Introduction
Numerous patients with RCC remain asymptomatic until
the late stages of the disease. The prevalence of the classic triad
of flank pain, gross haematuria and a palpable abdominal mass is
rare, while paraneoplastic syndromes occur in ~30% of patients with
symptomatic RCCs. A small number of patients present with symptoms
caused by metastatic RCC (1).
Metastatic disease at diagnosis is observed in ~25–30% of patients
with renal cell carcinoma (RCC), and <5% of patients exhibit
solitary metastasis. The most common distant RCC metastasis sites
are the lungs (50–60%), bone (30–40%), liver (30–40%) and brain
(5%); however, metastasis may involve virtually any organ site
(2). While adrenal metastasis from
RCC to the ipsilateral adrenal gland is common, synchronous
metastasis of RCC to the contralateral adrenal gland is rare, and
affects only 0.51% of patients (6/1,179) (3). Pancreatic metastases from RCC are
additionally rare (accounting for 1.5–3% of all metastatic RCC
cases), and these cases typically present a number of years
subsequent to the primary RCC diagnosis (4,5).
For localised RCC, surgery is the only curative
treatment with high-quality evidence and for metastatic RCC surgery
is curative only if the total tumor burden can be removed (1). As prognosis is directly associated with
the stage or degree of tumor dissemination, metastatic RCC
generally equates to a poor prognosis for patients; indeed,
response rates for treated patients remain low, at ~15–25%, with
