Introduction
The majority of patients with advanced ovarian
cancer will experience disease recurrence within a few years from
the time of diagnosis (1). Due to the
nature of the disease, with its location in the small pelvis and
spread in the form of diffuse carcinosis, there are no reliable
methods of detecting early recurrence using ultrasound, computed
tomography (CT) or magnetic resonance imaging (2,3).
Furthermore, it is well known that serum levels of carbohydrate
antigen 125 (CA125) have a considerable lead-time before clinically
detectable recurrence (4,5).
Currently, the clinical consequence of rising CA125
levels remains an issue of great debate, particularly as to whether
re-treatment should be initiated based only on biochemical CA125
recurrence. Rustin et al (6)
demonstrated that survival time was not improved when treatment was
based on biochemical recurrence alone. However, it should be noted
that this study investigated only women who experienced
normalization of CA125 during their first-line treatment.
Furthermore, the study has been criticized for its very diverse
relapse treatment, including different chemotherapy regimens, which
may not have been in accordance with the current standard. Also,
none of the patients were offered secondary cytoreductive surgery
that may have led to improved survival; this issue is currently
under intense discussion in the scientific community, and the
results of the ongoing randomized DESKTOP III (7) study are eagerly awaited to clarify
whether surgery for relapse will improve the outcome for patients
with recurrent disease. It may be highly relevant to practice
active monitoring of women after the end of treatment to detect
recurrence as early as possible, particularly if the DESKTOP III
study demonstrates a survival benefit. Furthermore, a retrospective
study by Fleming et al (8)
indicated that each week delay of treatment following the first
CA125 elevation in recurrent ovarian cancer correlated with a 3%
increased chance of suboptimal resection at secondary cytoreductive
surgery, and therefore CA125 surveillance increased optimal
resectability at secondary cytoreductive surgery. Thus, the current
situation calls for better methods for early detection of
recurrence with the perspective of curatively intended surgical
and/or non-surgical treatment. CA125 is insufficient for a number
of reasons, including the fact that it is not always elevated in
patients with mucinous tumors (9).
Human epididymis protein 4 (HE4) is a relatively new
biomarker approved by the United States Food and Drug
Administration (FDA) for monitoring of patients with epithelial
ovarian cancer. HE4 is encoded by the WFDC2 gene located on
chromosome 20q12-13.1 (10) and
belongs to the family of whey-acidic four-disulfide core proteins
with suspected trypsin-inhibitor properties (11). However, the biological role of HE4 has
not yet been identified (12). HE4 is
upregulated in ovarian cancer compared to other types of carcinomas
and benign ovarian tumors (13,14).
Recent studies have identified HE4 as a complementary marker for
ovarian cancer that can be elevated in some cases where CA125 is
not (15–21); however, its potential value in the
early detection of recurrence has not been elucidated.
The aim of the present study was to explore the
clinical value of serial measurements of HE4 and CA125 during
follow-up for the early detection of recurrence, and the additive
value of combining the two markers.
Materials and methods
Study population
The current study included patients with ovarian
cancer who had completed first-line combination chemotherapy with
paclitaxel (175 mg/m2 intravenously) and carboplatin
(AUC=5) every 3 weeks at two Danish Hospitals (Aalborg University
Hospital, Aalborg, Denmark; and Vejle Hospital, Vejle, Demark) and
attended follow-up according to national guidelines. Patients with
a serum sample drawn at the end of chemotherapy and ≥2
post-chemotherapy blood samples were included in the study.
All patients were entered as part of a translational
research protocol, with peripheral venous blood samples drawn at
the end of chemotherapy and at every scheduled follow-up visit:
Every 3 months for the first two years, every 6 months for the
third year, and once a year for the fourth and fifth years.
Clinical data were recorded in detailed case report forms. Detailed
patient characteristics have been given elsewhere (22).
The study was carried out in compliance with the
Helsinki II Declaration (23) and all
patients signed an informed consent form. The Danish Biomedical
Research Ethics Committee and the Danish Data Protection Agency
approved the study according to Danish law. Recurrence of disease
was defined according to the Gynecological Cancer Intergroup CA125
criteria (24,25) and/or radiological confirmation of
tumor recurrence, whichever occurred first. Biochemical recurrence
detected by CA125 required CT confirmation to verify the diagnosis
of recurrence.
Serum CA125 assay
The quantitative levels of serum CA125 were
determined using the commercially available CanAg CA125 Enzyme
Immunometric Assay (EIA) kit (cat. no.,400-10; Fujirebio
Diagnostics AB, Gothenburg, Sweden) with inter- and intra-assay
coefficients of variation (CV) of ≤10% and a sensitivity of 1.5
IU/ml. The assay is based on a direct sandwich technique using two
mouse monoclonal antibodies, Ov197 and Ov185, directed against two
independent epitopes of the protein core of the CA125 antigen. The
analysis was performed in 25 µl of serum and followed the
manufacturer's protocol.
Serum HE4 assay
HE4 serum levels were determined by the
enzyme-linked immunosorbent assay technique using a commercially
available FDA-approved kit (HE4 EIA kit, cat.no., 404-10; Fujirebio
Diagnostics AB). The analysis used 25 µl serum; the analytical
steps were conducted according to the manufacturer's instructions
and have been described in further detail in a previous publication
from our group (21).
HE4 control 1 and 2 were used for validation of each
assay series. The lyophilized controls contained HE4 antigen in a
human serum matrix and a non-azide antimicrobial preservative
included in the kit. The mean values of control duplicates and the
duplicate replicates of calibrators A-F were within the specified
ranges provided by the manufacturer for all runs. The total CV in
the present analysis was between 3.3 and 8.8% in the high and low
range of HE4 levels, respectively.
