Introduction
The invasion and metastasis of lung cancer is a
complex process that is regulated by multiple genes, and the
interaction between these genes has become a significant research
focus. Cyclooxygenase-2 (COX-2) is the key enzyme involved in the
occurrence and development of a variety of malignant tumors
(1,2).
COX-2 expression promotes proliferation, angiogenesis and
lymphangiogenesis within tumor cells, as well as promoting tumor
invasion and metastasis (3–5). Cluster of differentiation 44 variant 6
(CD44v6) is a splice variant of CD44, and its expression may alter
the composition and functioning of adhesion molecules on the tumor
cell surface, thereby contributing to the development of metastatic
potential in tumor cells (6,7). Matrix metalloproteinase-2 (MMP-2) is the
most widely distributed member of the MMP family, and is able to
degrade the extracellular matrix (ECM) and basement membrane, thus
participating in physiological and pathological processes including
tumor progression (8,9). Survivin is an important member of the
inhibitors of apoptosis proteins gene family, and is closely
associated with the differentiation, proliferation, angiogenesis,
invasion and metastasis of tumor cells (10,11). In
the present study, indomethacin, a cyclooxygenase inhibitor, was
used in combination with oxaliplatin, a chemotherapeutic drug, to
perform intervention treatment on lung cancer-nude mouse
transplanted tumors. The present study aimed to research the
association between the factors involved in the invasion and
metastasis of lung cancer, as well as the mechanism underlying the
prevention of tumor growth and metastasis caused by indomethacin
and oxaliplatin, thus seeking novel methods to assist with the
prevention of lung cancer.
Materials and methods
Cell culture
Ham's F-12K (Kaighn's) medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), containing 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences), 100 U/ml
penicillin (North China Pharmaceutical Group Corp., Shijiazhuang,
China) and 100 U/ml streptomycin (Shenzhen HuaYao South
Pharmaceutical Co., Ltd., Shijiazhuang, China), was used to
subculture A549 human lung cancer cells (gifted by Beijing
University, Beijing, China) at 37°C in an atmosphere of 5%
CO2 and saturated humidity. Cells were used when they
were in the logarithmic phase of growth. When microscopy revealed
>80% of cells demonstrated adherent growth, 0.25% trypsin
(HyClone; GE Healthcare Life Sciences) was used to digest and form
the single cell suspension.
Preparation of human lung cancer-nude
mouse transplanted tumor model
A total of 32 female BALB/c nude mice, at 4–5 weeks
old and weight 20–24 g (obtained from the Institute of Laboratory
Animal Science, Chinese Academy of Medical Sciences & Peking
Union Medical College Hospital, Beijing, China; License no. of
animal quality: SCXY Jing-2009-0003), were fed with sterile feed
and sterile purified water and were housed in individually
ventilated cages (8 mice per cage). The feeding environment was
specific pathogen-free, and the ambient temperature was 25 ± 2°C,
with a humidity of 45–50%. A549 cells in the logarithmic growth
phase were selected, digested and diluted with FBS-free Ham's F-12K
(Kaighn's) medium. Subsequently, a hemocytometer was used to adjust
the cell density to 1×107 cells/ml. The skin of the nude
mouse was disinfected, and 0.2 ml cell suspension was injected with
a 1 ml syringe subcutaneously into the left armpit of the nude
mouse, in order to establish the lung cancer-nude mouse
subcutaneous transplantation model.
Grouping and drug intervention
A total of 32 female BALB/c nude mice were randomly
divided into four groups, with 8 mice per group. The groups were as
follows: i) indomethacin (Sigma-Aldrich, St. Louis, MO, USA) group,
orally administered 2.5 mg/kg/day, combined with intraperitoneal
injection of saline; ii) oxaliplatin (Shandong Lukang Record
Pharmaceutical Co., Ltd., Jining, China) group, 10 mg/kg twice per
week, administered by intraperitoneal injection, combined with oral
administration of sterile distilled water; iii)
indomethacin-oxaliplatin combination group, orally administered
indomethacin 2.5 mg/kg/day, oxaliplatin 10 mg/kg twice per week,
administered by intraperitoneal injection; iv) control group,
intraperitoneally injected identical quantity of saline, as well as
orally administered sterile distilled water.
Calculation of tumor inhibition
rate
The growth of nude mice and tumors were observed,
and a caliper was used to measure the long diameter (a) and short
diameter (b) of the tumor every 7 days. The formula
V=ab2/2 was used to estimate the approximate tumor
volume. The following formula was used to calculate the percentage
tumor inhibition rate = [1 - (the average volume of each treatment
group prior to treatment - the average volume of each treatment
group at the conclusion of treatment)/(the average volume of the
control group prior to treatment - the average volume of the
control group at the conclusion of treatment)] × 100.
