Introduction
Multiple myeloma (MM) is a clonal plasma cell
malignancy associated with bone, renal, hematological and
neurological complications (1). Among
the most common symptoms of MM are ostealgia and multiple bone
lesions, which are also symptoms of bone marrow (BM) metastatic
tumors (2). Since these two diseases
are highly similar with regard to susceptible populations, clinical
features and BM cell morphology, it is often difficult to
differentiate between them in cases when the primary lesion is
unclear.
Oligodendroglioma is a rare type of tumor accounting
for ~5% of all primary brain tumors (3). Oligodendroglioma predominantly occurs in
adults, with peak incidence occurring in patients between the
fourth and sixth decades of life (4).
Among the various types of tumor involving the central nervous
system (CNS), oligodendroglioma is the least likely to metastasize
(5,6).
Metastasis of oligodendroglioma to the BM is even more rare, and
only a few cases have been reported to date (7–11). The
present study reports a case of oligodendroglioma, in which
metastasis to the BM was detected 5 years after craniotomy was
performed for the resection of the primary tumor; however, the
metastasis manifested as MM-like bone lesions, a small M component
and myeloma cell-like morphology in the BM, which made the
diagnosis particularly challenging. Written informed consent was
obtained from the patient's family for publication of this
study.
Case report
A 59-year-old male patient with chronic hepatitis B
presented to Beijing Chaoyang Hospital (Beijing, China) on January
20, 2014, with lower back pain that had persisted for 6 months and
multiple subcutaneous masses that had been apparent for 1 month.
The patient had a history of intracranial oligodendroglioma, which
had been treated with craniotomy and a gross total resection 5
years prior to admission. At 6 months prior to the current
admission, the patient presented to another hospital with lower
back pain accompanied by reduced activity after strenuous exercise.
A lumbar magnetic resonance imaging (MRI) scan (Signa HDxt 3.0T
scanner; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) was
performed in a different hospital, revealing a compression fracture
of the T12 vertebra. Subsequently, BM aspiration and biopsy were
scheduled. The aspiration detected the presence of 85.5%
myeloma-like cells in the BM, most of which tended to be
myeloblasts, while mitoses and degenerated tumor cells could also
be observed, according to the cell morphology. The biopsy indicated
hyperplastic BM proliferation with scattered infiltration of
lymphocytes and plasmacytes. The streptavidin-peroxidase method was
employed in paraffin sections for immunohistochemical staining (all
antibodies used were working solutions with no requirement for
dilution, and were mouse anti-human monoclonal, obtained from
Fuzhou Maixin Biotech. Co., Ltd., Fujian, China, unless otherwise
stated), which revealed clustered and scattered CD138 positivity
(catalog no. MAB-0200), minimal CD20 (catalog no. TA800385;
dilution, 1:150; OriGene Technologies, Rockville, MD, USA), ~15%
CD235a positivity (catalog no. MAB-0603) and CD3 positivity
(catalog no. RB-9039), scattered CD38 positivity (catalog no.
MAB-0341), negative CD56 reactivity (catalog no. ZM-0057; Zhongshan
Golden Bridge Biotechnology Co., Ltd., Beijing, China) and ~50%
myeloperoxidase (rabbit anti-human polyclonal; catalog no.
RAB-0379) positivity. Flow cytometric, chromosome and fluorescence
in situ hybridization (FISH) analyses were not performed, as
the BM aspiration was a dry tap at that time. Due to the patient's
history of oligodendroglioma, a brain MRI scan was performed;
evidence from the previous oligodendroglioma resection was observed
on the scan, but there were no significant findings. Serum
immunofixation electrophoresis revealed a small M component of
immunoglobulin G (IgG)-κ. IgG concentration was 14.3 g/l (normal
range, 7.5–15.6 g/l), and urine Bence-Jones protein was negative,
while peripheral blood leukocyte (4.87×109/l) and
platelet (144×109/l) counts, and hemoglobin
concentration (138 g/l) were within their normal ranges
(4–10×109/l, 100–300×109/l and 120–160 g/l,
respectively). Serum calcium and creatinine levels were normal,
while serum alkaline phosphatase (ALP) was markedly elevated (803
IU/l; normal range, 40–160 U/l).
