Introduction
The incidence of ovarian cancer in women is on the
increase. Statistics obtained in 2013 by the International
Anticancer Association (1) showed
that ovarian cancer was associated with cervical, uterine, and
breast cancer. The high incidence of these diseases accounts for
18.4% of the total number of female cancers (1). At least 137 million women are diagnosed
with ovarian cancer annually, of whom approximately 32.4% succumb
to ovarian cancer. The number of patients has increased
significantly at an annual rate of 0.32–0.48% (1). Statistics have shown that the proportion
of women with ovarian cancer in China has increased. It accounts
for 17.4% of female malignant tumors, its incidence rate was
approximately 26.4% and the mortality rate was approximately 35.4%,
slightly higher than the international average level (2). Therefore, the diagnosis and treatment of
ovarian cancer has become an important research focus. Previous
findings have shown that early detection and appropriate treatment
modalities for ovarian cancer are essential to ensure the
successful treatment of ovarian cancer (3,4).
Forkhead box transcription factor M1 (FoxM1), a
transcription factor identified to be associated with abnormal cell
proliferation and cancer (3), was
evaluated as the ‘Molecule for the Year 2010’ by the International
Society For Molecular and Cell Biology and Biotechnology Protocols
and Researches (4). He et al
(5) demonstrated that FoxM1 serves as
a type of transcription factor in the human body, which may
activate downstream target genes regulated by it (such as signaling
paths or key proteins relevant to cell proliferation) when the
human body received external stimuli or had some internal changes.
Wang et al (6) demonstrated
that FoxM1 was closely associated with the occurrence, development
and prognosis of some malignant tumors in the human body. For
example, the abnormal expression of FoxM1 gene was detected
in ovarian, liver and lung cancer (7). Zhang et al (8) revealed that higher FoxM1 expression
level was associated with tumor prognosis, while the 5-year
survival rate decreased significantly. Chen et al (9) identified that taxol had good curative
effects for various types of tumors and cancer. Zhu et al
(10) suggested that taxol was
beneficial in the treatment of colon cancer. Based on these
observations, the aim of the present study was ot examine the
mutual association between FoxM1 gene and ovarian
cancer.
Materials and methods
Materials
Clinical samples
The patients with ovarian cancer and their surgical
specimens were collected between 2011 and 2014 at the Sichuan
Provincial People's Hospital. The patients were aged 37–54 years,
with an average age of 43.5±4.6 years. The normal controls were
aged 39–55 years, with an average age of 44.3±4.2 years. The
subjects were randomly divided into the observation and control
groups. The observation group included 36 women with ovarian cancer
and the control group included 36 normal women.
Experimental medicines
An ovarian cancer detection kit was purchased from
Roche Diagnostics (Indianapolis, IN, USA). Other drugs were
purchased from Thermo Fisher Scientific, (Waltham, MA, USA). The
fluorescence quantitative primers were produced by Takara Bio
(Dalian, China), and FoxM1 antibody was provided by Acris
Antibodies (San Diego, CA, USA).
Methods
RNA extraction of ovarian carcinoma cells
Frozen tissue samples were removed from ~0.1 g
liquid nitrogen, thawed on ice and 0.45 ml RNA Plus (Beijing Ed
Biological Technology Co, Ltd., Beijing, China) was added.
Subsequently, the tissues were homogenized and 0.45 ml of RNA Plus
was added. Chloroform (200 µl) was then added and briefly mixed
followed by centrifugation at 8,000 × g for 15 min at <4°C. The
supernatant was transferred into an Eppendorf tube (Beijing Ed
Biological Technology Co, Ltd.) with an equal volume of isopropanol
and mixed, prior to centrifugation at 8,000 × g for 10 min at
<4°C. The supernatant was removed, 750 µl ethanol (75%) was
added to mix gently, and the mixture was centrifuged at 8,000 × g
for 10 min at 4°C. The supernatant and the remainder of the
residual ethanol were subsequently removed. An appropriate amount
of RNase-free water was added to the RNA pellet.
Fluorogenic quantitative polymerase chain
reaction (qPCR)
Fluorogenic quantitative polymerase chain reaction
was conducted according to the protocol for Takara Bio fluorescence
qPCR.
Detection of FoxM1 expression in serum with
ELISA
ELISA was carried out according to the
manufacturer's instructions (11).
The samples were diluted at a ratio of 1:200, and 100 µl serum
samples were added into each well. FoxM1 detection solution was
then added into the wells with 50 µl/well at 25°C for 1.5 h. TMB
substrate (50 µl) was then added into each well for color
development. The optical density was measured at 495 nm using a
fluorescent plate reader (Hewlett-Packard Development Company, Palo
Alto, USA) to calculate FoxM1 expression for each sample by
comparing with the standard curve.