5-year survival rates of 5–10% and overall median survival of <1
year (2). However, in certain cases,
radical resection of metastases may prolong survival. In the
present paper, a case of synchronous RCC metastasis to the
contralateral adrenal gland and pancreas is reported. The patient
remained alive at the final follow-up appointment, a total of 7
years subsequent to undergoing simultaneous right nephrectomy, left
adrenalectomy and segmental pancreatectomy.
Case report
A 55-year-old man with no relevant medical history
visited the Transplantation Center of The First Affiliated Hospital
at Wenzhou Medical University (Wenzhou, China) to undergo a routine
physical examination in March 2007. Abdominal magnetic resonance
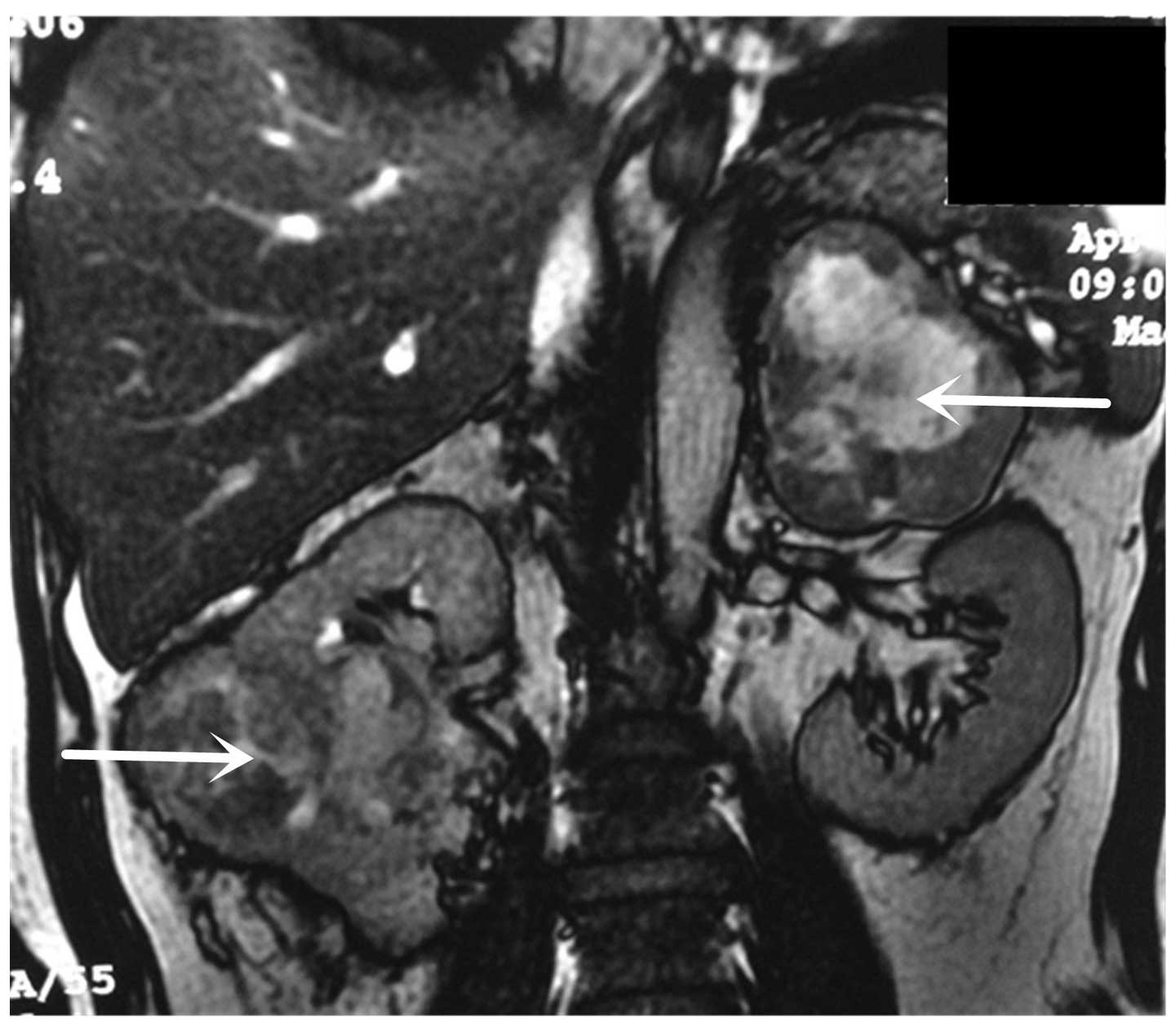
imaging (MRI; 1.5T; GE Healthcare, Little Chalfont, UK) revealed a
99×81 mm right renal tumor and a 94×72 mm left adrenal lesion
(Fig. 1). Thoracic computed
tomography (CT; LightSpeed Pro 16; GE Healthcare) and bone scans
were negative. The patient's full blood count, electrolytes, and
liver and renal function were within normal ranges. Metabolic
evaluation included 24-h urine collection for assessment of
17-ketosteroids, 17-hydrocorticoids, metanephrines, cortisol and
vanillylmandelic acid, the levels of which were normal.
During surgery (April 2007), a bilateral subcostal
incision was made. The right renal tumor measured 116×92×61 mm, and
the left adrenal lesion measured 96×79×57 mm. A 10×10 mm nodule on