Detection of CD44v6, MMP-2 and
survivin protein expression by immunohistochemical assay
The primary antibodies survivin rabbit anti-human
monoclonal antibody (dilution, 1:100; catalog no., ZA-0530), CD44v6
mouse anti-human monoclonal antibody (dilution, 1:300; catalog no.,
ZM-0052) and MMP-2 mouse anti-human monoclonal antibody (dilution,
1:300; catalog no., ZM-0330) and the monoclonal mouse anti-human
IgG secondary antibody (dilution, 1:100; catalog no., PV6002) were
purchased from Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd. (Beijing, China). Immunohistochemistry parvalbumin kit was
purchased from OriGene Technologies (Beijing, China). Paraffin
sections were conventionally deparaffinized, followed by high
pressure antigen repairing. Subsequently, the sections were
incubated with primary and secondary antibodies, followed by
3,3′-diaminobenzidine staining (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.), hematoxylin (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) restaining, differentiation,
blue-restoring, dehydration and mounting. Subsequently, the
sections were observed and images were captured under an optical
microscope (BX51; Olympus Corporation, Tokyo, Japan). The CD44v6
positive indicator was brown staining, located in the cell membrane
and/or cytoplasm. The MMP-2 positive indicator was brown staining,
located in the cell membrane and/or cytoplasm. The survivin
positive indicator was brown staining, located in the nucleus
and/or cytoplasm. A general observation of the tissue sections was
initially performed using a microscope, under low magnification,
and subsequently 10 high-power fields were randomly selected for
observation under magnification, ×200. The Beihang true color
pathological image analysis system (Beihang University, Beijing,
China) was used to calculate average integrated absorbance
values.
Detection of CD44v6, MMP-2 and
survivin mRNA expression by fluorescence reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay
TRIzol® reagent (RNA extraction solution)
was purchased from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Reverse transcriptase, RNasin®