Detection of FoxM1 in ovarian cancer tissues with
immunohistochemistry (IHC)
IHC for ovarian tissue samples was performed
according to the streptomycin affinity peroxidase (S-P) method
(12). Staining was calculated as
follows: <10% or negative, negative (−); only stained cell
membrane or >10% tumor cells, weak positive (+); >10% tumor
cells indicated weak or moderately complete staining, medium strong
positive (++); and >10% tumor cells indicated markedly complete
membrane staining, strong positive (+++).
Detection of FoxM1 in ovarian cancer tissues and
serum using western blotting
A Thermo Fisher Scientific animal cell protein
extraction kit was used to extract the total protein in the samples
(particular operation according to the specification) (13). Western blotting was conducted as
previously described (14). The
primary antibody was anti-FoxM1 (rabbit polyclonal antibody,
diluted 1:500, cat.no: sc-502; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and secondary antibody was horseradish
peroxidase-conjugated anti-rabbit IgG (diluted 1:5,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was
used to for the statistical analysis. The measurement data were
presented as mean ± standard deviation, while χ2 test
was used for the count data.
Results
mRNA expression of FoxM1 in patients
with or without ovarian cancer
In the present study, we selected ovarian tissue
samples of normal women and ovarian cancer patients. The RNA was
extracted from the tissue samples for fluorescence qPCR as
mentioned in Materials and methods. The primer sequences used were:
Forward 5′-TTTTGCTAGCTCAAGCCCTGTCAACTTTACC-3′, and reverse
5′-ATATAAGCTTTTGCTGCATCCCGCTCACCT-3′. Fig. 1 shows the electrophoretic result of
the PCR products. FoxM1 mRNA content in patients with
ovarian cancer was higher than that in the normal population (CK),
and its expression was not the same at the different time points.
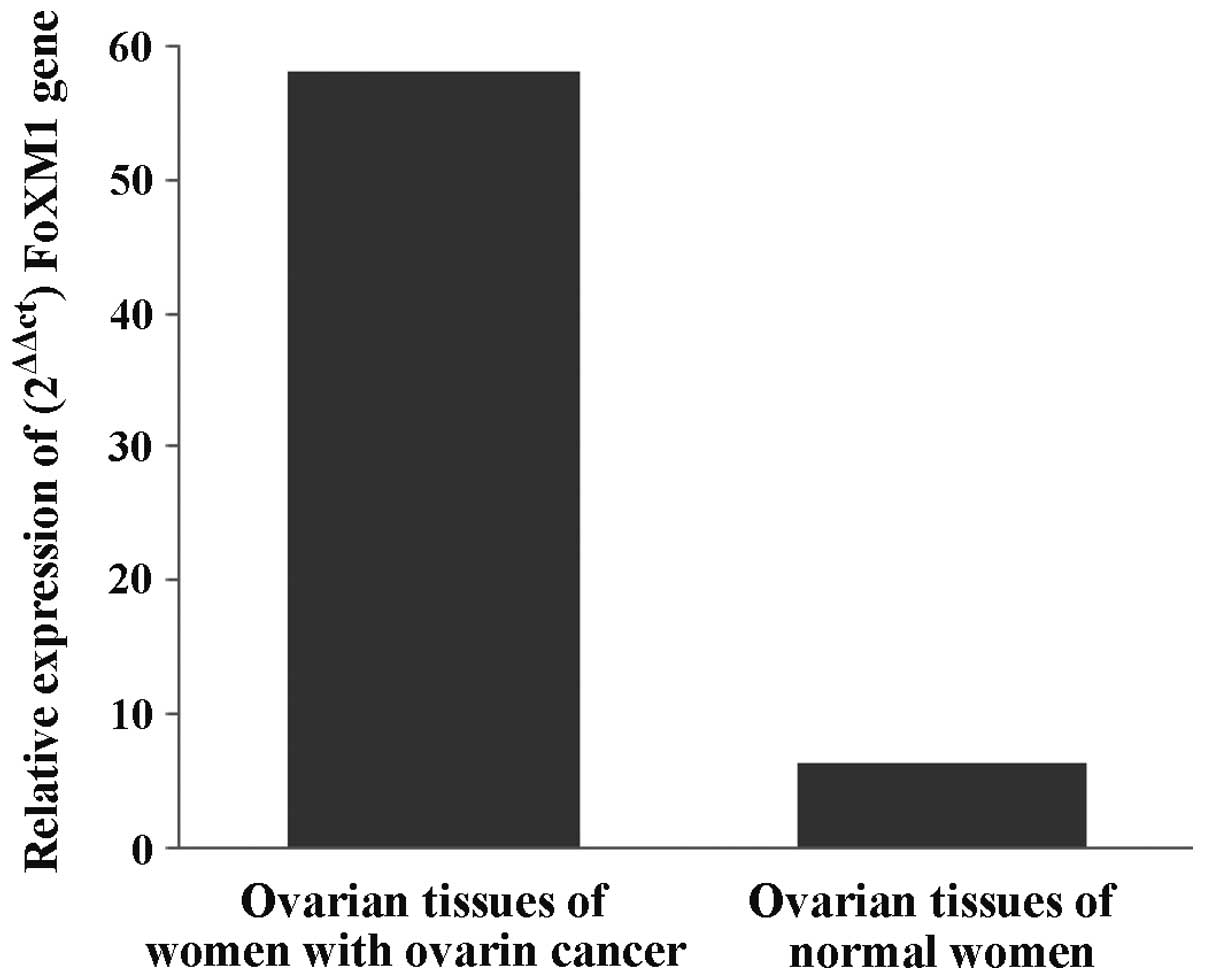
By comparing the expression of FoxM1 mRNA in the
experimental and control groups (Fig.