Statistical analyses
A validated HE4 threshold for monitoring during
follow-up has not been established, and the current study aimed to
investigate changes in HE4 and CA125 during follow-up compared to
their ‘baseline’ level at the end of adjuvant chemotherapy
treatment. At 3- and 6-month follow-up examinations, the HE4 and
CA125 levels were compared to the baseline end-of-treatment (EOT)
sample, and patients were divided into groups based on having an
increase above or below/equal to 50%. From this dichotomous
classification, a univariate Kaplan-Meier log-rank analysis was
performed to assess the association with progression-free survival
(PFS).
Multivariate analysis (Cox regression) was conducted
for the identification of independent factors predicting PFS.
The study also aimed to investigate whether the EOT
sample drawn at the end of chemotherapy (corresponding to the
beginning of follow-up) was able to predict disease recurrence at
this very early time point. It was decided a priori that the
data would be analyzed at a set sensitivity of 90% since the aim
was to investigate whether the markers were sensitive enough to
detect relapse from a serum sample taken just before the follow-up.
A sensitivity of 90% for detecting recurrence was achieved when the
threshold was 41 pmol/l for HE4, and 1 U/ml for CA125. The latter,
which is the lowest detectable level, was not meaningful in the
analysis of specificity, positive predictive value (PPV) or
negative predictive value (NPV) for CA125.
For the analysis of HE4 and CA125 thresholds, simple
and multiple regression analyses were used. Statistical analyses
were performed with NCSS software (version 2007; NCSS, Kaysville,
UT, USA; www.ncss.com) and STATA 13.1 (College
Station, TX, USA). The Mann-Whitney U test was used for the
comparison of medians. P<0.05 was considered to indicate
statistically significant differences.
Results
Patient characteristics
From May 2006 through August 2011, a total of 283
consecutive patients were enrolled in the translational research
protocol, and all had serum samples drawn during chemotherapy. In
July 2008, the protocol was amended and approved for collection of
blood samples during follow-up, and 88 patients were identified as
having a serum EOT sample and ≥2 sequential samples collected
during the follow-up period, according to the inclusion criteria.
The median follow-up time for patients still alive (n=52) was 47
months (range, 26–86 months). Of the 88 patients, 55 were diagnosed
with recurrence and 38 patients died during follow-up. More than
97.7% had ≥3 serial serum samples, and 72.7% had ≥4 serial samples
(maximum, 12). The median time between collection of the EOT sample
and the first follow-up sample at 3 months was 93 days (31–147
days) and the median time to the sample drawn at 6-month follow-up
was 187.5 days (68–266 days). In total, 547 serum samples were
analyzed: 83 EOT samples and 464 samples during the subsequent
follow-up period. Patient characteristics are presented in Table I.
 | Table I.Patient characteristics (n=88). |
Table I.
Patient characteristics (n=88).
| Clinicopathological
parameter | Value |
|---|
| Age, years |
|
|
Median | 64.0 |
|
Range | 28–77 |
| FIGO stage, n
(%) |
|
| I | 22 (25.0) |
| II | 7 (8.0) |
| III | 44 (50.0) |
| IV | 15 (17.0) |
| Gradea, n (%) |
|
| 1 | 11 (16.9) |
| 2 | 25 (38.5) |
| 3 | 29 (44.6) |
| Histological type, n
(%) |
|
|
Serous | 65 (73.9) |
|
Mucinous | 4 (4.5) |
|
Endometrioid | 9
(10.2) |
| Clear
cell | 6 (6.8) |
|
Otherb | 4 (4.5) |
| Residual tumor, n
(%) |
|
| 0
cm | 56 (63.6) |
| <1
cm | 11 (12.5) |
| ≥1
cm | 21 (23.9) |
Prediction of relapse from EOT samples
by CA125, HE4 and combined CA125/HE4 levels
The median CA125 serum level at the end of
first-line chemotherapy treatment (prior to the initiation of
follow-up) was 4 U/ml (95% CI, 1–5 U/ml; range 1–14 U/ml) for
patients without relapse and 5 U/ml (95% CI, 3–7 U/ml; range 1–116
U/ml) for patients with relapse (P=0.0985, Mann-Whitney U
test).
The median HE4 serum level at the end of first-line
chemotherapy treatment (prior to the initiation of follow-up) was
51 pmol/l (95% CI, 46–59 pmol/l; range, 15–127 pmol/l) for patients
without relapse and 67 pmol/l (95% CI, 60–81 pmol/l; range, 31–229
pmol/l) for patients with relapse (P=0.0013, Mann-Whitney U
test).