inhibitor, deoxynucleotides, TaqDNA polymerization enzyme
and agarose were purchased from Promega Corp. (Madison, WI, USA).
Random primers were synthesized by Sangon Biotech Co., Ltd
(Shanghai. China). RealMasterMix (SYBR Green) kit and DNAse was
purchased from Tiangen Biotech Co., Ltd. (Beijing, China). The
tumor tissue RNA was extracted using 2 µl DNAse per sample, and a
UV spectrophotometer (UV-2550 2450; Shimadzu, Corp., Kyoto, Japan)
was used to detect the optical density (OD) 260/OD280 ratio. If
this ratio was 1.8–2.0, it indicated that the extracted RNA was
slightly contaminated. Subsequently, reverse transcription was
performed to produce cDNA. The primers used were as follows:
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward,
5′-TGAACGGGAAGCTCACTGG-3′ and reverse,
5′-GCTTCACCACCTTCTTGATGTC-3′; CD44v6 forward,
5′-GGAGCCAAATGAAGAAAATGAA-3′ and reverse,
5′-TGAAATGGTGCTGGAGATAAAA-3′; MMP-2 forward,
5′-AACTACGATGATGACCGCAAG-3′ and reverse,
5′-GACAGACGGAAGTTCTTGGTG-3′; survivin forward,
5′-TTTCTCAAGGACCACCGCA-3′ and reverse, 5′-AGTCTGGCTCGTTCTCAGTG-3′.
The total PCR reaction volume was 20 µl, and the PCR thermal
cycling parameters were as follows: 95°C for 2 min, followed by
95°C for 15 sec, 59°C for 30 sec and 68°C for 40 sec for 40 cycles.
An ABI 7500 Real-Time PCR System (Thermo Fisher Scientific Inc.)
was used. The fluorescence signal was collected at the third step
(59°C for 30 sec) of each cycle. The experiment was performed 5
times. Following amplification, the results were analyzed, and the
target gene expression levels were normalized against GAPDH, thus
the relative quantification value (RQ value) of the target gene
expression was obtained. The RQ values were subsequently used for
statistical analysis, as described previously (12).
Statistical analysis
All obtained experimental data were statistically
analyzed with SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). The
data of each group were expressed as the mean ± standard deviation.
Intergroup comparison was performed using analysis of variance when
the data displayed homogeneity of variance, and if it did not a