2) the average level of FoxM1 mRNA expression in
patients with ovarian cancer was found to be 4.3- to 5.8-fold
significantly higher than that in the normal women. The result
identified a certain correlation between FoxM1 gene and
ovarian cancer.
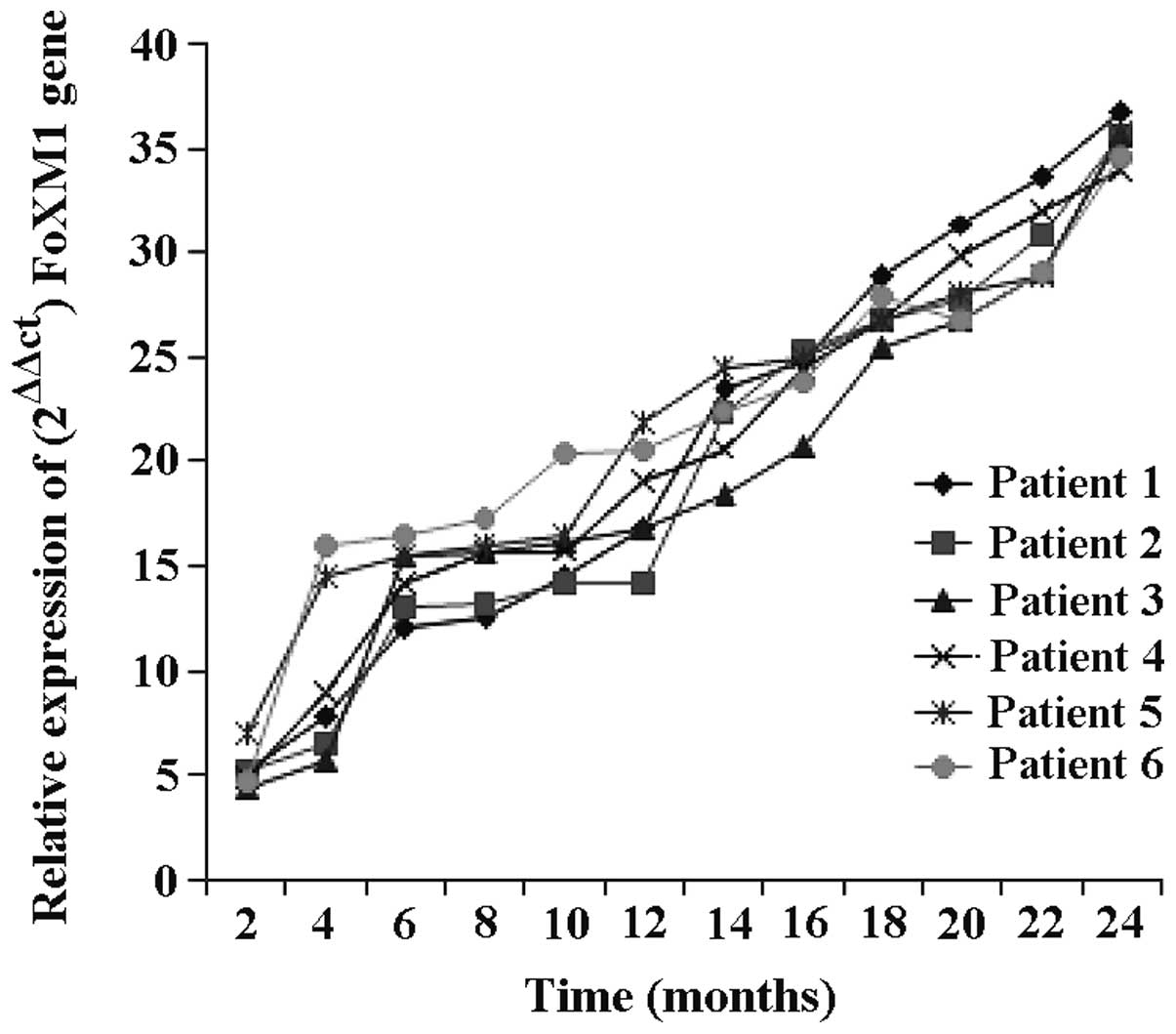
FoxM1 mRNA expression in ovarian
cancer patients at different stages
FoxM1 mRNA expression was detected in ovarian cancer
patients at different time points (Fig.
