Introduction
MicroRNAs (miRs) are small, non-coding RNAs that
have significant roles in a number of biological processes
(1). miRs are able to regulate the
expression of a wide variety of genes by binding the
3′-untranslated region (UTR), suppressing messenger (m)RNA
translation or degrading mRNA, thus modulating numerous cellular
activities (2). Emerging evidence has
demonstrated that a number of miRs act as tumor suppressor genes or
oncogenes during tumor development, suggesting that they may be
useful as novel diagnostic and therapeutic markers (1). Previous studies have demonstrated an
abnormal miR expression profile in ovarian cancer, suggesting miRs
may have a role in the development of ovarian cancer (3,4).
Ovarian cancer is the primary cause of
cancer-associated mortality in women, with an annual incidence of
238,719 and mortality rate of 151,917 individuals worldwide in 2012
(5). Due to a lack of efficient
screening and early diagnosis, an abundance of patients present
with distant metastases at the time of diagnosis; therefore,
ovarian cancer is a challenging public health issue (6). The traditional treatments for ovarian
cancer comprise a combination of surgery and chemotherapy (7); however, even following treatment, the
prognosis of late-stage patients is poor (8). Therefore, it is crucial to investigate
the molecular mechanisms of ovarian cancer in order to develop
improved methods of diagnosis.
miR-494 is located on chromosome 14q32.31 (9). It has been observed that aberrant
expression of miR-494 is involved in various stages of
tumorigenesis (10). Overexpression
of miR-494 promoted apoptosis and inhibited growth in
gastrointestinal stromal tumor cells and esophageal squamous cell
carcinoma cells (11). In A549 human
lung cancer cells, miR-494 was reported to suppress cell
proliferation and colony forming activity, and additionally induced
senescence (12). miR-494 was
observed to suppress the cell viability and angiogenic ability of
medulloblastoma cells (13). By
contrast, induced expression of miR-494 enhances cell migration and
invasion in glioma cells by targeting p190B RhoGTPase activating
protein (14). In human
hepatocellular carcinoma, miR-494 is overexpressed and increases
proliferation through an acceleration of G1/S transition by
targeting the mutated in colorectal cancer tumor suppressor
(15). However, to the best of our
knowledge, little has been reported regarding the function of
miR-494 in ovarian cancer.
In the present study, it was initially demonstrated
that miR-494 is underexpressed in ovarian cancer samples and cell
lines. Overexpression of miR-494 in ovarian cancer cell lines
inhibited their proliferation and induced cell apoptosis.
Furthermore, a luciferase reporter assay confirmed that fibroblast
growth receptor 2 (FGFR2) was a target of miR-494, implying that
miR-494 may suppress ovarian cancer cell proliferation via
targeting of FGFR2.
Materials and methods
Tissue specimens
A total of 25 pairs of ovarian cancer tissue and
adjacent non-tumor tissue were collected at Nanjing Medical
University Affiliated Wuxi Second Hospital (Wuxi, China) from
patients during resection. Paired non-tumor tissues (adjacent to
the tumor) were obtained from the same patient that the tumor
tissue samples were collected, during the same procedure. The
tissue samples were obtained during surgery and immediately frozen
in liquid nitrogen until RNA extraction. The mean age of the
patients was 51.5 years (range, 34.0–69.0 years). None of the
patients had received radiation or chemotherapy prior to surgery.
Written informed consent was obtained from all patients. The
present study was approved by the Ethical Committee of Nanjing
Medical University Affiliated Wuxi Second Hospital (Wuxi,
China).
Cell culture and transfection
Ovarian cancer cell lines (ES2, HO8910, OVCAR3,
A2780, SKOV3 and HeLa) were obtained from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). Cells were cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) and 10% fetal bovine
serum (GE Healthcare Life Sciences, Logan, UT, USA), at 37°C in a
humidified atmosphere with 5% CO2. miR-494 mimic and
negative control oligonucleotide sequences were obtained from
Shanghai GenePharma Co., Ltd., (Shanghai, China). The sequences of
miR-494 mimic and negative control were as follows: miR-494 mimic
forward, 5′-UGAAACAUACACGGGAAACCUC-3′ and reverse,
5′-GGUUUCCCGUGUAUGUUUCAUU-3′; the negative control forward,
5′-UUCUCCGAACGUGUCACGUTT−3′ and reverse, ACGUGACACGUUCGGAGAATT−3′.
