoid renal cell
carcinoma, and a follow up CT 6 months after surgery demonstrated
left retroperitoneal mass which was histologically revealed as a
retroperitoneal fibrosis rather than a relapse (4). Another case reports a 78-year-old male
diagnosed with clear cell carcinoma where biopsy of the resected
mass revealed stromal fibrosis (5),
and there was a case of a 43-year-old male with clear cell RCC,
which was accompanied by fibrosis of the peritoneum during the
surgery (6).
It has been widely accepted that fibrosis is an
inflammatory immunological reaction, and although diverse diseases
and molecular pathways initiate the fibrotic process, in all
instances the biochemical and cellular mechanisms contributing to
the final outcome are shared. Tissue injuries activate the immune
system and repair mechanisms, which lead to effective healing
pathways if a type 1 T helper (Th1) cell response is dominant,
whereas if a Th2 response is predominant, an increase in Th17 cells
will lead to chronic inflammation, ultimately resulting in fibrosis
(8). The chronic inflammatory
microenvironment has long been recognized as conducive to
tumorigenesis. Mesodermal tumor cells produce extracellular matrix
molecules involved in fibrosis, whereas ectoderm- or
endoderm-derived tumors can undergo epithelial-mesenchymal
transition (EMT) under inflammatory conditions, and EMT of
parenchymal tumor cells such as in RCC, is crucial for
tumor-associated fibrosis and metastasis (9).
Fromowitz and Miller (10) suggested fibrosis as an exaggerated
host response to tumor-associated antigens in RCC. The pathogenesis
of fibrosis associated with metastatic RCC is uncertain, although a
study by Terada (6) suggested that
local tissue responses to RCC may protect the surrounding tissue
from RCC invasion and growth, and thus fibrosis is considered a
favorable reaction for the patient.
The present case is of note since the biopsy of the
main mass on the RUL revealed fibrotic tissue, which made it
difficult to distinguish between primary lung cancer and metastatic
RCC. Few cases have reported on retroperitoneal and perirenal
fibrosis associated with RCC (4–6). However,
to the best of our knowledge, no cases of fibrosis associated with
pleural or lung metastasis of RCC have been reported. It was also a
possibility that the main mass could have been a benign fibrotic
mass in the present study, however, the PET-CT scan showed a lesion
with focal fluorodeoxyglucose uptake in the nodular mass,
surrounded by fibrotic tissue, suggesting malignancy and a fibrotic
response. The role of PET-CT for RCC has been limited to restaging
or the evaluation of metastasis (11–13).
However, PET-CT may have a complementary role as a problem-solving
tool in cases that are equivocal on conventional imaging.
In summary, the present report describes a patient
in whom recurrent RCC manifested in the thorax as lung and pleural
metastases, including fibrosis. It is important for physicians to
rule out primary lung cancer or mesothelioma for the differential
diagnosis through use of multimodalities such as
immunohistochemistry and PET-CT.
Acknowledgements
This study was supported by a grant from the Kangwon
National University (grant no. 20141482).
References
|
1
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sountoulides P, Metaxa L and Cindolo L:
Atypical presentations and rare metastatic sites of renal cell
carcinoma: A review of case reports. J Med Case Rep. 5:4292011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esquena S, Abascal JM, Trilla E, Torres I
and Morote J: Case report: Retroperitoneal fibrosis simulating
local relapse of sarcomatoid renal cell carcinoma. Int Urol
Nephrol. 38:463–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar Y, Bhatia A, Das A and Kathpalia AS:
Unusual appearance of perirenal fibrosis in renal cell carcinoma
simulating a tumour. Jpn J Clin Oncol. 39:677–681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terada T: Retroperitoneal fibrosis
associated with renal cell carcinoma. Int J Clin Exp Pathol.
6:1195–1196. 2013.PubMed/NCBI
|
|
7
|
Cohen HT and McGovern FJ: Renal cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wick G, Grundtman C, Mayerl C,
Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R and Wolfram D:
The immunology of fibrosis. Annu Rev Immunol. 31:107–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fromowitz FB and Miller F: Retroperitoneal
fibrosis as host response to papillary renal cell carcinoma.
Urology. 38:259–263. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramdave S, Thomas GW, Berlangieri SU,
Bolton DM, Davis I, Danguy HT, Macgregor D and Scott AM: Clinical
role of F-18 fluorodeoxyglucose positron emission tomography for
detection and management of renal cell carcinoma. J Urol.
166:825–830. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Safaei A, Figlin R, Hoh CK, Silverman DH,
Seltzer M, Phelps ME and Czernin J: The usefulness of F-18
deoxyglucose whole-body positron emission tomography (PET) for
re-staging of renal cell cancer. Clin Nephrol. 57:56–62. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang DE, White RL Jr, Zuger JH, Sasser HC
and Teigland CM: Clinical use of fluorodexoyglucose F 18 positron
emission tomography for detection of renal cell carcinoma. J Urol.
171:1806–1809. 2004. View Article : Google Scholar : PubMed/NCBI
|