3). The expression of FoxM1 gene gradually increased
with the progression of disease, the growth was gradual at the
early stage of disease and became rapid at the later stage (6
months later). Within 8–18 months of diagnosis of ovarian cancer,
the mRNA expression increased, indicating that FoxM1 gene is
associated with ovarian cancer, and there is a positive correlation
between the levels of FoxM1 and the severity of ovarian cancer
patients.
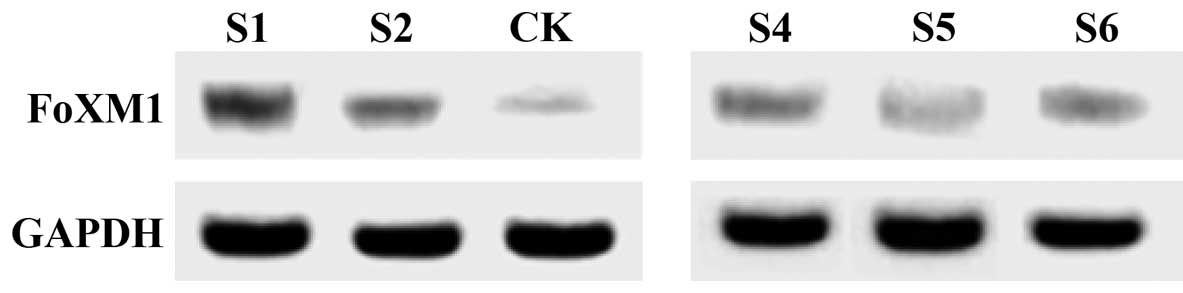
Expression of FoxM1 mRNA in serum of
ovarian cancer patients at different stages
FoxM1 protein expression in the serum of ovarian
cancer patients at different time points was detected using western
blotting (Fig. 4). The results showed
that the expression level of FoxM1 protein in serum gradually
increased as the disease progressed and the growth was
significantly increased after six months (Fig. 3). The results showed that FoxM1
gene is associated with ovarian cancer, and there is a positive
correlation between the expression levels of FoxM1 in serum and the
severity of ovarian cancer patients.