A total of 2×105 cells were seeded into a six-well plate
(Corning, Inc., Corning, NY, USA) and 100 nM miR-494 mimic and
negative control were transiently transfected into cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) once the cells had reached 70–90% confluence,
respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the tissue samples and cell lines was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription was performed to obtain complementary DNA
using a reverse transcription kit (PrimeScript™ RT reagent Kit with
gDNA Eraser; Takara Bio, Inc., Otsu, Japan), which also contained
DNase. Briefly, 1 µg RNA, 2 µl of 5X gDNA Eraser buffer and 1 µl of
gDNA Eraser and RNase Free dH2O were mixed and incubated
at 42°C for 2 min to remove the DNA. The RT reaction system
contained 10 µl of the aformentioned reaction solution, 1 µl of
PrimeScript RT Enzyme Mix I, 1 µl of RT Primer Mix, 4 µl of 5X
PrimeScript Buffer 2 and 4 µl of RNase Free dH2O. The
incubation conditions were 37°C for 15 min, followed by 85°C for 5
sec. A sample with no DNA was used as the negative control. All
reactions were performed with an ABI 7300 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
expression of miR-494 was analyzed by PCR using the
SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc.). U6 was
used for normalization. The primers were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China), and the sequences used were
as follows: miR-494 forward, 5′-TGACCTGAAACATACACGGGA−3′ and
reverse, 5′-TATCGTTGTACTCCACTCCTTGAC−3′; U6 forward,
5′-AAAGACCTGTACGCCAACAC-3′ and reverse,
5′-GTCATACTCCTGCTTGCTGAT−3′. The PCR cycling conditions were 95°C
for 3 min, followed by 40 cycles at 95°C for 30 sec, 62°C for 30
sec and 72°C for 30 sec.
The expression of FGFR2 was examined using a
SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc.). β-actin
was used as the internal control. Experiments were repeated three
times. The primer sequences were as follows (Genscript, Nanjing,
China): FGFR2 forward, 5′-TGACATTAACCGTGTTCCTGAG-3′ and reverse,
5′-TGGCGAGTCCAAAGTCTGCTAT-3′; β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The PCR cycling conditions were 94°C for 5 min, followed by 32
cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 45 sec. The
relative expression levels were evaluated by the 2−ΔΔCq
method (16).
Cell counting kit (CCK)-8 assay
Cell proliferation was assessed by CCK-8 assay.
Cells were seeded into 96-well plates (Corning, Inc.) and
transfected with miR-494 mimic or negative control as described
previously. A total of 24, 48, 72, 96 and 120 h subsequent to
transfection, 10 µl CCK-8 (Beyotime Institute of Biotechnology,
Haimen, China) was added into 100 µl medium. Following 2 h
incubation with the diluted CCK-8 in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.), the cells were lysed in
radioimmunoprecipitation assay buffer and centrifuged at 13,000 × g
for 10 min at 4°C. The absorbance of the supernatants was measured
at 450 nm on an imark microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Apoptosis assay using flow
cytometry
Cell apoptosis was analyzed using a propidium iodide
(PI)/Annexin V-fluorescein isothiocyanate (FITC) double staining
cell apoptosis detection kit (KeyGEN BioTECH, Nanjing, China),
according to the manufacturer's protocol, on a FACSCalibur™ (BD
Biosciences, Franklin Lakes, NJ, USA). A total of 3×105
cells were seeded into a 6-well plate (Corning, Inc.) and
transfected with miR-494 mimic or negative control. A total of 48 h
later, cells were collected and stained with Annexin V-FITC/PI, and
the percentage of apoptotic and viable cells was determined by flow
cytometry.
Dual luciferase assay
The wild-type and mutant 3′-UTRs of FGFR2 were
synthesized and cloned into the luciferase reporter vector pGL3
(catalog no., E1761; Promega Corp., Madison, WI, USA). HeLa cells
were seeded into 24-well plates (Corning, Inc.) and cultured until
they reached 70% confluence. Subsequently, 100 ng wild-type or
mutant pGL3-FGFR2-3′-UTR and 10 ng pRL-TK plasmid (E2241, Promega
Corp.), together with 30 nM miR-494 mimic or negative control were
cotransfected into cells using Lipofectamine 2000. The Renilla
luciferase activity was analyzed at 48 h post transfection using
the Dual-Luciferase® Reporter Assay System (Promega
Corp.) on a Lumat3 LB 9508 Single Tube Luminometer
(Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany).
Renilla luciferase activity was normalized to firefly luciferase
activity to control for transfection efficiency.
Western blot analysis
Following transfection, cells were washed twice with
ice-cold phosphate-buffered saline, harvested using trypsin (Gibco;
Thermo Fisher Scientific, Inc.) and lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Following 30 min of incubation, the lysed cells
were centrifuged at 13,000 × g for 10 min at 4°C, and the
supernatant was removed and frozen at −80°C. Total protein
extracted was quantified with a BCA Protein Assay kit (Beyotime
Institute of Biotechnology). Equal amounts of protein (80 µg) were
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis. The following primary antibodies were used: FGFR2
(dilution, 1:500; rabbit monoclonal; catalog no., #11835; Cell
Signaling Technology, Inc., Beverly, MA, USA) and β-actin
(dilution, 1:1,000; rabbit polyclonal; catalog no., BA0410; Wuhan
Boster Biological Technology, Ltd., Wuhan, China). Following
incubation with the primary antibodies overnight at 4°C, the
membranes were washed with TBST and incubated with secondary
antibody (dilution, 1:2,000; goat anti-rabbit; catalog no., BA1054;
Wuhan Boster Biological Technology, Ltd.) at room temperature for 2
h. Protein bands were detected using an enhanced chemiluminescence
detection system (Beyotime Institute of Biotechnology).