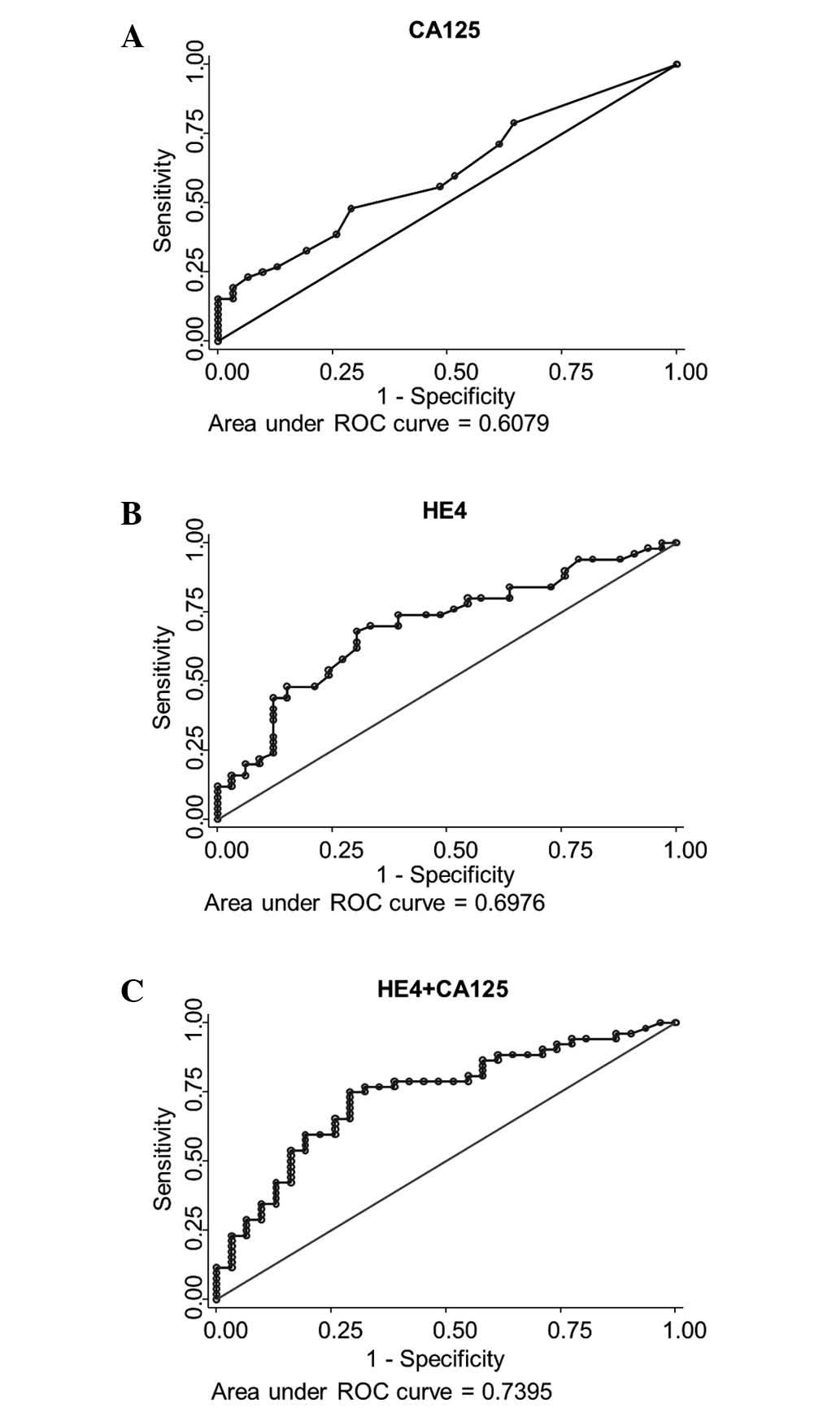
Fig. 1 illustrates the
receiver operating characteristic curve analysis for samples
collected at the end of first-line treatment prior to follow-up
(EOT sample), and prediction of recurrence with relapse (PFS) as
the endpoint.
HE4 values at the end of first-line treatment
classified 70 patients (84.3%) as being in the high risk of relapse
group, and 13 (15.7%) into the low-risk group, with a sensitivity
of 90.0% (95% CI, 79.0–96.8%), a specificity of 25.8% (95% CI,
11.9–44.6%), a PPV of 67.1% (95% CI, 54.9–77.9%) and an NPV of
61.5% (95% CI, 31.6–86.1%) (data not shown).