Kruskal-Wallis rank-sum test was performed. P<0.05 was
considered to indicate a statistically significant difference. The
correlation among the factors was analyzed using the Pearson
product-moment correlation coefficient, with P<0.01 considered
to indicate a statistically significant difference.
Results
Drug treatment reduces the growth of
human lung cancer-nude mouse transplanted tumors
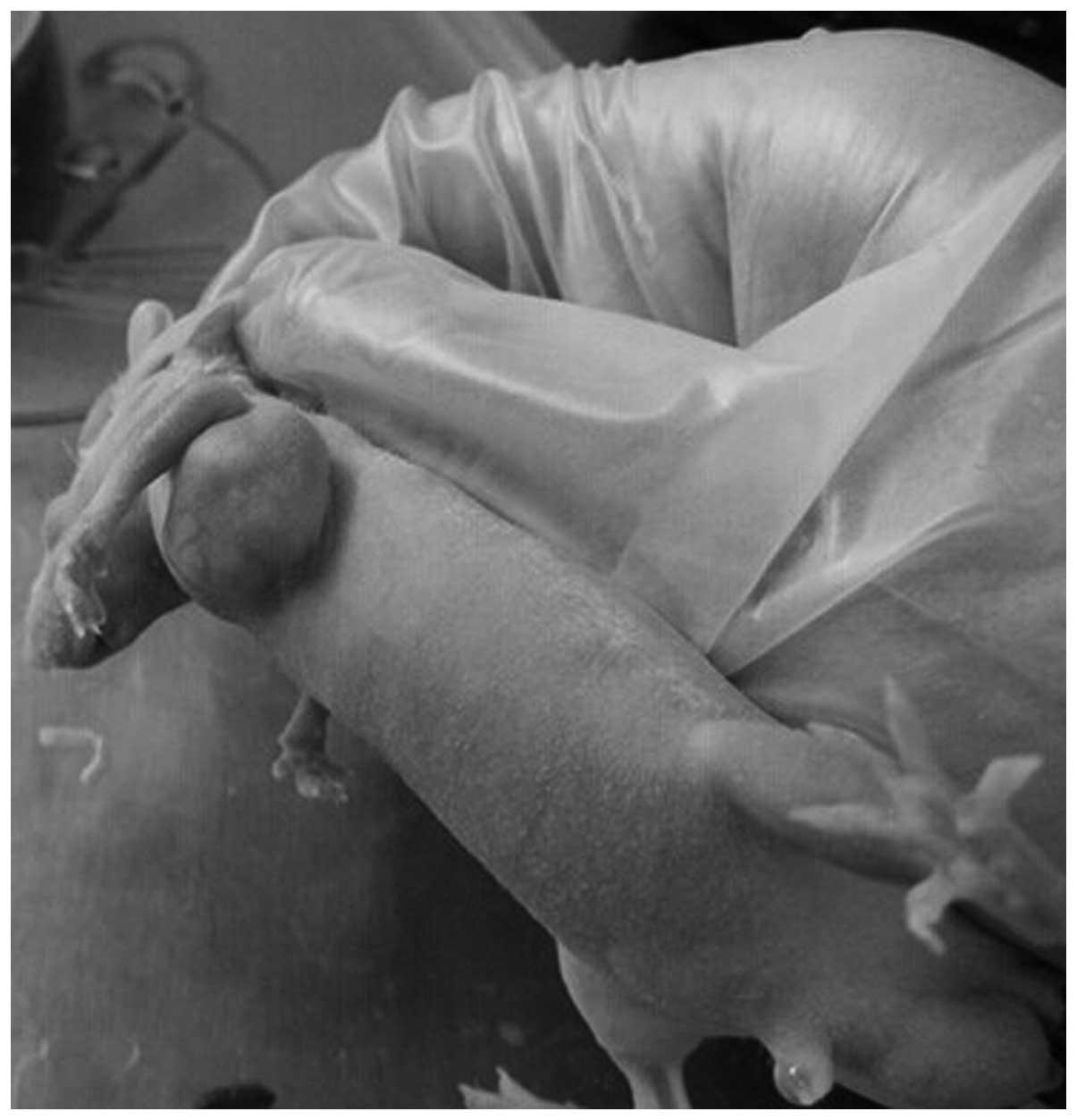
The 32 mice were all tumorigenic, with a tumor
formation rate of 100%. A total of 10 days subsequent to
transplantation, the average tumor diameter was 4 mm. Following
successful tumorigenesis, the early weight and activities of nude
mice did not change significantly; however, following tumor growth,
the nude mice exhibited weight loss and gradual decreased activity.
No mouse succumbed to disease during the experimental period
(Fig. 1).
Following treatment, the transplanted tumor volume
of the control group was 1,643.12±204.65 mm3, while
those of the indomethacin group, the oxaliplatin group and the
combination group were 1,450.29±133.20 mm3,
743.84±151.55 mm3 and 568.69±119.58 mm3,
respectively. Tumor volume comparison amongst the experimental
groups revealed a statistically significant difference (F=75.697;
P<0.01). The tumor inhibition rates of the indomethacin group,
the oxaliplatin group and the combination group were 26.67, 47.70
and 68.88%, respectively, and the tumor inhibition rate of the
combination group was significantly increased compared with the
monotherapy groups (P<0.05).
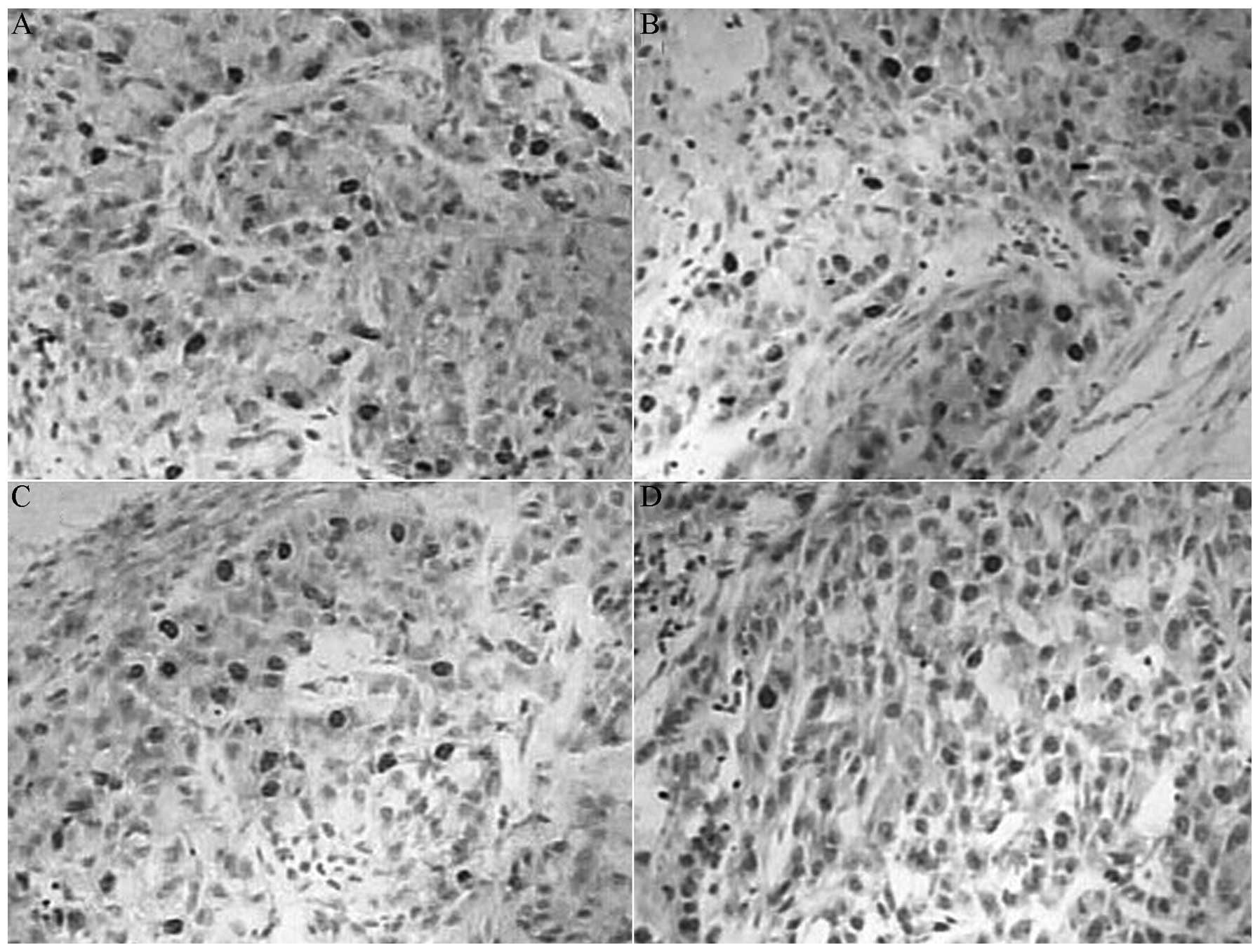
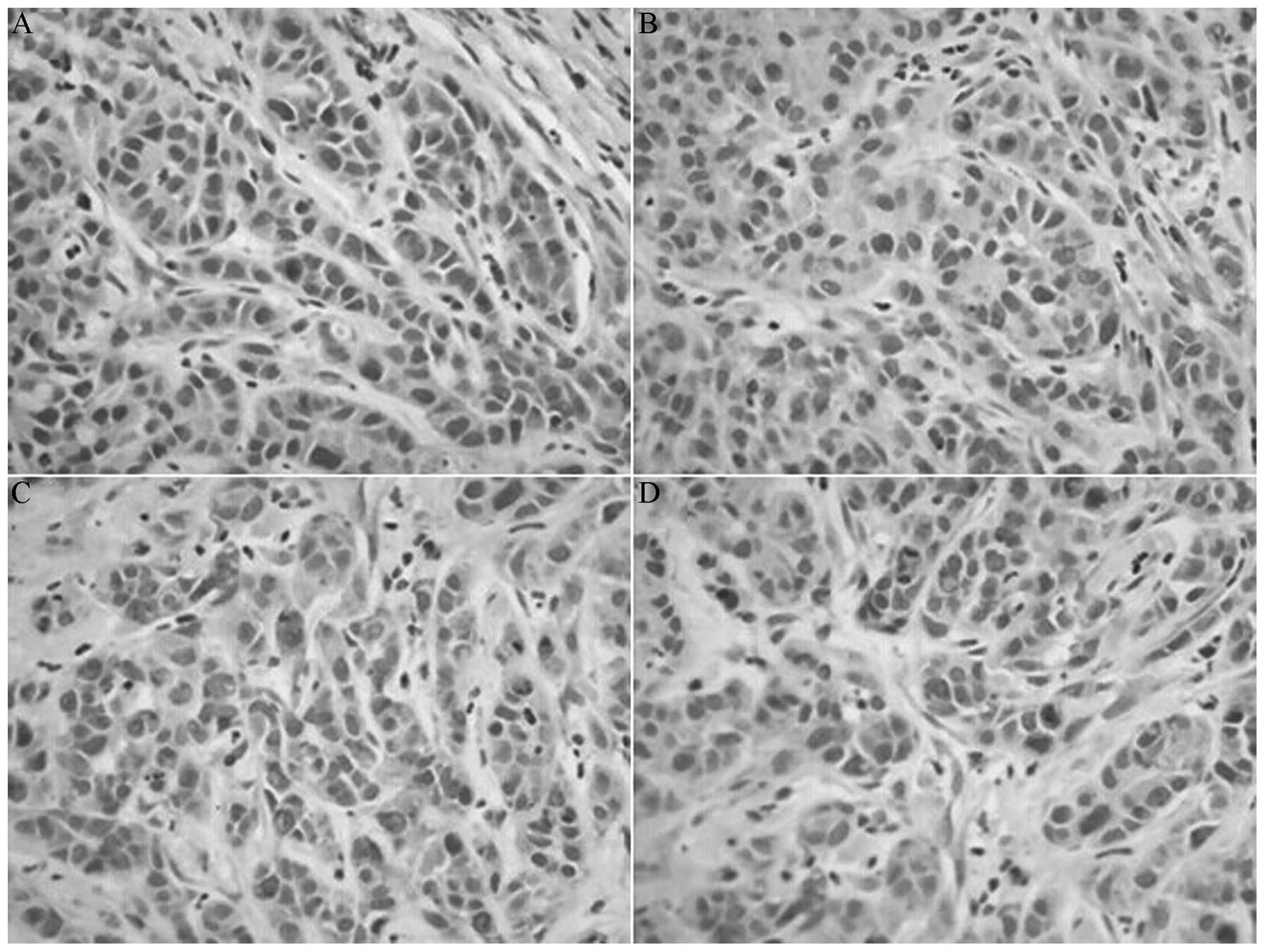
Drug treatment reduces the protein
expression of CD44v6 MMP-2 and survivin
The protein expression levels of CD44v6, MMP-2 and
survivin in the indomethacin group, the oxaliplatin group and the
combination group were reduced compared with the control group
(P<0.05), and the levels in the combination group were reduced
compared with the monotherapy groups (P<0.05). However, the
expression of CD44v6, MMP-2 and survivin proteins between the
indomethacin group and the oxaliplatin group did not exhibit a
significant difference (P>0.05; Table
I; Figs. 2–4).