Statistical analysis
The independent Student's t-test was used to compare
two preselected groups, which were expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression level of miR-494 in ovarian
cancer tissues and cell lines
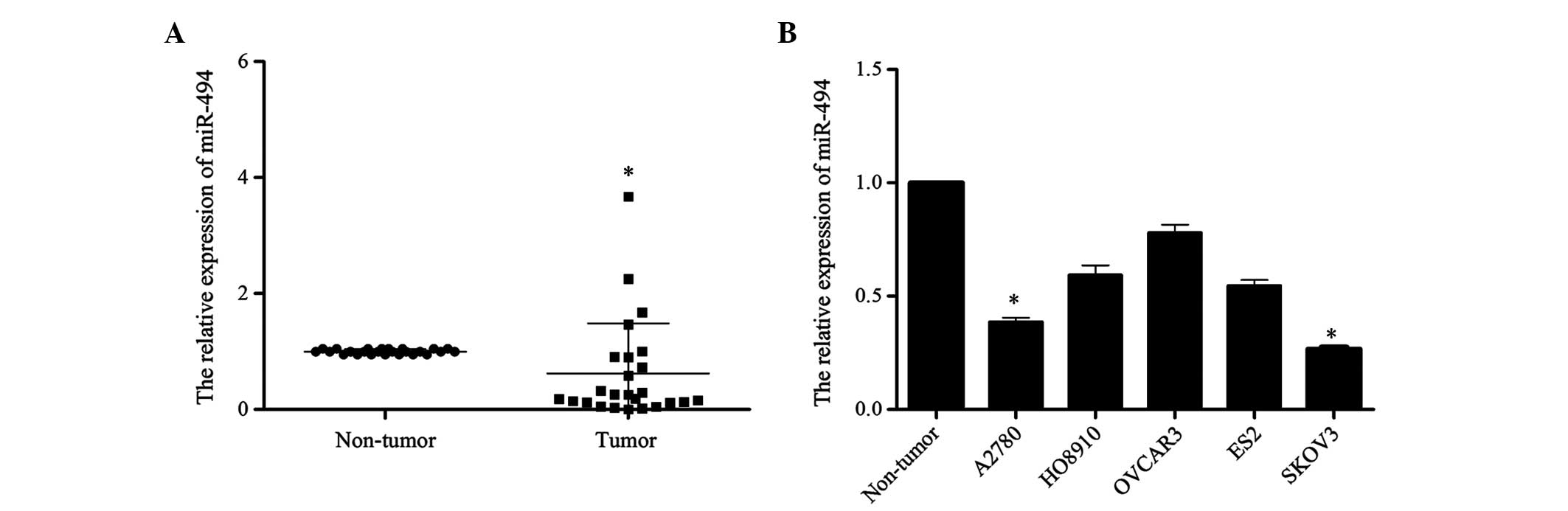
In order to investigate the potential role of
miR-494 in the development of ovarian cancer, the present study
detected the expression level of miR-494 in 25 ovarian cancer
tissues and 5 cell lines (ES2, HO8910, OVCAR3, A2780, SKOV3) by
RT-qPCR. The level of miR-494 expression in tumor tissues was
reduced compared with that in normal ovary tissues (P=0.012;
Fig. 1A). Similarly, compared with
the three normal ovarian tissues that were pooled and used as the
normal control, the expression of miR-494 in the 5 ovarian cancer
cell lines was reduced at different extent (A2780 vs. Non-tumor,
P=0.017; HO8910 vs. Non-tumor, P=0.089; OVCAR3 vs. Non-tumor,
P=0.28; ES2 vs. Non-tumor, P=0.057; SKOV3 vs. Non-tumor, P=0.015;
Fig. 1B). Taken together, these
results suggest that miR-494 may be involved in the development of
human ovarian cancer.