When combining HE4 and CA125, 69 patients (83.1%)
were in the high-risk and 14 (16.9%) in the low-risk group,
resulting in a sensitivity of 90.0% (95% CI, 79.0–96.8%), a
specificity of 29% (95% CI, 14.2–48.0%), a PPV of 68.1% (95% CI,
55.8–78.8%) and an NPV of 64.3% (95% CI, 35.1–87.2%) (data not
shown).
Prediction of relapse during
follow-up
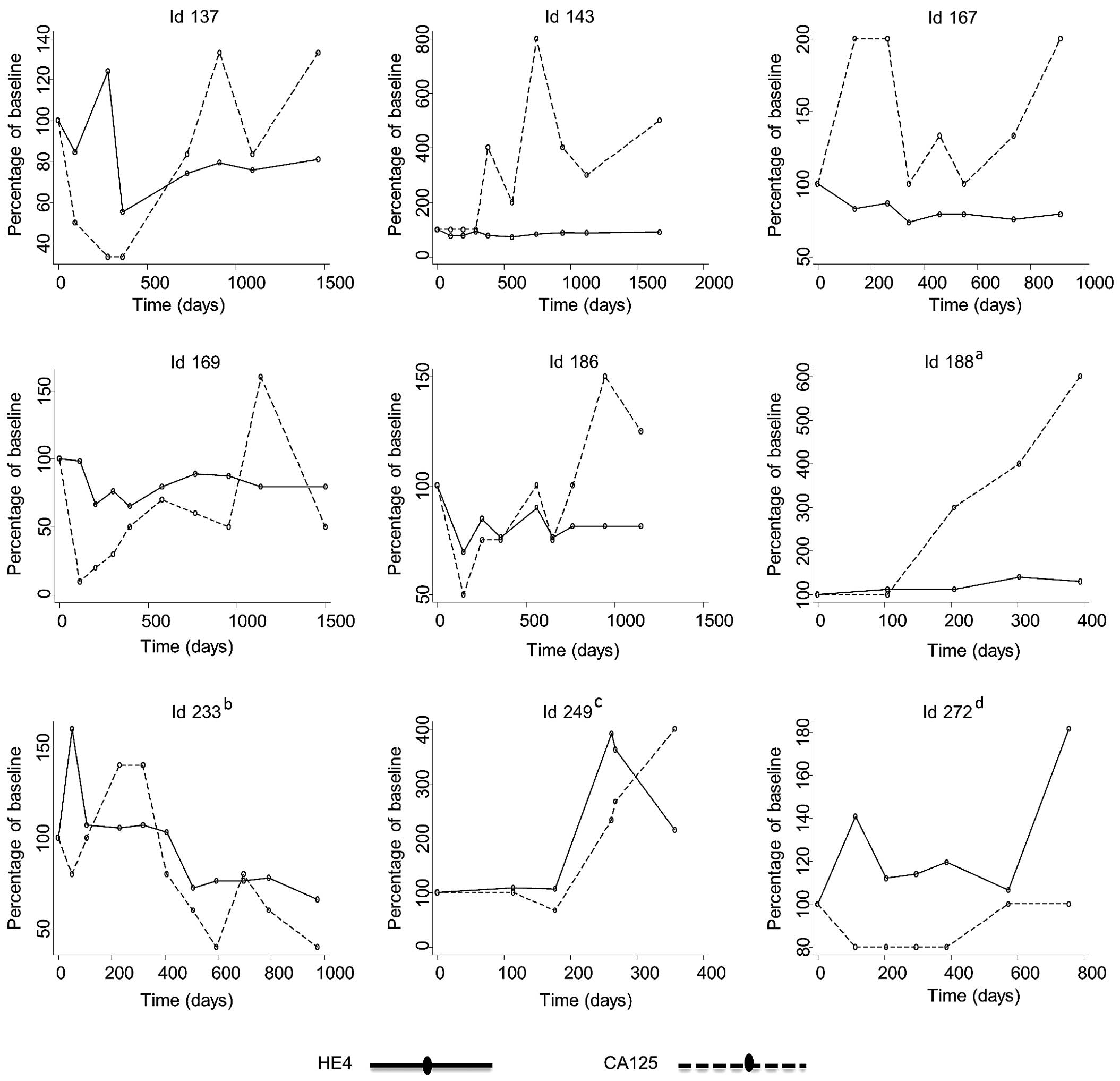
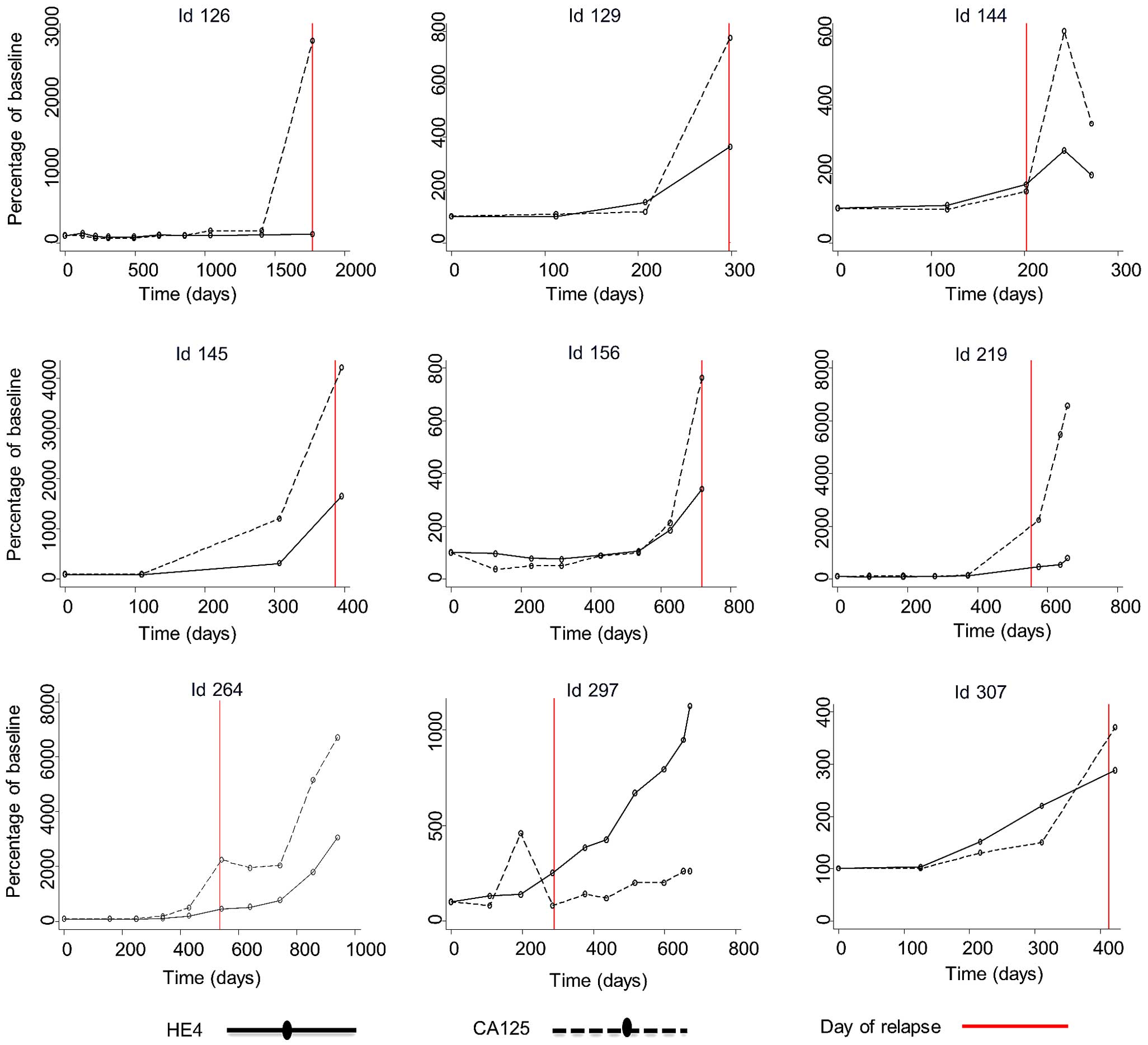
Fig. 2 (patients
without relapse) and Fig. 3 (patients
with relapse) illustrate selected cases during the follow-up
process, in which the first serum sample is the EOT sample and
subsequent samples were obtained during the follow-up visits.