 | Table I.Comparison of integral absorbance
values of CD44v6, MMP-2 and survivin proteins among the
experimental groups (n=8). |
Table I.
Comparison of integral absorbance
values of CD44v6, MMP-2 and survivin proteins among the
experimental groups (n=8).
| Variable | CD44v6 | MMP-2 | Survivin |
|---|
| Control | 9080 ± 698 | 16963 ± 698 | 17754 ± 2505 |
| Oxaliplatin | 7737 ±
477a,b | 14225 ±
926a,b | 11945 ±
1646a,b |
| Indomethacin | 7706 ±
504a,b | 14398 ±
670a,b | 12768 ±
1478a,b |
| Combination | 5227 ±
1497a | 9941 ±
231a | 7317 ±
1009a |
| P-value | <0.01 | <0.01 | <0.01 |
Drug treatment reduces the mRNA
expression of CD44v6 MMP-2 and survivin
The mRNA expression levels of CD44v6, MMP-2 and
survivin in the indomethacin group, the oxaliplatin group and the
combination group were reduced compared with the control group
(P<0.05), and the levels in the combination group were reduced
compared with the monotherapy group (P<0.05). However, the
expression of CD44v6, MMP-2 and survivin mRNAs between the
indomethacin group and the oxaliplatin group did not exhibit a
significant difference (P>0.05; Table
II).
 | Table II.Comparison of relative quantitative
expression values of CD44v6, MMP-2 and survivin mRNAs among the
experimental groups (n=8). |
Table II.
Comparison of relative quantitative
expression values of CD44v6, MMP-2 and survivin mRNAs among the
experimental groups (n=8).
| Variable | Survivin mRNA | CD44v6 mRNA | MMP-2 mRNA |
|---|
| Control | 1.073 ± 0.078 | 1.026 ± 0.037 | 1.008 ± 0.017 |
| Oxaliplatin | 0.617 ±
0.017a,b | 0.697 ±
0.011a,b | 0.771 ±
0.012a,b |
| Indomethacin | 0.616 ±
0.018a,b | 0.707 ±
0.010a,b | 0.783 ±
0.011a,b |
| Combination | 0.426 ±
0.024a | 0.469 ±
0.012a | 0.520 ±
0.011a |
| P-value | <0.01 | <0.01 | <0.01 |
Correlation analysis results
In the combination group, it was found that MMP-2
protein and MMP-2 mRNA expression were positively correlated
(r=0.958; P<0.01). CD44v6 protein and CD44v6 mRNA expression
were positively correlated (r=0.906; P<0.01). Survivin protein
and survivin mRNA expression were positively correlated (r=0.931;
P<0.01). MMP-2 protein and CD44v6 protein expression were
positively correlated (r=0.907; P<0.01). Survivin protein and
MMP-2 protein expression were positively correlated (r=0.841;
P<0.01). Survivin protein and CD44v6 protein expression were
positively correlated (r=0.857; P<0.01).
Discussion
Among various types of lung cancer, the expression
intensity of COX-2 has been observed to be highest in non-small
cell lung cancer, followed by squamous cell carcinoma, large cell
lung cancer and certain cases of small cell lung cancer (13). Previous studies have identified that
routine administration of nonsteroidal anti-inflammatory drugs
(NSAIDs) may reduce the risk of occurrence of colon, breast, lung,
prostate and other types of cancer (14). Treatment with the COX-2 inhibitor
indomethacin exerts antitumor effects, as well as chemotherapy
sensitization, and may regulate the body's immune status (15). Oxaliplatin, as a third-generation
anticancer drug, inhibits the synthesis and replication of DNA,
thereby preventing the development of tumor cells, and it has been
observed to be effective for the treatment of tumors that were
resistant to cisplatin (16).
Therefore, studying the impact of NSAIDs combined with
chemotherapeutic drugs on the tumor growth of a lung cancer-nude
mouse transplanted tumor model, as well as the expression of
survivin, CD44v6 and MMP-2, may provide a novel method for the
treatment and prevention of lung cancer.
The results of the present study demonstrated that
the tumor volumes of the drug-treated groups were reduced compared
with the control group. The tumor inhibition rates of the
indomethacin group and the oxaliplatin group were 26.67 and 47.70%,
respectively, while that of the combination group was 71.14%, which
was significantly increased compared with the monotherapy groups.
This indicated that the combination of NSAIDs and chemotherapeutic
drugs may provide significant synergy for the inhibition of tumor
growth in a lung cancer-nude mouse model. Furthermore, indomethacin
has few side effects, its clinical application is wide and it is
additionally easy to obtain. Therefore, the combination of low-dose
indomethacin with chemotherapeutic drugs may reduce the significant
side effects observed when chemotherapeutic drugs are used alone,
meaning that patient tolerance may be improved.