Overexpression of miR-494 inhibits
ovarian cancer cell growth and promotes apoptosis
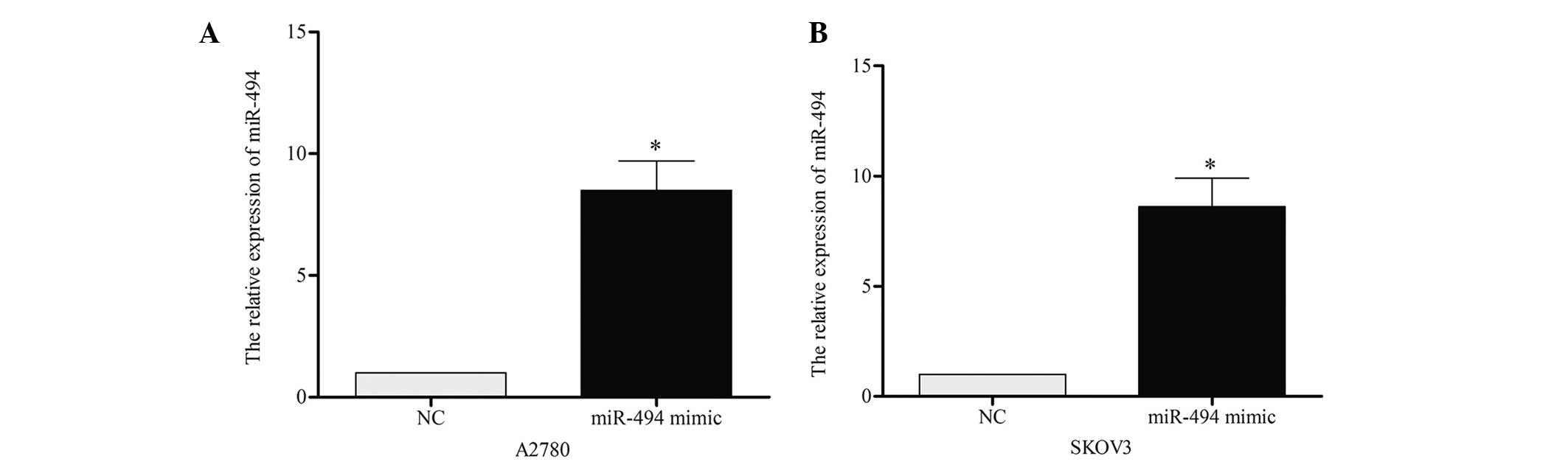
To investigate the role of miR-494 on cell growth
and apoptosis, the present study performed a gain-of-function
analysis using A2780 and SKOV3 cell lines. miR-494 mimic or
negative control was transiently transfected into the two cell
lines, and RT-qPCR was performed to confirm the effect 48 h later.
As demonstrated in Fig. 2A and B, the
expression of miR-494 in A2780 [miR-494 vs. negative control (NC),
P=0.019] and SKOV3 (miR-494 vs. NC, P=0.021) cells was
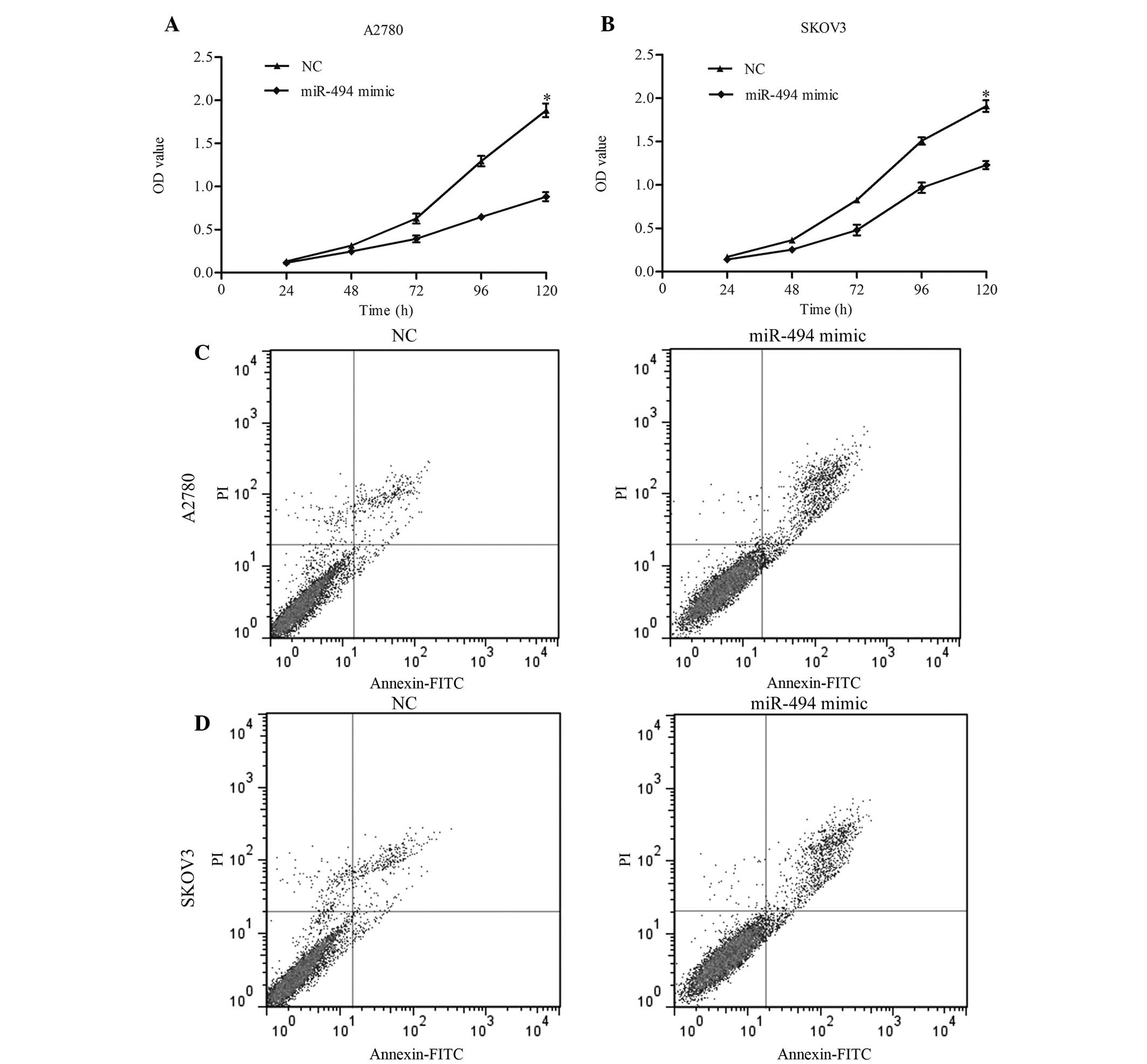
significantly increased following transfection. CCK-8 was performed
to detect the growth ability of cells. The results of this assay
indicated that overexpression of miR-494 resulted in a significant
decrease in cell proliferation in 120 h (Fig. 3A, miR-494 mimic vs. NC, P=0.011;
Fig. 3B, miR-494 mimic vs. NC,
P=0.038). The present study additionally examined the apoptotic
changes following miR-494 transfection with miR-949 mimic. The
results revealed that the apoptotic rate in miR-494-transfected
cells was increased compared with the control (Fig. 3C, miR-494 vs. NC, P=0.017; Fig. 3D, miR-494 vs. NC, P=0.024). Taken
together, these data suggest that miR-494 may inhibit ovarian
cancer cell growth by inducing apoptosis.
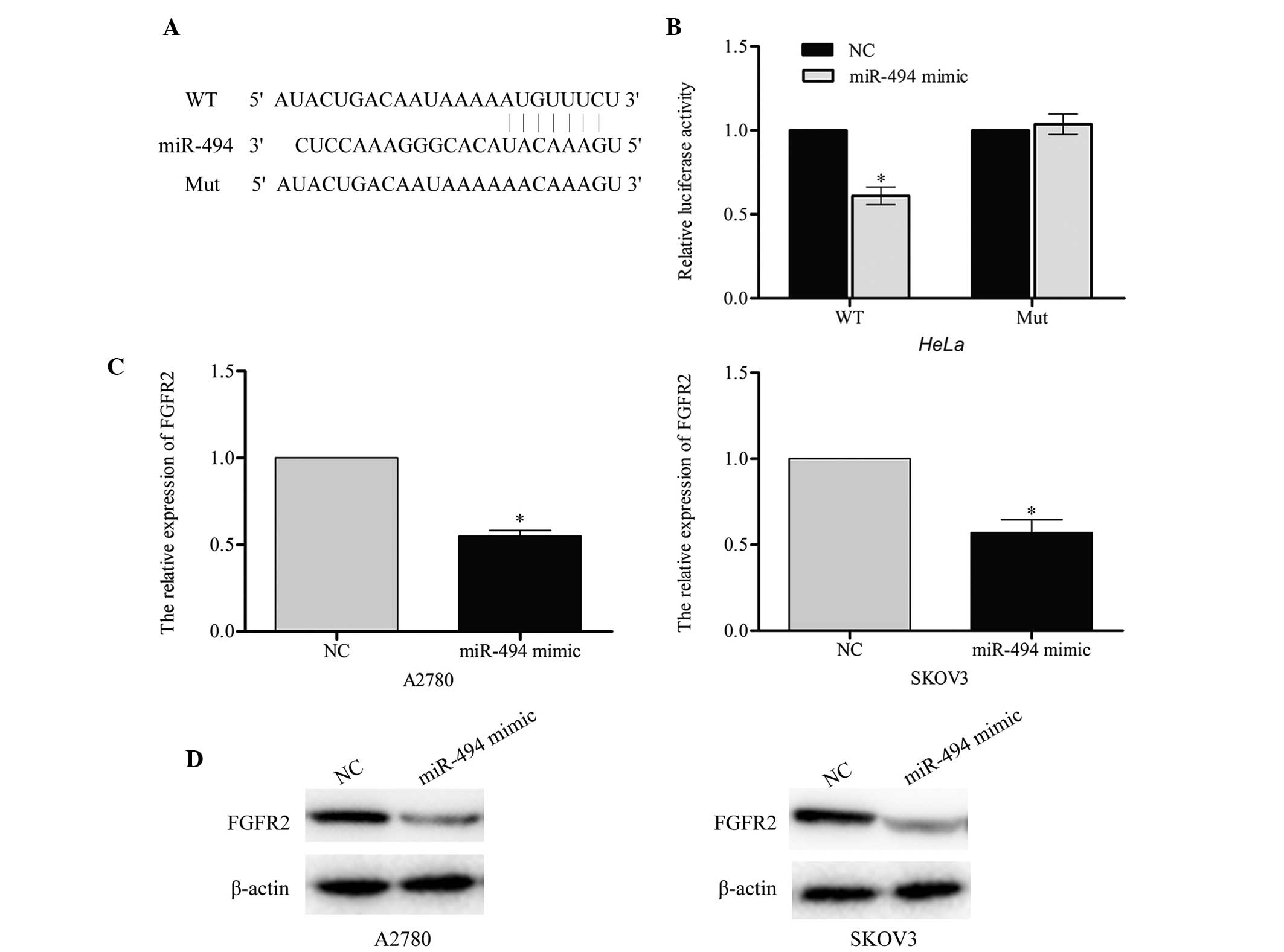
FGFR2 is a target gene of miR-494
According to the predictions made by the online