the lower edge of the body of the pancreas was observed. The nodule
exhibited a good plane for dissection, which permitted complete
excision by segmental pancreatectomy. The nodule was fixed in 10%
fixed in formalin, dehydrated in graded alcohol, embedded in
paraffin, sliced to a thickness of 5 µm and deparaffinized in
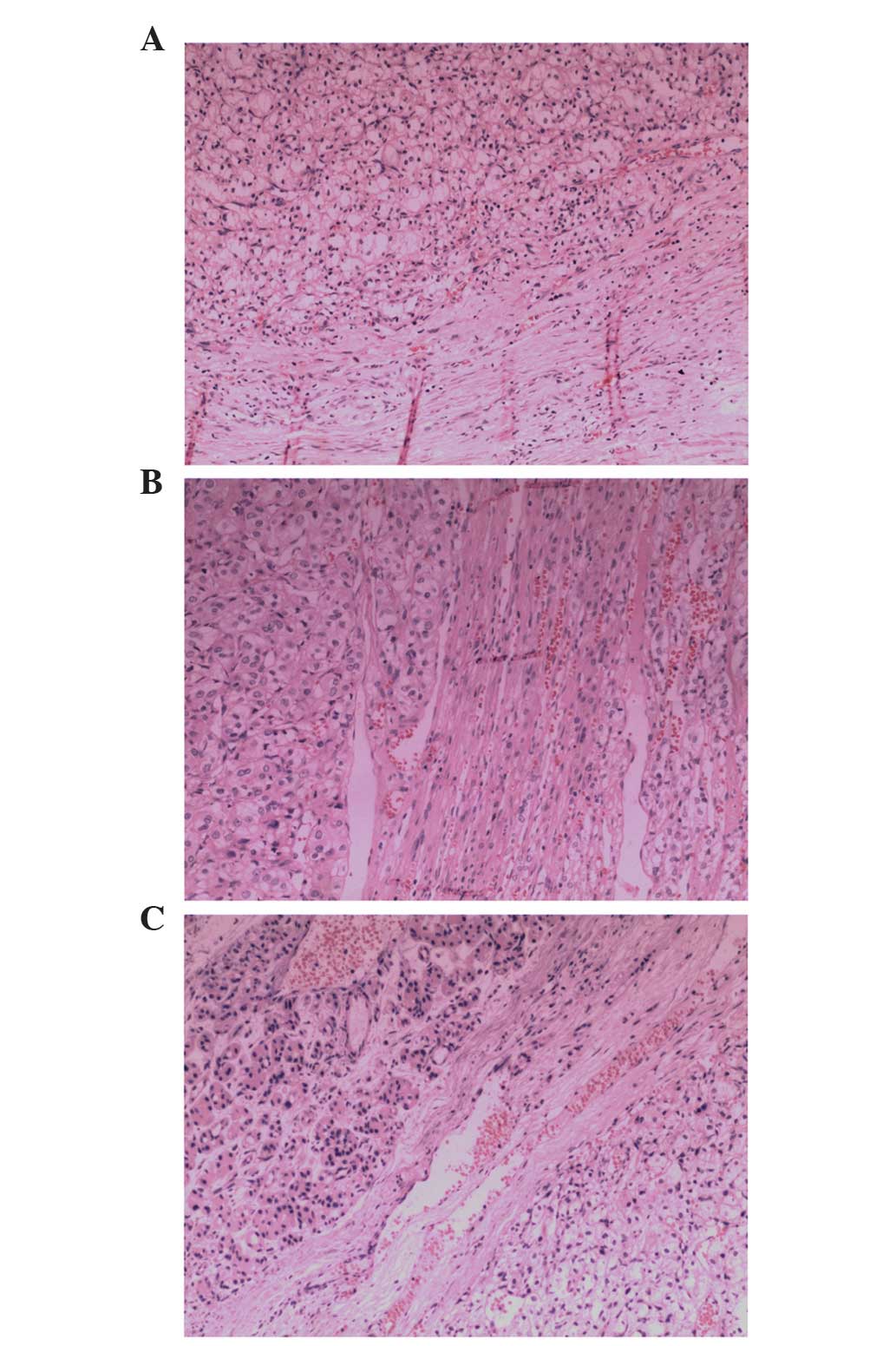
xylene. Histopathological examination (hematoxylin and eosin
staining) revealed that the contralateral adrenal tumor and
pancreatic nodule were pathologically identical to the renal tumor.
The histopathological specimens were also used to determine a
diagnosis of clear cell RCC (Fig. 2).
All surgical margins were tumor-free. It was determined that the
clinicopathological stage was pT2bN0M1 (6).
The patient recovered 12 days subsequent to surgery
and was discharged from hospital. He was administered interferon
immunotherapy (intrahepatic injection with 6 MIU interferon alfa-2b
iinjection 3 times per week) for 6 months. CT at 24 months
post-surgery identified no evidence of recurrence or new metastatic
lesions. However, abdominal CT 51 months subsequent to surgery
revealed 3 lesions in the bilateral adrenal area. A total of 66
months later, the patient exhibited 7 metastatic nodules in the
bilateral lungs, including 4 in the left lung and 3 in the right.
The patient refused additional treatment and remained alive with
these metastatic lesions during the annual follow-up appointments
for 7 years. The patient succumbed on November 30, 2015.