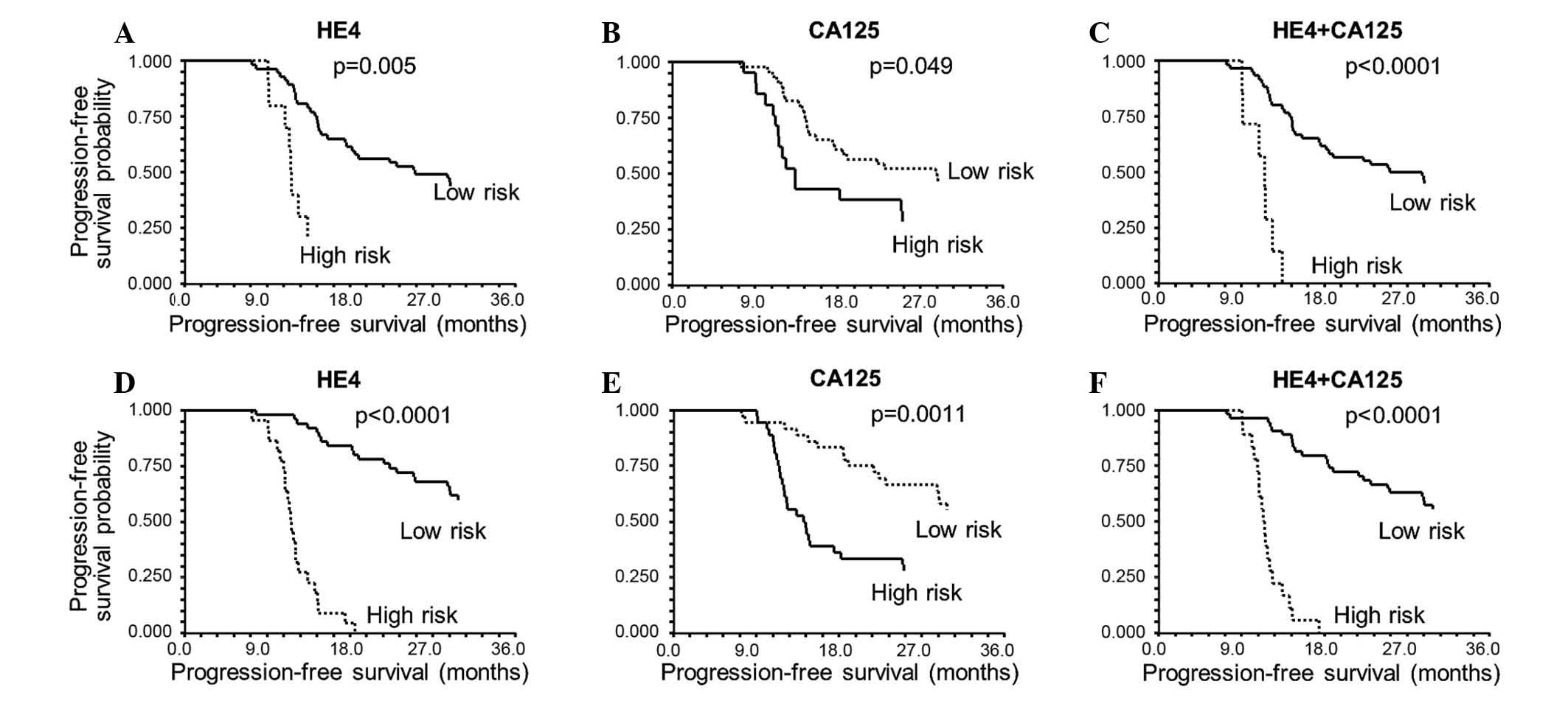
Analysis of HE4 and CA125 levels at 3 months after EOT (Fig. 4A and B) revealed that an increase of
≥50% (high-risk patients) relative to the level at EOT is
associated with a significant worsening of PFS. In particular, a
HE4 increase >50% was correlated with significant worsening of
PFS time [hazard ratio (HR), 2.82; 95% CI, 0.91–8.79; P=0.0052,
log-rank test]. Additionally, increased CA125 (>50%) was
associated with poorer PFS time (HR, 1.86; 95% CI, 0.90–3.80;
P=0.0487, log-rank test).
The median PFS was 25.1 months (95% CI, 17.7–56.8)
if HE4 did not increase >50% compared to the EOT sample, while
it was 11.1 months (95% CI, 11.0–12.4) for an increase >50% at
the 3-month follow-up.
For CA125 at 3 months, the median PFS was 28.7
months (95% CI, 17.4–56.8) if stable, and 13.4 months (95% CI,
11.5–24.9) if increased >50%.
The impact of increased HE4 became more clear after
6 months of follow-up (HR, 7.71; 95% CI, 3.03–19.58; P<0.0001,
log-rank test) (Fig. 4D and E), with
a median PFS time of 56.8 months (95% CI, 28.7–63.2) if stable,
compared with 11.4 months (95% CI, 10.8–12.1) when HE4 increased
>50%. The corresponding median PFS values were 56.8 months (95%
CI, 28.7–63.2) vs. 14.2 months (95% CI, 12.0–7.4) for CA125 (HR,
2.55; 95% CI, 1.39–4.68, P=0.0011, log-rank test).
Combining the two markers and classifying the
patients into a high-risk group if both markers had increased
>50% revealed similar results: P<0.0001 with a median PFS of
25.1 months (95% CI, 17.7–56.8) if both markers were stable, and
11.5 months (95% CI, 9.2–11.6) if both markers increased ≥50% at 3
months (Fig. 4C). However, this group
of high-risk patients was small at the 3-month follow-up (n=7). At
6 months, the combination of both markers also predicted PFS
P<0.0001 (Fig. 4F), with a median
PFS of 56.8 months (95% CI, 28.7–63.2) if both markers were stable,
and 11.4 months (95% CI, 10.9–11.9) if both markers increased
≥50%.