software TargetScan (www.targetscan.org), Pictar (pictar.mdc-berlin.de/)
and miRanda (www.microrna.org), a large number of
genes were identified as direct targets of miR-494. The present
study selected FGFR2 as a likely regulator of cell apoptosis. A
luciferase reporter assay was performed in order to investigate
whether FGFR2 was a target for miR-494. The wild-type
(pGL3-FGFR2-WT) and mutant (pGL3-FGFR2-Mut) binding site sequences
of miR-494 are listed in Fig. 4A. The
results of this assay demonstrated that the luciferase activity of
pGL3-FGFR2-WT in the two cell lines was markedly reduced compared
with the mutant pGL3-FGFR2-Mut (miR-494 mimic vs. NC, P=0.043;
Fig. 4B), which suggested that
miR-494 may directly target FGFR2.
In order to confirm the endogenous regulatory role
of miR-494 in relation to FGFR2 in ovarian cancer cells, the
present study measured the level of FGFR2 in A2780 and SKOV3 cells
following transfection with miR-494 mimic or negative control. The
results of this investigation indicated that the FGFR2 mRNA and
protein level were significantly downregulated in A2780 (miR-494
mimic vs. NC, P=0.031) and SKOV3 (miR-494 mimic vs. NC, P=0.037)
cells that had been transfected with miR-494 mimic (Fig. 4C and D). Taken together, these results
indicate that FGFR2 may be a direct target of miR-494.
Discussion
Due to the changes to the human lifestyle, the
occurrence of reproductive-, diet- and hormone-associated types of