Written informed consent was obtained from the
patient for the publication of the present case report and
accompanying images, and ethical approval was obtained from the
ethics committee of the First Affiliated Hospital of Wenzhou
Medical University.
Discussion
To the best of our knowledge, the present study
describes the first reported case of synchronous metastasis to the
contralateral adrenal gland and pancreas from RCC. The fact that
this patient remained alive at the final follow-up appointment, a
total of 7 years subsequent to the surgical resection of the
primary tumor and metastases, is encouraging for treatment of
similar cases in the future.
RCC is known for its multiple modes of presentation
and its propensity to metastasize to almost every organ (2). Metastasis of RCC to the pancreas may
occur via the hematogenous route (involving the draining collateral
veins from the primary RCC lesion) or via the lymphatic route
(involving the retrograde lymph flow through the retroperitoneal
nodes), and direct spread to the pancreas is rare (7). The pathway through which metastasis to
the contralateral adrenal gland occurs remains to be elucidated,
although the antegrade hematogenous route may be involved (8). Notably, previous studies have
demonstrated that contralateral adrenal metastases are able to grow
to a considerable size, even when originating from only a small
number of metastatic RCC cells, and without metastasizing to other
organs; therefore, the adrenal gland may have an increased affinity
for RCC spread compared with other organs (9,10).
Diagnosis of adrenal metastases from RCC typically
relies on findings from CT and ultrasonography examinations
(11). Even with these advanced
imaging technologies, it can be difficult to differentiate adrenal
metastases from primary tumors of the adrenal gland, for example,
adrenal cortical adenoma (12). It is
generally considered that radiological findings of a solitary
adrenal mass in the absence of elevated serum adrenocortical
hormones is highly suggestive of an adrenal metastatic lesion, and
the present case confirmed this (13). As conservative treatments, including
chemotherapy, hormone therapy and radiotherapy, have not proven
effective in the treatment of metastatic RCC, surgical removal of
solitary metastatic lesions remains the only treatment option for
such patients (14). Surgical
resection of adrenal metastases is safe, and additionally promoted
by the development of a laparoscopic technique, using either a
retroperitoneal or a transperitoneal approach (15). In the treatment of the current case,
the ipsilateral adrenal gland was spared during radical nephrectomy
in order to avoid causing postoperative adrenal insufficiency; this
strategy was designed in accordance with previous findings that
have suggested that a routine ipsilateral adrenalectomy is not
recommended when there are no suspicions of tumor spread (16).
If the pancreatic metastases from RCC are small and
isolated, patients may be asymptomatic (17). However, a larger pancreatic tumor may
cause abdominal pain, jaundice and weight loss (18). In the present case, the pancreatic
metastatic nodule was small and not detected during preoperative
examination. As pancreatic metastases from RCC are typically
isolated lesions, they are more amenable to surgical treatment
(19). The type of pancreatic
metastasectomy selected for treatment, including
pancreaticoduodenectomy middle-segment or distal pancreatectomy,
depends upon the location of the tumor within the pancreas, and the
decision should be made with the goal of achieving clear margins of
resection (12). Current pancreatic
resections have low rates of mortality and morbidity, particularly
when performed at large healthcare facilities (20).
In conclusion, metastatic RCC is generally
associated with a poor prognosis (2);
few patients experience long-term survival following surgery, and
the clinical application of targeted drugs, including interferon
immunotherapy, may improve patient survival (21). In the current case, the patient
underwent simultaneous right nephrectomy, left adrenalectomy and
segmental pancreatectomy, followed by receipt of interferon
immunotherapy for 6 months. The patient survived for >7 years
after undergoing treatment. Thus, complete surgical excision of the
primary tumor and metastases may be the best available treatment
for certain patients.
Acknowledgements
The present study was funded by the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY12H10004).
References
|
1.