On multivariate analysis, CA125 was non-significant
at 3- and 6-month follow-up, while HE4 was highly significant at
6-month follow-up, with an HR of 8.23 (95% CI, 3.28–20.9;
P<0.0001, Cox regression) (Table
II). For the combination of HE4 and CA125 on multivariate
analysis, there were too few high-risk patients (n=7) with both
markers positive at 3 months for the analysis to be conducted;
whereas the HR at 6 months was 7.43 (95% CI, 2.92–18.9;
P<0.0001) (data not shown) when both biomarkers increased
≥50%.
 | Table II.Multivariate progression-free
survival analysis for 3 and 6 months of follow-up. |
Table II.
Multivariate progression-free
survival analysis for 3 and 6 months of follow-up.
|
| 3-month
follow-up | 6-month
follow-up |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 0.97 | 0.94–1.00 | 0.081 | 0.96 | 0.93–1.00 | 0.034 |
| FIGO stage |
|
|
I/II | 1.00 |
| Ref | 1.00 |
| Ref |
|
III/IV | 9.99 | 2.70–36.9 | <0.001 | 5.90 | 1.89–18.4 | 0.002 |
| Tumor grade |
|
| 1 | 1.00 |
| Ref | 1.00 |
| Ref |
| 2/3/not
graded | 2.54 | 0.86–7.71 | 0.093 | 1.42 | 0.48–4.15 | 0.524 |
| Histology |
|
|
Serous | 1.00 |
| Ref | 1.00 |
| Ref |
|
Non-serousa | 1.33 | 0.52–3.40 | 0.546 | 1.12 | 0.44–2.85 | 0.816 |
| Residual tumor |
|
| 0
cm | 1.00 |
| Ref | 1.00 |
| Ref |
| <1
cm | 3.88 | 1.50–10.0 | 0.005 | 2.06 | 0.84–5.02 | 0.112 |
| ≥1
cm | 2.17 | 0.92–5.11 | 0.078 | 2.24 | 0.94–5.35 | 0.069 |
| HE4 |
|
| Below
cut-off | 1.00 |
| Ref | 1.00 |
| Ref |
| Above
cut-off | 1.31 | 0.46–3.72 | 0.612 | 8.28 | 3.28–20.9 | <0.0001 |
| CA125 |
|
| Below
cut-off | 1.00 |
| Ref | 1.00 |
| Ref |
| Above
cut-off | 1.29 | 0.60–2.76 | 0.513 | 1.45 | 0.67–3.17 | 0.348 |
Discussion
In June 2008, the HE4 EIA kit (Fujirebio Diagnostics
AB) was approved by the FDA to monitor recurrence or progressive
disease in patients with epithelial ovarian cancer. In September
2011, the FDA approved marketing of the HE4 test (Fujirebio
Diagnostics, Malvern, Pennsylvania) in combination with the CA125
test in the Risk of Ovarian Malignancy Algorithm (ROMA™) as a
diagnostic tool for determining the likelihood of malignancy at the
time of surgery in women presenting with an ovarian adnexal mass.
ROMA™ is a qualitative serum test that combines the results of HE4
EIA, ARCHITECT CA125 II™ (not the same CA125 test used by the
present study) and menopausal status into a numerical value and
classifies women as being at low- or high-risk for malignant
disease. This risk is given as an adjunct to the two test results
for CA125 and HE4.
Another possible application of HE4 is during
follow-up, which has only been sparsely investigated (26–31). A
simple approach is to analyze the marker at the EOT in an effort to
identify a group of patients at high risk of early recurrence, an
important aspect prompting for further investigation. The results
presented herein indicate that analyzing HE4 at this stage is
insufficient for reliable classification with regard to the risk of
recurrence.
In the current study, measurements of HE4 at the 3-
and 6-month follow-ups demonstrated a significant difference in PFS
compared with CA125, with considerably higher hazard ratios for
HE4. Combination of the two markers, with classification of the
patients into a high-risk group if both markers increased ≥50%, did
not provide additional value. By reviewing the Kaplan-Meier curves,
it appears that HE4 levels are responsible for delineating the
large difference in PFS. This was also found to be true on
multivariate analysis: CA125 proved not to be significant at 3- or
6-month follow-up, whereas HE4 was highly significant at 6-month
follow-up with an HR of 8.23. Similarly, combining HE4 and CA125 in
the multivariate analysis, HE4 was revealed to be the important
marker, while CA125 appeared not to complement the prognostic value
of HE4 during follow-up.
A study by Havrilesky et al (26) monitored 27 patients with advanced
ovarian cancer subsequent to chemotherapy and evaluated a biomarker
panel of which HE4 was one. All 27 patients experienced recurrence
following initial response to treatment. The sensitivity for
predicting recurrence was 100% for the biomarker panel and 96% for
CA125. In 15 patients (56%), ≥1 panel biomarkers were elevated
earlier (range, 6–69 weeks) than CA125 and prior to other clinical
evidence of recurrence. However, a drawback of this study is the
lack of a control group to enable comparison of marker behavior
during follow-up in patients with and without recurrence. The
current study also demonstrated that, compared to CA125, more
patients had elevated HE4 at relapse (or during follow-up), but
elevated HE4 was also present in the control group of patients with
no clinical detection of relapse.
A study by Plotti et al (27) investigated serum CA125 and HE4 levels
in 34 patients with radiological suspicion of recurrence and in 34
patients with benign ovarian tumors. The CA125 sensitivity and
specificity for detecting recurrent ovarian cancer were 35.29 and
58.82%, respectively. The HE4 sensitivity values were 73.53 and
26.47% when using 70 and 150 pmol/l cut-offs, respectively. HE4
specificity was 100% (all patients in the ovarian cancer group had
relapse of ovarian cancer). When combining CA125 and HE4 at a
cut-off of 70 pmol/l, the sensitivity in detecting recurrent
ovarian cancer was 76.47% with a specificity of 100%. It is
difficult to compare these results with the current study, since
Plotti et al used a patient group with benign tumors for
comparison and therefore likely achieved a higher specificity
compared to the current results, for which the control group was
ovarian cancer patients without recurrence during follow-up.