cancer is increasing (17).
Accumulating evidence has revealed that miRs participate in
tumorigenesis, tumor development, drug resistance and other
pathological processes of cancer (18). miRs affect malignant cellular
behaviors by silencing a multitude of target genes, and regulating
the downstream signaling pathways (19). By targeting various genes, miR may
have opposing roles in different types of cancer (20). Similarly, miR-494 demonstrates
contrasting functions in various types of cancer. In
gastrointestinal stromal tumor cells, lung cancer cells (12), medulloblastoma cells (21), oral cancer cells (22), esophageal squamous cell carcinoma
cells (23), gastric cancer (24) and cholangiocarcinoma (21), miR-494 functions as a tumor suppressor
gene. By contrast, in glioma cells, oral squamous cell carcinoma
(25), bronchial cancer (26) and hepatocellular carcinoma (15), miR-494 functions as an oncogene. In
the present study, the expression profile of miR-494 was evaluated
in 25 pairs of tumor and adjacent non-tumor samples. The results of
this investigation revealed that the expression of miR-494 was
reduced in cancer tissues compared with normal tissues, and that
the five ovarian cancer cell lines exhibited a decreased miR-494
expression level compared with the normal control. These results
are consistent with previous studies in ovarian cancer tissues and
cell lines (27,28), suggesting that miR-494 may have a
significant role in ovarian cancer.