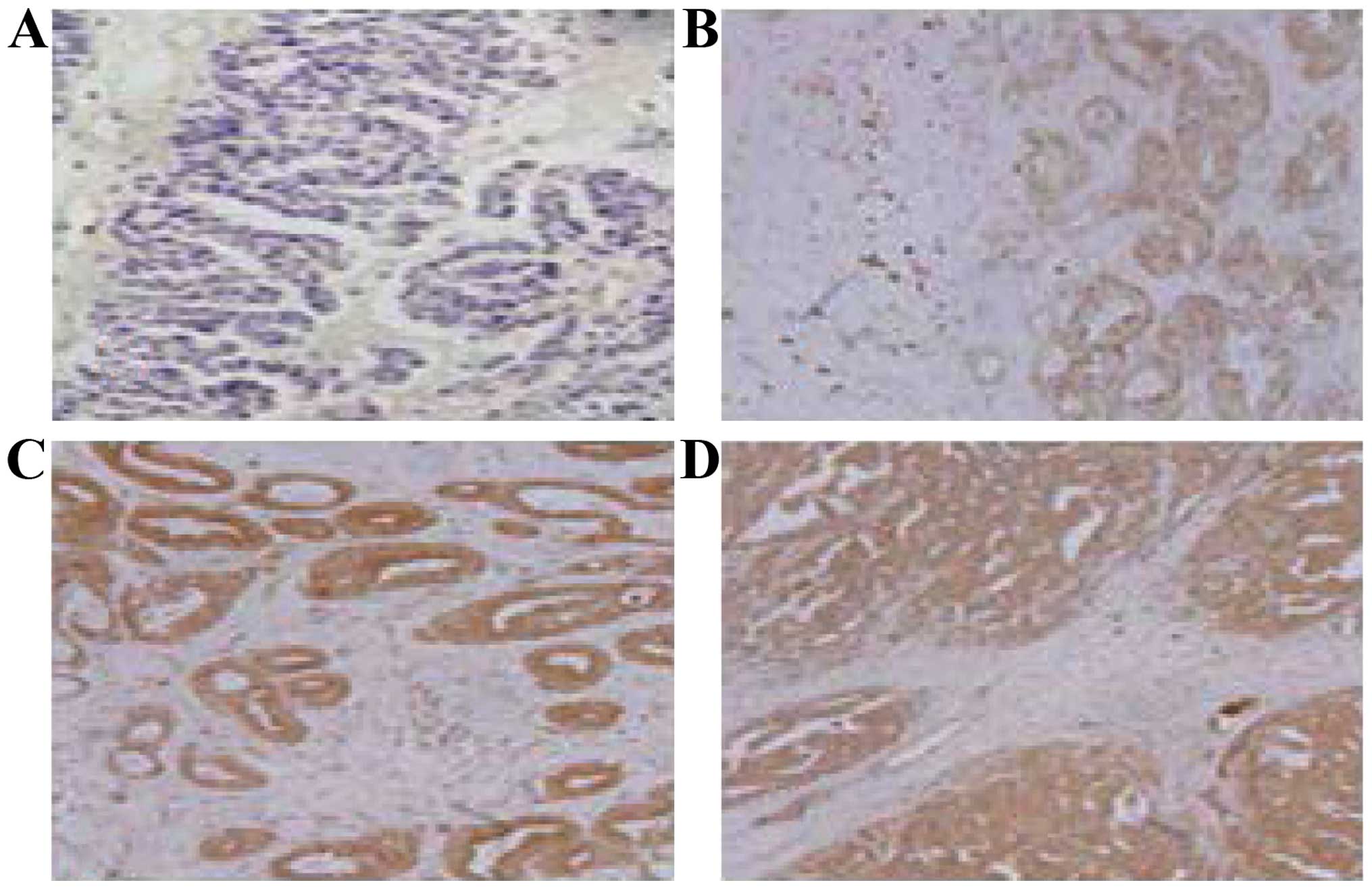
IHC results of FoxM1 in ovarian cancer tissues
(Fig. 5) demonstrated that positive
staining of FoxM1 expression was mainly concentrated in the
ovarian cancer cell membrane (B-D), and the expression was shown
as: i) Negative (−); ii) weak positive (+); iii) moderately strong
positive (++); and iv) strong positive (+++). The main features of
positive staining were the uneven size of brown small particles,
while there were not found in the normal ovarian tissues. The
content of FoxM1 in ovarian cancer patients was higher than that in
the normal population and mainly concentrated in the ovarian
tissue.
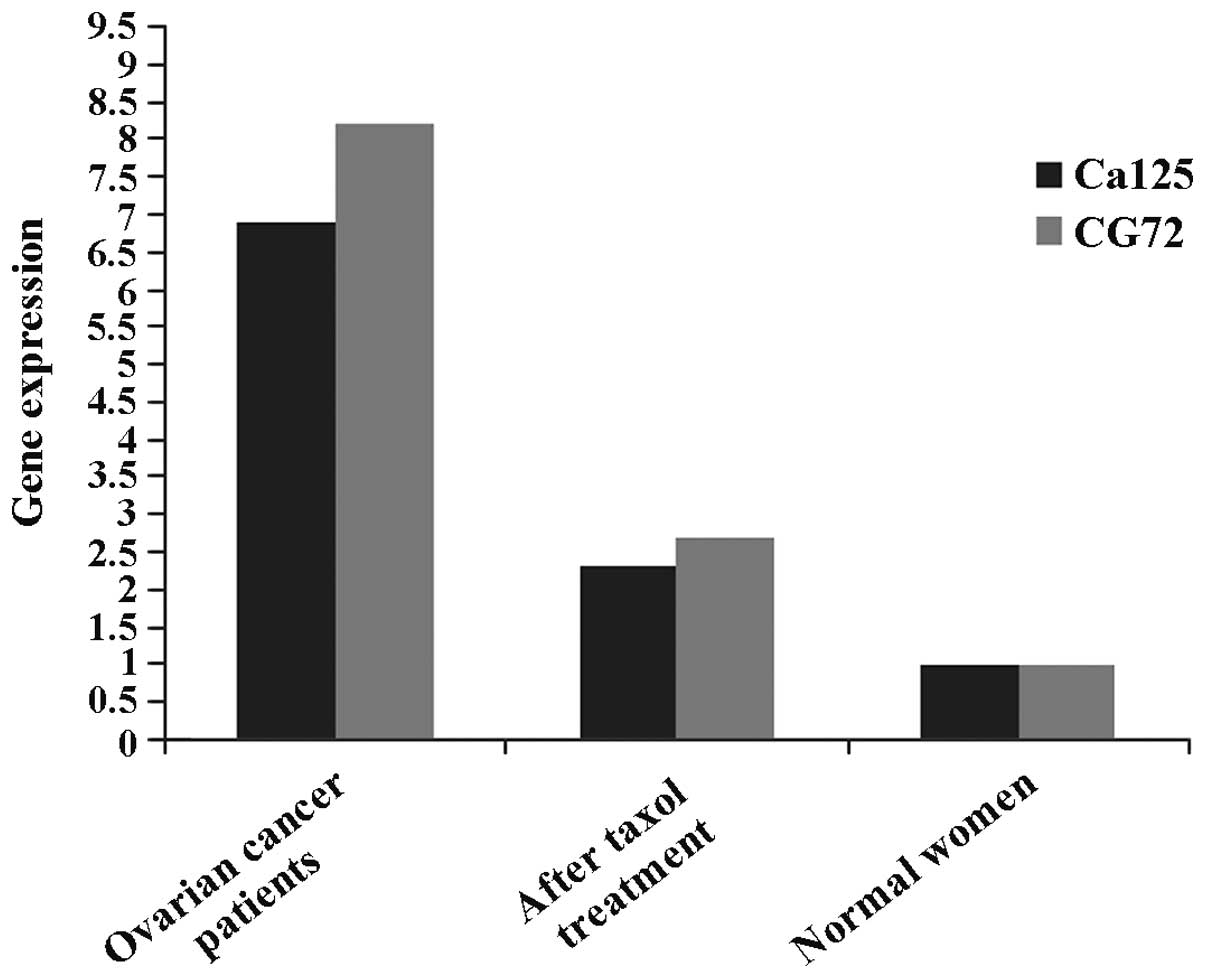
Curative effects of taxol on ovarian
cancer patients
In the present study, we preliminarily examined the
curative effects of taxol on ovarian cancer patients. The results
revealed corresponding sensitive markers of ovarian cancer (CA125,
AG72), and contents of the above sensitive markers in ovarian
cancer patients of the experimental group administered taxol
significantly decreased, suggesting that taxol has curative effects