Based on these findings, the patient was diagnosed
with MM IgG-κ subtype at Durie-Salmon stage III according to the
diagnostic criteria defined by the International Myeloma Working
Group (12), and subsequently
received 5 cycles of melphalan + prednisolone + thalidomide (MPT)
combination therapy (6 mg melphalan on days 1–7; 60 mg prednisolone
on days 1–7; and 200 mg/day thalidomide, every day, for a 28-day
cycle), followed by 1 cycle of melphalan + prednisolone +
cyclophosphamide combination therapy (6 mg melphalan on days 1–7;
60 mg prednisolone on days 1–7; 0.2 g cyclophosphamide on day 1 and
1.0 g on day 4; and 200 mg/day thalidomide, every day, for a 28-day
cycle). Prior to the third cycle of MPT therapy, an additional BM
aspiration was performed, in order to evaluate the efficiency of
the treatment. The BM aspirate was diluted, which revealed that the
BM proliferation was markedly reduced, and the ‘myeloma cells’ were
rarely observable. A BM biopsy revealed a few scattered plasma
cells, which were partially positive for CD138, and κ (catalog no.
MAB-0356) and λ (catalog no. MAB-0357) light chains following
immumohistochemical staining. Flow cytometry did not detect any
abnormal plasma cells. Quantitative polymerase chain reaction
showed that the expression of MYC was slightly elevated
(21.33%) compared with ABL proto-oncogene 1. In addition, prior to
the administration of the third cycle of MPT therapy, the patient
suddenly presented with aconuresis, logagnosia and paralysis of the
right limbs. A brain MRI scan was performed, which indicated
cerebral infarction. Furthermore, the location of the
oligodendroglioma resection showed no obvious changes, while the
areas of skull destruction had clearly increased and expanded
compared with the MRI scan performed 2 months prior. These symptoms
were relieved following the administration of anticoagulants and
antiplatelet drugs.
After the 6 cycles of two different combination
therapies, the patient's lower back pain was relatively relieved;
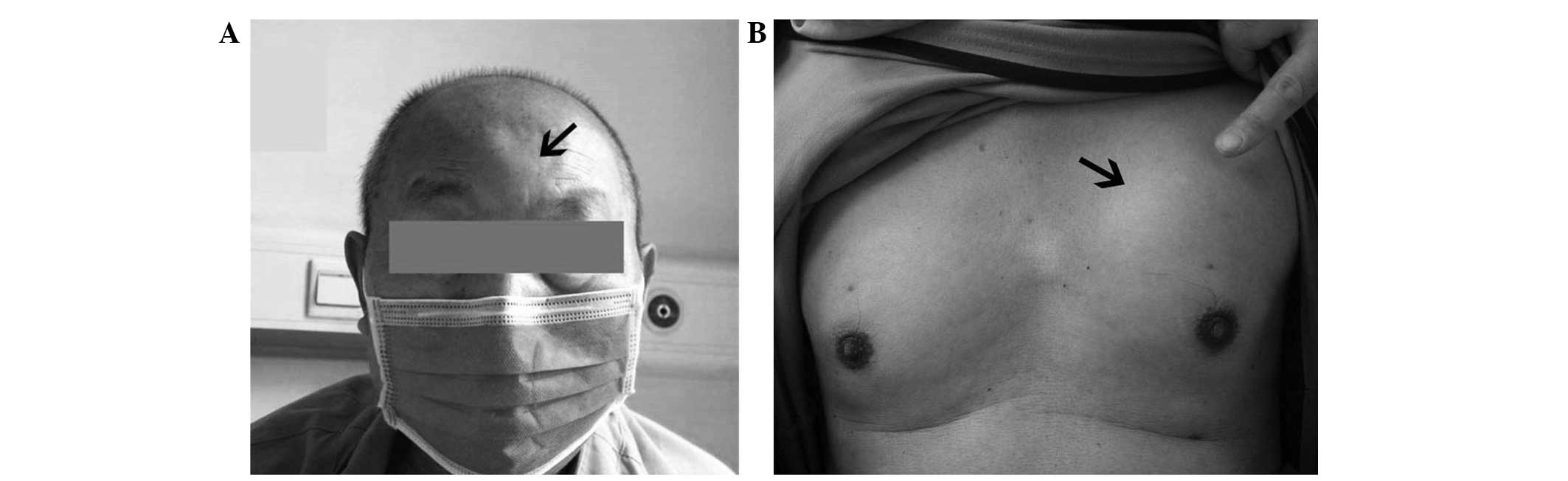
however, he gradually developed multiple painless and immovable
subcutaneous masses on his forehead and the left side of his chest
(Fig. 1). The patient was admitted
Beijing Chaoyang Hospital for further diagnosis and treatment. Upon
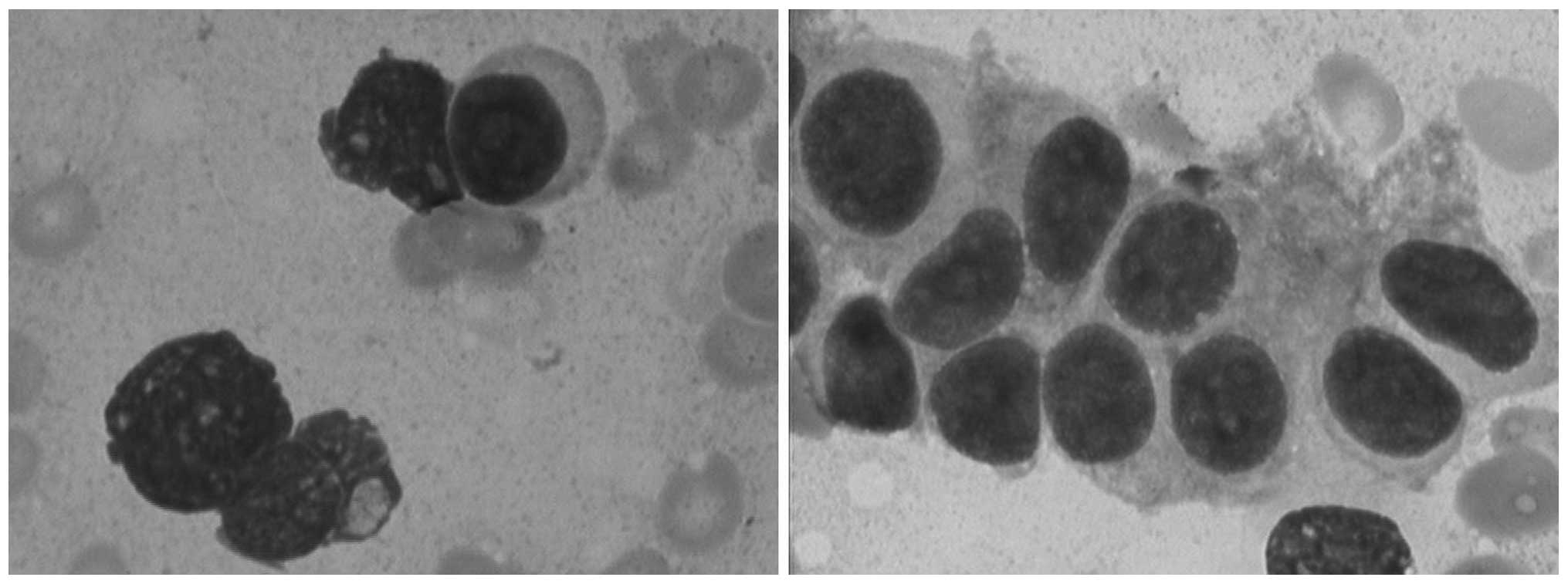
the current admission, key laboratory tests were repeated. BM