To elucidate the role and potential underlying
mechanism of miR-494 in ovarian cancer, a gain-of-function study
was performed on the A2780 and SKOV3 cell lines. The results of the
present study indicated that miR-494 inhibited ovarian cancer cell
proliferation and promoted cell apoptosis. Previous studies have
shown that miR-494 participates in the regulation of cell apoptosis
in human glioblastoma cells, non-small cell lung cancer and
esophageal squamous cell carcinoma by targeting various genes
(23,29,30). Wang
et al (31) reported that
miR-494 was able to target pro-apoptotic and anti-apoptotic
proteins, resulting in cardioprotective effects against
ischemia/reperfusion-induced injury. To additionally investigate
the pro-apoptotic role of miR-494 in ovarian cancer, the present
study predicted the target gene of miR-494. It was confirmed by
dual luciferase assay that FGFR2 was a target gene of miR-494.
Subsequently, western blotting revealed that the mRNA and protein
expression levels of FGFR2 were reduced in miR-494 overexpression
A2780 and SKOV3 cell lines compared with control cells. This
indicated that miR-494 may directly target FGFR2.
FGFR2 belongs to the FGFR family, and is comprised
of 2 isoforms: FGFR2-IIIb and FGFR2-IIIc (32). FGFRs are transmembrane tyrosine kinase
receptor proteins, which have crucial roles in embryonic
development, cell growth, tumorigenesis, invasiveness, motility and
angiogenesis by binding matching FGFs (33). In colorectal cancer, FGFR2 is highly
expressed and correlates with tumor growth, metastasis and
angiogenesis (34). A reduction in
FGFR2 expression was observed to inhibit proliferation of ovarian
cancer cell lines in vitro and additionally reduced the half
maximal inhibitory concentration of cisplatin (35). FGFR2 has been implicated in the
regulation of apoptosis in several types of cancer. Overexpression
of FGFR2 in breast cancer is correlated with a lower rate of
apoptosis (36). Restoration of FGFR2
expression in human prostate cancer cell lines suppresses cell
growth and tumorigenicity concurrent with increased cell
differentiation and apoptosis (37).
The results of the present study suggest that miR-494 induces
apoptosis through targeting FGFR2.
In conclusion, the present study demonstrates that
miR-494 is downregulated in ovarian cancer tissues. miR-494 may
inhibit the proliferation of ovarian cancer cells by inducing
apoptosis, potentially via targeting the anti-apoptotic gene FGFR2.
The findings of the present study provide evidence for the clinical
value of miR-494 as a target for ovarian cancer therapy, and the
precise regulatory mechanisms underlying these findings are
recommended for additional study in the future.
References
|
1
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen W, Song M, Liu J, Qiu G, Li T, Hu Y
and Liu H: MiR-26a promotes ovarian cancer proliferation and
tumorigenesis. PLoS One. 9:e868712014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tung CS, Wong KK and Mok SC: Biomarker
discovery in ovarian cancer. Womens Health (Lond Engl). 4:27–40.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen D, Zhang Y, Wang J, Chen J, Yang C,
Cai K, Wang X, Shi F and Dou J: MicroRNA-200c overexpression
inhibits tumorigenicity and metastasis of CD117+CD44+ ovarian
cancer stem cells by regulating epithelial-mesenchymal transition.
J Ovarian Res. 6:502013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Kim S and Kim IM: Regulation of
metastasis by microRNAs in ovarian cancer. Front Oncol. 4:1432014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song L, Liu D, Wang B, He J, Zhang S, Dai
Z, Ma X and Wang X: miR-494 suppresses the progression of breast
cancer in vitro by targeting CXCR4 through the Wnt/β-catenin
signaling pathway. Oncol Rep. 34:525–531. 2015.PubMed/NCBI
|
|
10
|
Shen PF, Chen XQ, Liao YC, Chen N, Zhou Q,
Wei Q, Li X, Wang J and Zeng H: MicroRNA-494-3p targets CXCR4 to
suppress the proliferation, invasion, and migration of prostate
cancer. Prostate. 74:756–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim WK, Park M, Kim YK, Tae YK, Yang HK,
Lee JM and Kim H: MicroRNA-494 downregulates KIT and inhibits
gastrointestinal stromal tumor cell proliferation. Clin Cancer Res.
17:7584–7594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohdaira H, Sekiguchi M, Miyata K and
Yoshida K: MicroRNA-494 suppresses cell proliferation and induces
senescence in A549 lung cancer cells. Cell Prolif. 45:32–38. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asuthkar S, Velpula KK, Nalla AK, Gogineni
VR, Gondi CS and Rao JS: Irradiation-induced angiogenesis is
associated with an MMP-9-miR-494-syndecan-1 regulatory loop in
medulloblastoma cells. Oncogene. 33:1922–1933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwak SY, Yang JS, Kim BY, Bae IH and Han
YH: Ionizing radiation-inducible miR-494 promotes glioma cell
invasion through EGFR stabilization by targeting p190B rhoGAP.
Biochim Biophys Acta. 1843:508–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim L, Balakrishnan A, Huskey N, Jones KD,
Jodari M, Ng R, Song G, Riordan J, Anderton B, Cheung ST, et al:
MicroRNA-494 within an oncogenic microRNA megacluster regulates
G1/S transition in liver tumorigenesis through suppression of
mutated in colorectal cancer. Hepatology. 59:202–215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Webb PM: Environmental (nongenetic)
factors in gynecological cancers: Update and future perspectives.