A relatively recent study by Manganaro et al
(28) investigated three consecutive
serum samples drawn at 3-month intervals from 21 patients with
advanced ovarian cancer. In the 9 patients with relapse, an
increase in HE4 (>150 pmol/l) was noted in 22, 78 and 89% of the
patients according to the time interval from surgery (1–3 months
from surgery, 4–6 months and 7–10 months from surgery,
respectively). Only 44% of the patients with relapse showed CA125
levels >35 U/ml at 7–10 months from surgery. None of the 12
patients with stable disease had HE4 levels >150 pmol/l, whereas
4 patients had CA125 levels >35 U/ml. These results are in
agreement with those of the current study, wherein the predictive
value of HE4 also increased with the time interval.
Only a few other studies, which have included ≤20
patients, have investigated HE4 during follow-up, with similar
results (29–31).
All of the previous studies are substantially
smaller than the current study and do not have a control group of a
reasonable number of patients without clinical
recurrence/progressive disease. For certain of the studies, it is
not clear when follow-up blood samples were taken, and some samples
appear to be drawn during the chemotherapy. The material used in
the present study was prospectively collected and retrospectively
analyzed as part of a prospective marker protocol, and blood tests
were recorded regularly during follow-up. The patients were not
retrospectively identified, since their recurrence was already
known, and a group of patients with no clinical relapse were
available for marker comparison. We have previously published the
dynamics of HE4 and CA125 during chemotherapy, and therefore this
was not in the scope of the present study (32).
The largest of the previously described studies is
the study by Plotti et al (27). As a criterion for inclusion, these
patients had radiological signs of relapse at the time of serum
sample collection. Therefore, the sample was drawn at time of
diagnosis for recurrent disease and not as part of a follow-up
study. The 100% specificity is obvious when all included patients
had recurrent disease at sample collection. Furthermore, no
comparisons with patients without relapse were performed, and only
comparisons with a control group ~30 years younger than an average
ovarian cancer cohort, and in which every individual had a benign
ovarian tumor.
Early treatment of recurrence may not lead to an
improved overall survival time based on therapies available at
present (33). Nevertheless, the
majority of ovarian cancer patients with advanced disease at
diagnosis will relapse after primary treatment, with a dismal
prognosis (34). Therefore,
investigation of the level of serum markers in patients under
monitoring may be essential in distinguishing patients at risk of
relapse from those with less aggressive disease. HE4 appears to be
a sensitive and specific marker for the detection of recurrence
and, in some cases, has the potential to detect recurrence in
patients in whom CA125 is negative. However, based on the present
results, investigating consecutive blood samples in comparison to a
single blood test drawn at the time of diagnosis is not as simple
as previously described in the literature. The picture also looks
different when marker levels are compared with those of ovarian
cancer patients without known relapse, instead of with a group of
healthy women. What is clear from the current study and other
studies is that there will be cases in which CA125 is not workable
and in which HE4 may be a better marker of recurrence.
In conclusion, the results presented here indicate
that an early increase of >50% of HE4 in the follow-up period
relative to the EOT suggests a high risk of recurrence. This opens
the perspective of early treatment. However, the results call for
confirmation in a larger number of patient samples.
Acknowledgements
The author appreciate the skilled work by laboratory
technicians Camilla Davidsen and Sara Egsgaard, who handled all the
blood samples and performed the CA125 and HE4 analyses. The authors
also wish to thank the study nurse at Vejle Hospital, Yvette
Schandorf Sørensen; and the study nurses at Aalborg Hospital,
Kirsten Lambæk and Janni Møldrup, who helped to keep track of the
included patients and patient data.
The study was supported by grants from Vejle
Hospital and The Cancer Foundation. The present paper was also
supported by Fujirebio Diagnostics AB, who kindly provided the kits
for the CA125 and HE4 immunoassays.
References
|
1
|
Siegel R, Naishadham MA and Jemal A:
Cancer statistics 2012. CA Cancer J Clin. 62:10–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu PY, Alberts DS, Monk BJ, Brady M, Moon
J and Markman M: An early signal of CA-125 progression for ovarian
cancer patients receiving maintenance treatment after complete
clinical response to primary therapy. J Clin Oncol. 25:3615–3620.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vergote I, Rustin GJ, Eisenhauer EA,
Kristensen GB, Pujade-Lauraine E, Parmar MK, Friedlander M,
Jakobsen A and Vermorken JB: Re: New guidelines to evaluate the
response to treatment in solid tumors [ovarian cancer]. Gynecologic
Cancer Intergroup. J Natl Cancer Inst. 92:1534–1535. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rustin GJ, Nelstrop AE, Tuxen MK and
Lambert HE: Defining progression of ovarian carcinoma during
follow-up according to CA 125: A north thames ovary group study.
Ann Oncol. 7:361–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuxen MK, Sölétormos G and Dombernowsky P:
Serum tumor marker CA 125 for monitoring ovarian cancer during
follow-up. Scand J Clin Lab Invest. 62:177–188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rustin GJ, van der Burg ME, Griffin CL,
Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C,
Qian W, et al: Early versus delayed treatment of relapsed ovarian
cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet.
376:1155–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harter P: DESKTOP III (AGO-OVAR OP.4): A
randomized trial evaluating cytoreductive surgery in patients with
platinum-sensitive recurrent ovarian cancer. http://www.gcig.igcs.org/ClinicalTrials.html
|
|
8
|
Fleming ND, Cass I, Walsh CS, Karlan BY
and Li AJ: CA125 surveillance increases optimal resectability at
secondary cytoreductive surgery for recurrent epithelial ovarian
cancer. Gynecol Oncol. 121:249–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Einhorn N, Bast RC Jr, Knapp RC, Tjernberg
B and Zurawski VR Jr: Preoperative evaluation of serum CA 125
levels in patients with primary epithelial ovarian cancer. Obstet
Gynecol. 67:414–416. 1986.PubMed/NCBI
|
|
10
|
Clauss A, Lilja H and Lundwall A: A locus
on human chromosome 20 contains several genes expressing protease
inhibitor domains with homology to whey acidic protein. Biochem J.