aspiration detected the presence of 43% ‘immature plasma cells’
(Fig. 2), and the BM biopsy yielded
mostly cortical bone and did not reveal any hemopoietic tissue. As
the BM aspiration was again a dry tap, flow cytometric, chromosome
and FISH analyses could not be performed. The serum immunofixation
electrophoresis showed no M component or Bence-Jones protein, and
the concentration of IgG was 8.46 g/l. Chest X-ray (Gendex-Del
ATC525 X-ray Generator; Del Medical Inc., Harrison, NY, USA) showed
a diffusely increased bone density and multiple bone lesions on the
ribs and vertebrae. Several soft tissue masses were visible on the
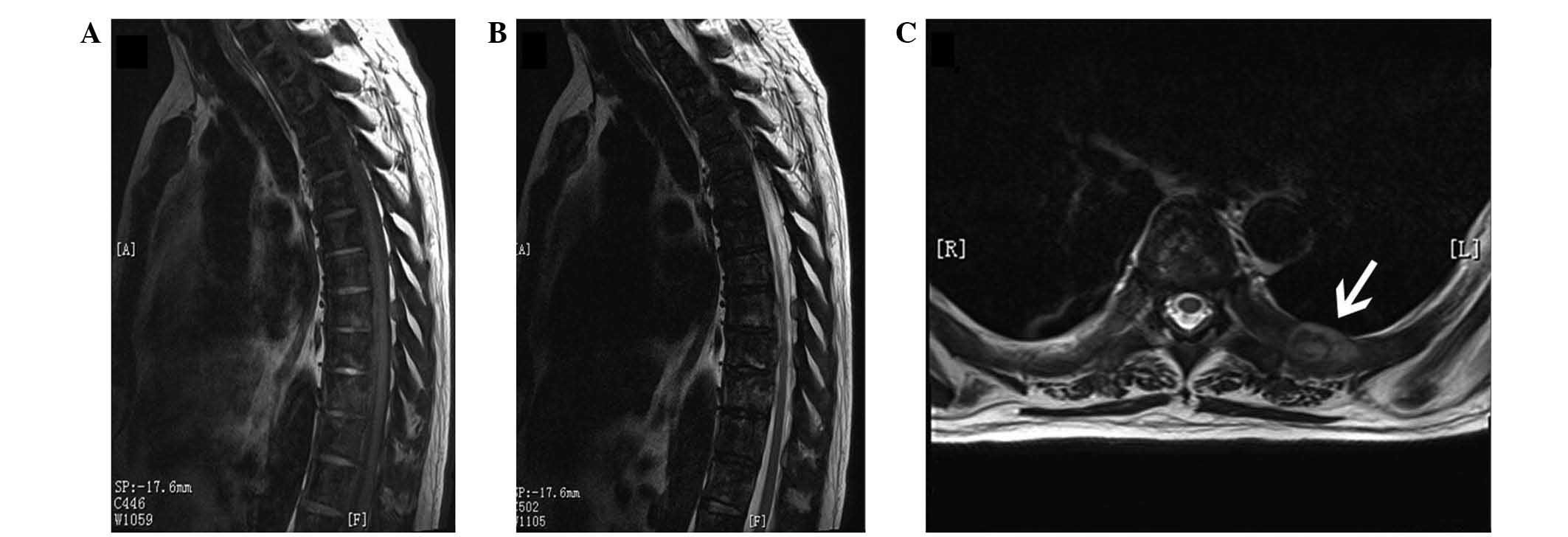
scan. On MRI of the thoracic spine, heterogeneous signals were
detected in the thoracic vertebrae, and a soft tissue mass was
found on the left 7th rib (Fig. 3).
In addition, the patient was diagnosed with moderate anemia
(hemoglobin, 88 g/l) upon admission. Serum calcium and creatinine
were normal, while the ALP levels remained elevated (473 IU/l).
Neuron-specific enolase was also found to be increased at 60.67
ng/ml (normal range, 0–16.3 ng/ml), while β2 microglobulin was
normal (2.49 mg/l; normal range, 1.09–2.53 mg/l).