on ovarian cancer to a certain extent (Fig. 6).
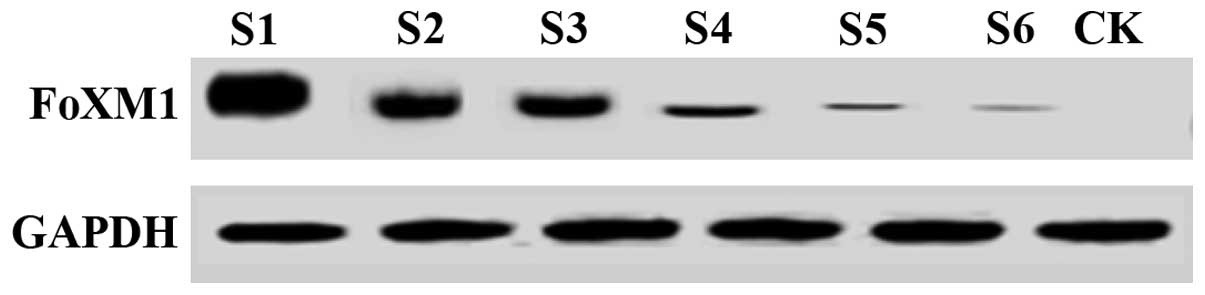
Expression of FoxM1 in ovarian cancer
patients prior to and following taxol treatment
In the present study, the patients with ovarian
cancer were treated with taxol. Prior to and following therapy, the
expression of FoxM1 in the lesion tissues of the experimental
samples was measured by ELISA, and results revealed that the
average content of FoxM1 in the serum of normal women was
approximately 4.19±0.63 ng/ml. The results for the 36 female
ovarian cancer patients revealed that the average content of FoxM1
in the serum prior to treatment was 12.1±21.21 ng/ml, and that
after treatment with taxol the serum level was approximately
5.73±0.39 ng/ml. The content in the serum of ovarian cancer
patients (12.12±1.21 ng/ml) was significantly higher than that in
the serum of the controls (4.19±0.63 ng/ml). Compared with
conditions prior to treatment, the expression of FoxM1 in ovarian
cancer patients treated with taxol was significantly decreased,
indicating that taxol exerts certain effects on ovarian cancer.