368:233–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bouchard D, Morisset D, Bourbonnais Y and
Tremblay GM: Proteins with whey-acidic-protein motifs and cancer.
Lancet Oncol. 7:167–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bouchard D, Morisset D, Bourbonnais Y and
Tremblay GM: Proteins with whey-acidic-protein motifs and cancer.
Lancet Oncol. 7:167–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galgano MT, Hampton GM and Frierson HF Jr:
Comprehensive analysis of HE4 expression in normal and malignant
human tissues. Mod Pathol. 19:847–853. 2006.PubMed/NCBI
|
|
14
|
Drapkin R, von Horsten HH, Lin Y, Mok SC,
Crum CP, Welch WR and Hecht JL: Human epididymis protein 4 (HE4) is
a secreted glycoprotein that is overexpressed by serous and
endometrioid ovarian carcinomas. Cancer Res. 65:2162–2169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hellström I, Raycraft J, Hayden-Ledbetter
M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N and
Hellström KE: The HE4 (WFDC2) protein is a biomarker for ovarian
carcinoma. Cancer Res. 63:3695–3700. 2003.PubMed/NCBI
|
|
16
|
Hellstrom I and Hellstrom KE: SMRP and HE4
as biomarkers for ovarian carcinoma when used alone and in
combination with CA125 and/or each other. Adv Exp Med Biol.
622:15–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hellstrom I and Hellstrom KE: fTwo novel
biomarkers, mesothelin and HE4, for diagnosis of ovarian carcinoma.
Expert Opin Med Diagn. 5:227–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montagnana M, Lippi G, Ruzzenente O,
Bresciani V, Danese E, Scevarolli S, Salvagno GL, Giudici S,
Franchi M and Guidi GC: The utility of serum human epididymis
protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal.
23:331–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moore RG, Brown AK, Miller MC, Skates S,
Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P,
Granai CO and Bast RC Jr: The use of multiple novel tumor
biomarkers for the detection of ovarian carcinoma in patients with
a pelvic mass. Gynecol Oncol. 108:402–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moore RG, McMeekin DS, Brown AK,
DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC
Jr and Skates SJ: A novel multiple marker bioassay utilizing HE4
and CA125 for the prediction of ovarian cancer in patients with a
pelvic mass. Gynecol Oncol. 112:40–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steffensen KD, Waldstrøm M, Brandslund I
and Jakobsen A: Prognostic impact of prechemotherapy serum levels
of HER2, CA125, and HE4 in ovarian cancer patients. Int J Gynecol
Cancer. 21:1040–1047. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steffensen KD, Waldstrøm M and Jakobsen A:
The relationship of platinum resistance and ERCC1 protein
expression in epithelial ovarian cancer. Int J Gynecol Cancer.
19:820–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
World Medical Association Declaration of
Helsinki: ethical principles for medical research involving human
subjects. JAMA. 310:2191–2194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rustin GJ: Use of CA-125 to assess
response to new agents in ovarian cancer trials. J Clin Oncol.
21(Suppl 10): S187–S193. 2003. View Article : Google Scholar
|
|
25
|
Rustin GJ, Quinn M, Thigpen T, du Bois A,
Pujade-Lauraine E, Jakobsen A, Eisenhauer E, Sagae S, Greven K,
Vergote I, et al: Re: New guidelines to evaluate the response to
treatment in solid tumors (ovarian cancer). J Natl Cancer Inst.
96:487–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Havrilesky LJ, Whitehead CM, Rubatt JM,
Cheek RL, Groelke J, He Q, Malinowski DP, Fischer TJ and Berchuck
A: Evaluation of biomarker panels for early stage ovarian cancer
detection and monitoring for disease recurrence. Gynecol Oncol.
110:374–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plotti F, Capriglione S, Terranova C,
Montera R, Aloisi A, Damiani P, Muzii L, Scaletta G,
Benedetti-Panici P and Angioli R: Does HE4 have a role as biomarker
in the recurrence of ovarian cancer? Tumour Biol. 33:2117–2123.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manganaro L, Michienzi S, Vinci V,
Falzarano R, Saldari M, Granato T, Viggiani V, Frati L and Anastasi
E: Serum HE4 levels combined with CE CT imaging improve the
management of monitoring women affected by epithelial ovarian
cancer. Oncol Rep. 30:2481–2487. 2013.PubMed/NCBI
|
|
29
|
Anastasi E, Marchei GG, Viggiani V,
Gennarini G, Frati L and Reale MG: HE4: A new potential early
biomarker for the recurrence of ovarian cancer. Tumour Biol.
31:113–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schummer M, Drescher C, Forrest R, Gough
S, Thorpe J, Hellström I, Hellström KE and Urban N: Evaluation of
ovarian cancer remission markers HE4, MMP7 and Mesothelin by
comparison to the established marker CA125. Gynecol Oncol.
125:65–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Granato T, Midulla C, Longo F, Colaprisca
B, Frati L and Anastasi E: Role of HE4, CA72.4, and CA125 in
monitoring ovarian cancer. Tumour Biol. 33:1335–1339. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steffensen KD, Waldstrøm M, Brandslund I,
Petzold M and Jakobsen A: The prognostic and predictive value of
combined HE4 and CA-125 in ovarian cancer patients. Int J Gynecol
Cancer. 22:1474–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rustin GJ, van der Burg ME, Griffin CL,
Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C,
Qian W, et al: Early versus delayed treatment of relapsed ovarian
cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet.
376:1155–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
National Cancer Institute: Cancer
statistics: SEER stat fact sheets, ovary cancer. http://seer.cancer.gov/statfacts/html/ovary.htmlAccessed.
April 14–2016
|