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Antonelli A, Cozzoli A, Simeone C, Zani D,
Zanotelli T, Portesi E and Cunico Cosciani S: Surgical treatment of
adrenal metastasis from renal cell carcinoma: A single-centre
experience of 45 patients. BJU Int. 97:505–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sellner F, Tykalsky N, De Santis M, Pont J
and Klimpfinger M: Solitary and multiple isolated metastases of
clear cell renal carcinoma to the pancreas: An indication for
pancreatic surgery. Ann Surg Oncol. 13:75–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zacharoulis D, Asopa V, Karvounis E and
Williamson RC: Resection of renal metastases to the pancreas: A
surgical challenge. HPB (Oxford). 5:137–141. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kim SP, Alt AL, Weight CJ, Costello BA,
Cheville JC, Lohse C, Allmer C and Leibovich BC: Independent
validation of the 2010 American Joint Committee on Cancer TNM
classification for renal cell carcinoma: Results from a large,
single institution cohort. J Urol. 185:2035–2039. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sotiropoulos GC, Lang H, Liu C, Brokalaki
EI, Molmenti E and Broelsch CE: Surgical treatment of pancreatic
metastases of renal cell carcinoma. JOP. 6:339–343. 2005.PubMed/NCBI
|
|
8.
|
Moslemi MK, Saghafi H and Firoozabadi MH:
Renal cell carcinoma with simultaneous bilateral adrenal
metastasis: Ipsilateral radical nephrectomy with contralateral
adrenal preservation. Case Rep Oncol. 3:372–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sagalowsky AI, Kadesky KT, Ewalt DM and
Kennedy TJ: Factors influencing adrenal metastasis in renal cell
carcinoma. J Urol. 151:1181–1184. 1994.PubMed/NCBI
|
|
10.
|
Dieckmann KP, Wullbrand A and Krolzig G:
Contralateral adrenal metastasis in renal cell cancer. Scand J Urol
Nephrol. 30:139–143. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Israel GM and Bosniak MA: How I do it:
Evaluating renal masses. Radiology. 236:441–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lau WK, Zincke H, Lohse CM, Cheville JC,
Weaver AL and Blute ML: Contralateral adrenal metastasis of renal
cell carcinoma: Treatment, outcome and a review. BJU Int.
91:775–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stein A, Mecz Y, Sova Y, Lurie M and Lurie
A: Synchronous and metachronous contralateral adrenal metastases
from primary renal carcinoma. Urol Int. 58:58–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sakorafas GH, Milingos D, Revelos K,
Siafakas J, Kontopoulos P and Peros G: Isolated metachronous
contralateral adrenal metastasis from renal cell carcinoma. Mt
Sinai J Med. 73:822–824. 2006.PubMed/NCBI
|
|
15.
|
Shonkwiler RJ and Lee JA: Laparoscopic
retroperitoneal adrenalectomy. Surg Laparosc Endosc Percutan Tech.
21:243–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Peters I, Hora M, Herrmann TR, von Klot C,
Wegener G, Stransky P, Hes O, Kuczyk MA and Merseburger AS:
Incidence of synchronous and metachronous adrenal metastases
following tumor nephrectomy in renal cell cancer patients: A
retrospective bi-center analysis. Springerplus. 2:2932013.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ballarin R, Spaggiari M, Cautero N, De
Ruvo N, Montalti R, Longo C, Pecchi A, Giacobazzi P, De Marco G,
D'Amico G, et al: Pancreatic metastases from renal cell carcinoma:
The state of the art. World J Gastroenterol. 17:4747–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gilani SM, Tashjian R, Danforth R and
Fathallah L: Metastatic renal cell carcinoma to the pancreas:
diagnostic significance of fine-needle aspiration cytology. Acta
Cytol. 57:418–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lavu H and Yeo CJ: Metastatic renal cell
carcinoma to the pancreas. Gastroenterol Hepatol (NY). 7:699–700.
2011.
|
|
20.
|
Wente MN, Kleeff J, Esposito I, Hartel M,
Müller MW, Fröhlich BE, Büchler MW and Friess H: Renal cancer cell
metastasis into the pancreas: A single-center experience and
overview of the literature. Pancreas. 30:218–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Singer EA, Gupta GN and Srinivasan R:
Update on targeted therapies for clear cell renal cell carcinoma.
Curr Opin Oncol. 23:283–289. 2011. View Article : Google Scholar : PubMed/NCBI
|