CD44 is a cell adhesion molecule, which primarily
mediates cell-cell and cell-matrix adhesion; therefore, it may have
a significant role in lymphocyte homing, blood cell generation,
migration and metastasis of tumor cells (17). CD44v6 is a CD44 isoform that contains
a mutated exon 11, and its expression may alter the composition and
functioning of adhesion molecules on the tumor cell surface,
enhancing their adhesive abilities (18). This may alter the physiochemical
properties of cells, and may contribute to tumor cells obtaining
metastatic potential. In the present study, indomethacin treatment
alone or in combination with oxaliplatin reduced the expression of
CD44v6 protein and mRNA. CD44v6 may be involved in tumor lymphatic
metastasis via the following mechanism: Tumor cells that express
CD44v6 on the cell membrane may imitate the homing process of
activated lymphocytes, transferring from the original tumor to the
lymph nodes, and thus obtaining the camouflage of lymphocytes
(19). Furthermore, CD44v6 may bind
distal lymphatic ligands, meaning that transferred tumor cells are
able to escape recognition and killing by the human immune system,
and are able to easily enter the lymph nodes to contribute to the
formation of metastases (19). CD44v6
is involved in adhesion among cancer cells and basal layer adhesion
protein, collagen, fibronectin and type I collagenase (MMP-2 and
MMP-9), thus creating conditions for the cancer cells to degrade
the ECM (20). As a hyaluronic acid
receptor on the T cell surface, CD44v6′s NH-2-terminal functional
area may connect the hyaluronate of the ECM and basement membrane,
anchoring it onto the host's ECM and basement membrane (21), thus regulating cell morphology and
movement (22), and laying the
foundation for the invasion of tumor cells.
MMP-2 hydrolyzes type IV and V collagen and
fibronectin, which are components of the cell basement membrane and
ECM, leading to destruction of the basement membrane. Subsequently,
this allows tumor cells to more easily break through the
ECM-composed barrier structure, and invade surrounding tissues or
enter the lymphatic system. Cells are subsequently able to migrate
through the lymphatic system, evade immune surveillance, enter
distant lymphatic vessels and implant onto the distal lymphatic
duct, resulting in the occurrence of distant metastases (23). Furthermore, MMP-2 is able to degrade
the vascular basement membrane, causing the production of ECM
degradation products, resulting in the production of chemotactic
molecules by vascular endothelial cells and allowing invasion of
vessels (24). In addition, MMP-2 may
activate growth factors, regulate cell-cell and cell-matrix
adhesion and regulate and promote angiogenesis, therefore promoting
the invasion and metastasis of tumor cells (24). In the present study, treatment with
NSAIDs alone or in combination with chemotherapeutic drugs reduced
the expression of MMP-2 in lung cancer xenografts, indicating that
NSAIDs were able to inhibit MMP-2. The potential mechanism through
which this was achieved may have been caused by NSAIDs inhibiting
the activity and expression of COX-2, thus preventing the
activation of secreted MMP-2, and reducing the expression of MMP-2.
This would hinder ECM hydrolysis, resulting in a reduction in
invasion and metastasis activities. The combination treatment group
exhibited significantly reduced MMP-2 expression levels, suggesting
that treatment with the two-drug combination led to a synergistic
effect on the inhibition of MMP-2 expression.
CD44v6 expression reduces adhesion among tumor
cells, and these cells are subsequently prone to leaving the
primary lesion (25). CD44v6 also
mediates adhesion among tumor cells and the ECM (6,17). MMP-2
degrades the ECM, forming a local dissolution zone, and thus
providing an approach towards the metastasis of tumor cells
(24). CD44v6 expression activates
certain signal transduction pathways in vivo, leading to
secretion of proteolytic enzymes and acceleration of ECM
degradation (26). Therefore,
adhesion and matrix degradation may occur simultaneously during the
process of lymph node metastasis, thus having synergistic
effects.
Survivin is the strongest apoptosis inhibitor
identified to the best of our knowledge, and its tissue
distribution demonstrates clear cell selectivity (27). Survivin is not expressed in normal
adult differentiated tissues (except for the thymus and placenta),
while it is expressed in lung, stomach, colorectal, liver,
pancreatic, breast and other types of cancer (28). Survivin is primarily expressed in the
G2/M phase of the cell cycle (29),
and is the regulation gene of this phase, therefore may be capable
of preventing the induction of G2/M phase apoptosis, and promoting
the abnormal proliferation of transformed cells via mitosis.
Primarily via inhibition of the activities of apoptosis-regulation
terminal effectors (caspase-3 and caspase-7), survivin may be
capable of blocking various stimuli that induce apoptosis, thus
playing the role of anti-apoptotic agent (30).
p21 may be released from the cyclin-dependent kinase
4-survivin composite body, and bind caspase-3 and inhibit its
activity, thus reducing apoptosis (31). Furthermore, survivin may reduce the
release of cytochrome c, thus demonstrating an
anti-apoptotic role. The results of the present study suggested
that NSAIDs may reduce the expression of the survivin protein and
gene, which may be due to inhibition of the activity of COX-2, and
its combination with oxaliplatin demonstrated a synergistic effect
towards inhibition of the expression of survivin.