Therefore, the taxol may be used to treat ovarian cancer by
reducing the FoxM1 gene expression in patients.
Discussion
Previous findings by Francis et al (15) revealed that FoxM1 is an important
transcription factor, and is closely associated with tumor
occurrence and development. The study by Yoshida et al
(16) demonstrated that FoxM1
comprised 10 exons located at 12 p13-3 positions of the 20 kb
chromosome. The expression amount of FoxM1 genes in cancer
cells has been found to be significantly higher than that in the
normal cells, and it can be taken as a marker for certain types of
tumor and cancer (16). The
percentage of female ovarian cancer in China has increased annually
(17,18), and the rapid diagnosis of ovarian
cancer has become a hot research topic. Chen et al (19) suggested that genes associated with the
occurrence and deterioration of ovarian cancer can be classified as
those associated with ovarian cancer at the mRNA level and those
associated with ovarian cancer at the protein level. Gong et
al (20) identified 61 genes
possibly associated with ovarian cancer, although those authors did
not examine the mechanism involved in mRNA and protein expression
levels in a detailed manner.
Taxol was identified in 1971 by Wani et al
(21) who separated it from the bark
of short-leaf taxus brevifolia. Wang et al (22) previously demonstrated that it taxol
may serve as a broad-spectrum antitumor drug. Schiff et al
proved that taxol had a unique anticancer mechanism (23,24). For
instance, it acted on cell microtubules and to a certain extent
induced protein separation in relevant proteins by interplaying
with the amino acid at the 31st position at the N end of the
microtubule and the amino acid at the 217th-231st position. Further
blocking cells in the G2PM period and ultimately causing
abnormality or ceasing of mitosis, apoptosis of cancer cells, owing
to the inability of the cells to multiply, can be used for
treatment of the cancer (24). In the
present study, we found that FoxM1 gene was associated with
the onset of some tumors and cancer, and demonstrated that it was
correlated with ovarian cancer. The expression of FoxM1 in
ovarian cancer patient serum and ovarian tissue was significantly
higher than that in normal women. To the best of our knowledge, for
the first time, we confirmed by experiment that, taxol also had
certain therapeutic effects on female ovarian cancer. Compared with
conditions prior to treatment, contents of relevant sensitive
markers (CA125 and AG72) of ovarian cancer in patients decreased
significantly, By comparing the expression amount of FoxM1
gene in ovarian cancer patients prior to and following treatment
with taxol, we found that the expression amount of FoxM1
gene significantly decreased following treatment.
In conclusion, we preliminarily evaluated treatment
of female ovarian cancer with taxol, and subsequently, to the best
of our knowledge, identified a new gene, FoxM1, which was
associated with female ovarian cancer. By comparing the expression
amount of FoxM1 gene in the observation and control groups,
it was found that in comparison to the normal women, the FoxM1
levels in patients with ovarian tumor significantly increased
(P<0.05). Additionally, the results showed that FoxM1
gene expression was reduced in ovarian cancer patients after
treatment with taxol. Thus, the curative effects of female ovarian
cancer were preliminarily evaluated. Compared with the control
group without taxol treatment, contents of relevant sensitive
markers of ovarian cancer (CA125 and AG72) in ovarian patients
decreased significantly, suggesting that taxol exerted a
therapeutic effect on ovarian cancer. Measuring the expression
amount of FoxM1 gene in ovarian cancer patients prior to and
following taxol treatment showed that the expression of FoxM1 with
taxol significantly decreased ovarian cancer patients. The finding
suggests that taxol is capable of reducing FoxM1 levels in ovarian
cancer patients. Therefore, taxol has promising implications for
the treatment of ovarian cancer and may be developed as a
therapeutic agent.
References
|
1
|
Zheng RS, Zhang SW, Wu LY, Li GL, Zhao P,
Hao J and Chen WQ: Report of incidence and mortality from China
cancer registries in 2008. China Cancer. 21:1–12. 2012.
|
|
2
|
Pei GJ, Fu L, Cui YL, Gao LH, Wang WL and
Lu WQ: Meta-analysis of risk factors of breast cancer in Chinese
women. China Cancer. 21:1–12. 2012.
|
|
3
|
Laoukili J, Stahl M and Medema RH: FoxM1:
At the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.PubMed/NCBI
|
|
4
|
Yap KL, Fraley SI, Thiaville MM, Jinawath
N, Nakayama K, Wang J, Wang TL, Wirtz D and Shih IeM: NAC1 is an
actin-binding protein that is essential for effective cytokinesis
in cancer cells. Cancer Res. 72:4085–4096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He SY, Shen HW, Xu L, Zhao XH, Yuan L, Niu
G, You ZS and Yao SZ: FoxM1 promotes tumor cell invasion and
correlates with poor prognosis in early-stage cervical cancer.