Future Oncol. 11:295–307. 2015. View Article : Google Scholar
|
|
18
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Ann Biol Clin (Paris).
68:263–272. 2010.(In French). PubMed/NCBI
|
|
21
|
Yamanaka S, Campbell NR, An F, Kuo SC,
Potter JJ, Mezey E, Maitra A and Selaru FM: Coordinated effects of
microRNA-494 induce G2/M arrest in human
cholangiocarcinoma. Cell Cycle. 11:2729–2738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Libório-Kimura TN, Jung HM and Chan EK:
miR-494 represses HOXA10 expression and inhibits cell proliferation
in oral cancer. Oral Oncol. 51:151–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Chen X, Zhang S, Zhang X, Li T,
Liu Z, Wang J, Zang W, Wang Y, Du Y and Zhao G: Upregulation of
miR-494 inhibits cell growth and invasion and induces cell
apoptosis by targeting cleft lip and palate transmembrane 1-like in
esophageal squamous cell carcinoma. Dig Dis Sci. 60:1247–1255.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He W, Li Y, Chen X, Lu L, Tang B, Wang Z,
Pan Y, Cai S, He Y and Ke Z: miR-494 acts as an anti-oncogene in
gastric carcinoma by targeting c-myc. J Gastroenterol Hepatol.
29:1427–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ries J, Vairaktaris E, Agaimy A, Kintopp
R, Baran C, Neukam FW and Nkenke E: miR-186, miR-3651 and miR-494:
Potential biomarkers for oral squamous cell carcinoma extracted
from whole blood. Oncol Rep. 31:1429–1436. 2014.PubMed/NCBI
|
|
26
|
Duan H, Jiang Y, Zhang H and Wu Y: MiR-320
and miR-494 affect cell cycles of primary murine bronchial
epithelial cells exposed to benzo[a]pyrene. Toxicol In Vitro.
24:928–935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim YW, Kim EY, Jeon D, Liu JL, Kim HS,
Choi JW and Ahn WS: Differential microRNA expression signatures and
cell type-specific association with Taxol resistance in ovarian
cancer cells. Drug Des Devel Ther. 8:293–314. 2014.PubMed/NCBI
|
|
28
|
Yuan J, Wang K and Xi M: miR-494 inhibits
epithelial ovarian cancer growth by targeting c-Myc. Med Sci Monit.
22:617–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al: miR-494-3p
Regulates Cellular proliferation, invasion, migration, and
apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell
Mol Neurobiol. 35:679–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romano G, Acunzo M, Garofalo M, Di Leva G,
Cascione L, Zanca C, Bolon B, Condorelli G and Croce CM: MiR-494 is
regulated by ERK1/2 and modulates TRAIL-induced apoptosis in
non-small-cell lung cancer through BIM down-regulation. Proc Natl
Acad Sci USA. 109:16570–16575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amann T, Bataille F, Spruss T, Dettmer K,
Wild P, Liedtke C, Mühlbauer M, Kiefer P, Oefner PJ, Trautwein C,
et al: Reduced expression of fibroblast growth factor receptor
2IIIb in hepatocellular carcinoma induces a more aggressive growth.
Am J Pathol. 176:1433–1442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang J, Chen P, Hu Z, Zhou X, Chen L, Li
M, Wang Y, Tang J, Wang H and Shen H: Genetic variants in
fibroblast growth factor receptor 2 (FGFR2) contribute to
susceptibility of breast cancer in Chinese women. Carcinogenesis.
29:2341–2346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsuda Y, Ueda J and Ishiwata T:
Fibroblast growth factor receptor 2: Expression, roles, and
potential as a novel molecular target for colorectal cancer.
Patholog Res Int. 2012:5747682012.PubMed/NCBI
|
|
35
|
Cole C, Lau S, Backen A, Clamp A, Rushton
G, Dive C, Hodgkinson C, McVey R, Kitchener H and Jayson GC:
Inhibition of FGFR2 and FGFR1 increases cisplatin sensitivity in
ovarian cancer. Cancer Biol Ther. 10:495–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tannheimer SL, Rehemtulla A and Ethier SP:
Characterization of fibroblast growth factor receptor 2
overexpression in the human breast cancer cell line SUM-52PE.
Breast Cancer Res. 2:311–320. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yasumoto H, Matsubara A, Mutaguchi K, Usui
T and McKeehan WL: Restoration of fibroblast growth factor
receptor2 suppresses growth and tumorigenicity of malignant human
prostate carcinoma PC-3 cells. Prostate. 61:236–242. 2004.
View Article : Google Scholar : PubMed/NCBI
|