The Wnt signaling pathway is involved in a number of
physiological processes in vivo, and its abnormal activation
may lead to the occurrence of cancer, fibrosis and other diseases
(32). In lung cancer, the Wnt
signaling pathway is highly active. Survivin is a downstream target
gene of the Wnt signaling pathway, exhibiting dual roles as an
inhibitor of apoptosis and promoter of cell proliferation (33). As a downstream target gene of the Wnt
signaling pathway, the Wnt-induced-secreted-protein-1 (WISP-1) is
associated with the occurrence of a number of malignancies
(34). WISP-1 may promote tumor
formation via inhibition of the p53-modulating apoptotic signaling
pathway. WISP-1 may induce the expression and secretion of MMP-2,
and as MMP-2 may be an intermediary of the WISP-1 response, its
effect of degrading the ECM may lead to the consequent movement and
metastasis of cancer cells (35). The
correlation analysis in the present study revealed that survivin
protein expression was positively correlated with MMP-2 protein
expression, and was additionally associated with lymph node
metastasis, suggesting that NSAIDs may reduce the expression of
downstream molecules of the Wnt signaling pathway. This may inhibit
the activity of the Wnt signaling pathway, and promote the
apoptosis of tumor cells, which ultimately may result in a
reduction of tumor invasiveness, as well as inhibition of the
occurrence of lymph node metastasis. Combination treatment with
indomethacin and oxaliplatin demonstrated good synergy.
In addition, the specificity protein 1 (Sp1) may
upregulate the expression of MMP-2 and survivin (36), thus participating in the regulation of
proliferation, apoptosis, angiogenesis and other processes in
various tumor cells (37). NSAIDs may
inhibit the activity of Sp1, thus reducing the expression of
survivin and MMP-2; however, the specific mechanism underlying this
process remains to be elucidated.
In conclusion, indomethacin treatment alone or in
combination with oxaliplatin may significantly inhibit tumor growth
inside a lung cancer-nude mouse transplanted tumor model, and is
additionally capable of reducing the expression levels of survivin,
CD44v6 and MMP-2. The combination of indomethacin and oxaliplatin
treatment caused a synergistic antitumor effect via various
mechanisms, thus providing potential novel strategies for the
treatment of lung cancer.
References
|
1
|
Di Nicolantonio JJ, McCarty MF, Chatterjee
S, Lavie CJ and O'Keefe JH: A higher dietary ratio of long-chain
omega-3 to total omega-6 fatty acids for prevention of
COX-2-dependent adenocarcinomas. Nutr Cancer. 66:1279–1284. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han H, Yang S, Lin SG, Xu CS and Han ZH:
Effects and mechanism of downregulation of COX-2 expression by RNA
interference on proliferation and apoptosis of human breast cancer
MCF-7 cells. Mol Med Rep. 10:3092–3098. 2014.PubMed/NCBI
|
|
3
|
Li M, Tan SY and Wang XF: Paeonol exerts
an anticancer effect on human colorectal cancer cells through
inhibition of PGE2 synthesis and COX-2 expression. Oncol
Rep. 32:2845–2853. 2014.PubMed/NCBI
|
|
4
|
Harris RE, Casto BC and Harris ZM:
Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J
Clin Oncol. 5:677–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shao Y, Sun K, Xu W, Li XL, Shen H and Sun
WH: Helicobacter pylori infection, gastrin and
cyclooxygenase-2 in gastric carcinogenesis. World J Gastroenterol.
20:12860–12873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Athanassiou-Papaefthymiou M, Shkeir O, Kim
D, et al: Evaluation of CD44 variant expression in oral, head and
neck squamous cell carcinomas using a triple approach and its
clinical significance. Int J Immunopathol Pharmacol. 27:337–349.
2014.PubMed/NCBI
|
|
7
|
Zhao S, He JL, Qiu ZX, Chen NY, Luo Z,
Chen BJ and Li WM: Prognostic value of CD44 variant exon 6
expression in non-small cell lung cancer: A meta-analysis. Asian
Pac J Cancer Prev. 15:6761–6766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hua D, Kong W, Zheng X, et al: Potent
tumor targeting drug release system comprising MMP-2 specific
peptide fragment with self-assembling characteristics. Drug Des
Devel Ther. 8:1839–1849. 2014.PubMed/NCBI
|
|
9
|
Kamyab-Hesari K, Mohtasham N, Aghazadeh N,
Biglarian M, Memar B and Kadeh H: The expression of MMP-2 and Ki-67
in head and neck melanoma, and their correlation with
clinic-pathologic indices. J Cancer Res Ther. 10:696–700.
2014.PubMed/NCBI
|
|
10
|
Budak M, Korpinar MA, Kalkan T and Tuncel
H: Mutation detection in the promoter region of survivin gene on
N-methyl-N-nitrosourea induced colon tumor model in experiment.
Bratisl Lek Listy. 115:554–556. 2014.PubMed/NCBI
|
|
11
|
Abd El-Hakim TF, El-Shafie MK, Abdou AG,
Azmy RM, El-Naidany SS and Badr El-Din MO: Value of urinary
survivin as a diagnostic marker in bladder cancer. Anal Quant
Cytopathol Histpathol. 36:121–127. 2014.PubMed/NCBI
|
|
12
|
Faibish D, Suzuki M and Bartlett JD:
Appropriate real-time PCR reference genes for fluoride treatment
studies performed in vitro or in vivo. Arch Oral Biol. 62:33–42.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turk HM, Camci C, Sevinc A, Bukyukberber
S, Sari I and Adli M: Cyclooxygenase-2 expression is not a marker
of poor survival in lung cancer. Asian Pac J Cancer Prev.
13:315–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ranger GS: Current concepts in colorectal
cancer prevention with cyclooxygenase inhibitors. Anticancer Res.