Gynecol Oncol. 127:601–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: A novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeasmin S, Nakayama K, Rahman MT, Rahman
M, Ishikawa M, Katagiri A, Iida K, Nakayama N, Otuski Y, Kobayashi
H, et al: Biological and clinical significance of NAC1 expression
in cervical carcinomas: A comparative study between squamous cell
carcinomas and adenocarcinomas/adenosquamous carcinomas. Hum
Pathol. 43:506–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Cheng Y, Ren X, Hori T,
Huber-Keener KJ, Zhang L, Yap KL, Liu D, Shantz L, Qin ZH, et al:
Dysfunction of nucleus accumbens-1 activates cellular senescence
and inhibits tumor cell proliferation and oncogenesis. Cancer Res.
72:4262–4275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YZ, Chen KZ and Zeng JJ: Observation
and nursing oftaxol in the treatment of cancer chemotherapy. J Qilu
Nurs. 15:49–50. 2009.(In Chinese).
|
|
10
|
Zhu HC, Zhao BZ, Zheng XZ and Zhu YT: The
effect of taxol on the expression of Bcl-2 and Bax in colon
carcinoma HT-29 cells. Chin Med Mod Dist Educ Chin. 10:141–142.
2012.(In Chinese).
|
|
11
|
Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL,
Wu H, Patel R, Liu D, Qin ZH, Shih IM and Yang JM: NAC1 modulates
sensitivity of ovarian cancer cells to cisplatin by altering the
HMGB1-mediated autophagic response. Oncogene. 31:1055–1064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okazaki K, Nakayama N, Nariai Y, Nakayama
K, Miyazaki K, Maruyama R, Kato H, Kosugi S, Urano T and Sakashita
G: Nuclear localization signal in a cancer-related transcriptional
regulator protein NAC1. Carcinogenesis. 33:1854–1862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu PH, Hung SH, Ren T, Shih IeM and Tseng
Y: Cell cycle-dependent alteration in NAC1 nuclear body dynamics
and morphology. Phys Biol. 8:0150052011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Padda RS, Gkouvatsos K, Guido M, Mui J,
Vali H and Pantopoulos K: A high-fat diet modulates iron metabolism
but does not promote liver fibrosis in hemochromatotic
Hjv−/− mice. Am J Physiol Gastrointest Liver Physiol.
308:G251–G261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Francis RE, Myatt SS, Krol J, Hartman J,
Peck B, McGovern UB, Wang J, Guest SK, Filipovic A, Gojis O, et al:
FoxM1 is a downstream target and marker of HER2 overexpression in
breast cancer. Int J Oncol. 35:57–68. 2009.PubMed/NCBI
|
|
16
|
Yoshida Y, Wang IC, Yoder HM, Davidson NO
and Costa RH: The forkhead box M1 transcription factor contributes
to the development and growth of mouse colorectal cancer.
Gastroenterology. 132:1420–1431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan XD and Pan LY: Comparative proteomic
analysis of platinum drug resistance related proteins in ovarian
cancer and the research on the function of drug resistance related
protein Annexin A3. Chin J Obstet Gynecol. 41:584–587. 2006.(In
Chinese).
|
|
18
|
Sun P and Bai P: An analysis of prognostic
factors in 32 cases with transitional cell carcinoma of the ovary.
J Chin Oncol. 17:97–100. 2011.(In Chinese).
|
|
19
|
Chen LL: Ovarian epithelium carcinoma
induce abnormal differentiation of dendritic cell precursors
(unpublished PhD thesis). Zhejiang University. 2009.
|
|
20
|
Gong YQ, Han FJ and Wu XK: Advances in
etiology of epithelial ovarian cancer. J Med Res. 18–20. 2010.
|
|
21
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI. The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang ZH, Zeng HX and Chang XH: A
experiment study of effects on glycometabolism after the
application of cortisol in chemotherapy includes taxoll. Prog
Obstet Gynecol. 19:206–209. 2010.(In Chinese).
|
|
23
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schiff PB and Horwitz SB: Taxol stabilizes
microtubulesin mouse fibroblast cells. Proc Natl Acad Sci USA.
77:1561–1565. 1980. View Article : Google Scholar : PubMed/NCBI
|