34:6277–6282. 2014.PubMed/NCBI
|
|
15
|
Quidville V, Segond N, Pidoux E, Cohen R,
Jullienne A and Lausson S: Tumor growth inhibition by indomethacin
in a mouse model of human medullary thyroid cancer: implication of
cyclooxygenases and 15-hydroxyprostaglandin dehydrogenase.
Endocrinology. 145:2561–2571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Francesco AM, Ruggiero A and Riccardi
R: Cellular and molecular aspects of drugs of the future:
Oxaliplatin. Cell Mol Life Sci. 59:1914–1927. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Avoranta ST, Korkeila EA, Syrjänen KJ,
Pyrhönen SO and Sundström JT: Lack of CD44 variant 6 expression in
rectal cancer invasive front associates with early recurrence.
World J Gastroenterol. 18:4549–4556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dommann SN, Ziegler T, Dommann-Schener CC,
Meyer J, Panizzon R and Burg G: CD44v6 is a marker for systemic
spread in cutaneous T-cell lymphomas. A comparative study between
nodal and cutaneous lymphomas. J Cutan Pathol. 22:407–412. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang YZ, Fang TY, Xu HG and Zhuo ZQ:
Expression of CD44v6 and Livin in gastric cancer tissue. Chin Med J
(Engl). 125:3161–3165. 2012.PubMed/NCBI
|
|
20
|
Protin U, Schweighoffer T, Jochum W and
Hilberg F: CD44-deficient mice develop normally with changes in
subpopulations and recirculation of lymphocyte subsets. J Immunol.
163:4917–4923. 1999.PubMed/NCBI
|
|
21
|
Matsumura Y and Tarin D: Significance of
CD44 gene products for cancer diagnosis and disease evaluation.
Lancet. 340:1053–1058. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Indinnimeo M, Cicchini C, Giarnieri E,
Stazi A, Mingazzini PL and Stipa V: Evaluation of CD44 variant 6
expression and clinicopathological factors in pulmonary metastases
from colon carcinoma. Oncol Rep. 10:1875–1877. 2003.PubMed/NCBI
|
|
23
|
Röcken M: Early tumor dissemination, but
late metastasis: Insights into tumor dormancy. J Clin Invest.
120:1800–1803. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maehara Y, Kabashima A, Koga T, Tokunaga
E, Takeuchi H, Kakeji Y and Sugimachi K: Vascular invasion and
potential for tumor angiogenesis and metastasis in gastric
carcinoma. Surgery. 128:408–416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko YH, Won HS, Jeon EK, Hong SH, Roh SY,
Hong YS, Byun JH, Jung CK and Kang JH: Prognostic significance of
CD44s expression in resected non-small cell lung cancer. BMC
Cancer. 11:3402011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skubitz AP: Adhesion molecules. Cancer
Treat Res. 107:305–329. 2002.PubMed/NCBI
|
|
27
|
Cho HJ, Kim HR, Park YS, Kim YH, Kim DK
and Park SI: Prognostic value of survivin expression in stage III
non-small cell lung cancer patients treated with platinum-based
therapy. Surg Oncol. 24:329–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao YF, Lu J, Du XJ, Sun LC, Zhao X, Peng
L, Cao L, Xiao PF, Pang L, Wu D, et al: Survivin selective
inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor cells. BMC
Cancer. 12:6192012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andersson SE, Svensson MN, Erlandsson MC,
Dehlin M, Andersson KM and Bokarewa MI: Activation of Fms-like
tyrosine kinase 3 signaling enhances survivin expression in a mouse
model of rheumatoid arthritis. PLoS One. 7:e476682012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jarrin M, Mansergh FC, Boulton ME, Gunhaga
L and Wride MA: Survivin expression is associated with lens
epithelial cell proliferation and fiber cell differentiation. Mol
Vis. 18:2758–2769. 2012.PubMed/NCBI
|
|
31
|
Liu W, Zhu F, Jiang Y, Sun D, Yang B and
Yan H: siRNA targeting survivin inhibits the growth and enhances
the chemosensitivity of hepatocellular carcinoma cells. Oncol Rep.
29:1183–1188. 2013.PubMed/NCBI
|
|
32
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bongiovanni L, D'Andrea A, Porcellato I,
Ciccarelli A, Malatesta D, Romanucci M, Della Salda L, Mechelli L
and Brachelente C: Canine cutaneous melanocytic tumours:
significance of β-catenin and survivin immunohistochemical
expression. Vet Dermatol. 26:270–e59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katsube K, Sakamoto K, Tamamura Y and
Yamaguchi A: Role of CCN, a vertebrate specific gene family, in
development. Dev Growth Differ. 51:55–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hou CH, Chiang YC, Fong YC and Tang CH:
WISP-1 increases MMP-2 expression and cell motility in human
chondrosarcoma cells. Biochem Pharmacol. 81:1286–1295. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang L, Luo RY, Yang J and Cheng YX:
Knockdown of survivin contributes to antitumor activity in
cisplatin-resistant ovarian cancer cells. Mol Med Rep. 7:425–430.
2013.PubMed/NCBI
|
|
37
|
Mao Y, Chen H, Lin Y, Xu X, Hu Z, Zhu Y,
Wu J, Xu X, Zheng X and Xie L: microRNA-330 inhibits cell motility
by downregulating Sp1 in prostate cancer cells. Oncol Rep.
30:327–333. 2013.PubMed/NCBI
|