Based on the aforementioned findings, the patient
was diagnosed with refractory non-secretory MM, without excluding
extramedullary plasmocytoma. Subsequently, 1 cycle of
cyclophosphamide + thalidomide + daunorubicin + dexamethasone
combination therapy (1.2 g cyclophosphamide on day 1; 100 mg/day
thalidomide, every day; 20 mg daunorubicin on days 1–4; and 20 mg
dexamethasone on days 1–4, for a 28-day cycle) was administered,
followed by 1 cycle of cisplatin + etoposide + cyclophosphamide +
dexamethasone + thalidomide (15 mg cisplatin on days 1–4; 60 mg
etoposide on days 1–4; 0.4 g cyclophosphamide on days 1–4; 20 mg
dexamethasone on days 1–4; and 100 mg/day thalidomide, every day,
for a 28-day cycle). After these 2 courses, the symptoms of
subcutaneous masses and anemia showed no improvement. At this time,
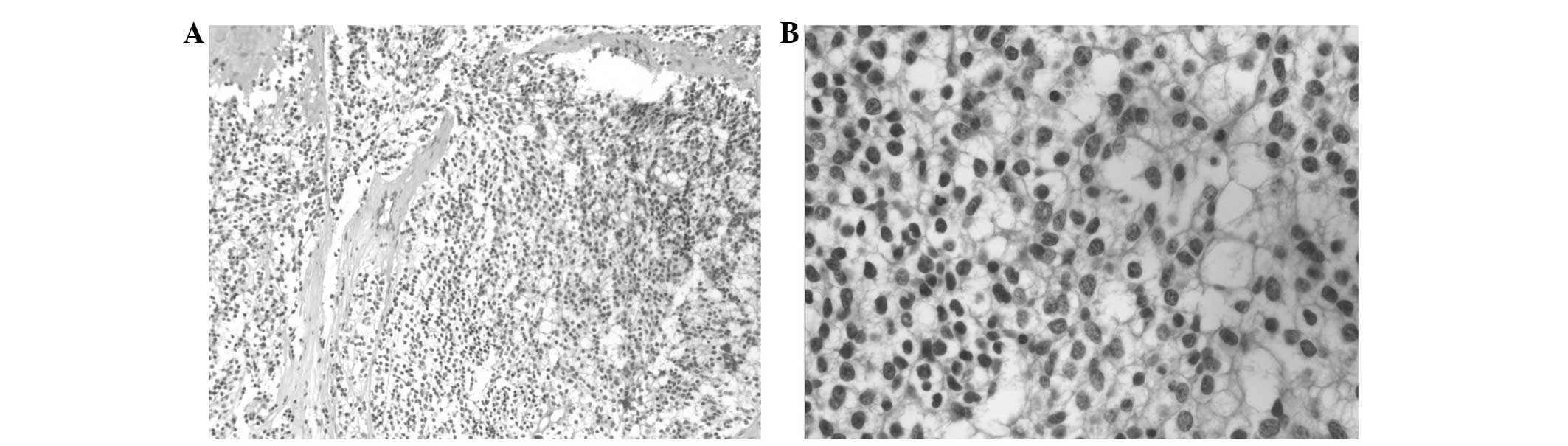
a biopsy of the subcutaneous mass found in the left side of the
chest was performed. Microscopy showed fibrous tissue with tumor
cell infiltration; cells were consistent in size and had a
transparent cytoplasm (Fig. 4).
Immunohistochemical staining showed the following characteristics:
Negative reactivity for vimentin (catalog no. TA801297; dilution,
1:150; OriGene Technologies), cytokeratin (CK; catalog no.
MAB-0671), H-CK (catalog no. Kit-0020), CK-L (catalog no.
MAB-0051), CD38 (catalog no. MAB-0341), CD138 (catalog no.
MAB-0200), κ (catalog no. MAB-0356), λ (catalog no. MAB-0357),
multiple myeloma oncogene 1 (catalog no., ZA-0853; Zhongshan Golden
Bridge Biotechnology Co., Ltd.), human melanoma black 45 (catalog
no. ab787; Abcam, Cambridge, UK), epithelial membrane antigen
(catalog no. Kit-0011), synaptophysin (catalog no. RM-9111),
chromogranin A (catalog no. MAB-0202), glial fibrillary acidic
protein (catalog no. ZM-0118; Zhongshan Golden Bridge Biotechnology
Co., Ltd.) and calcitonin (catalog no. ZA-0578; Zhongshan Golden
Bridge Biotechnology Co., Ltd.), and posiivity for CD56 (catalog
no. ZM-0057; Zhongshan Golden Bridge Biotechnology Co., Ltd.) and
S-100 (catalog no. MAB-0697), with a Ki-67 labeling index (catalog
no. RB-9043) of <5%.
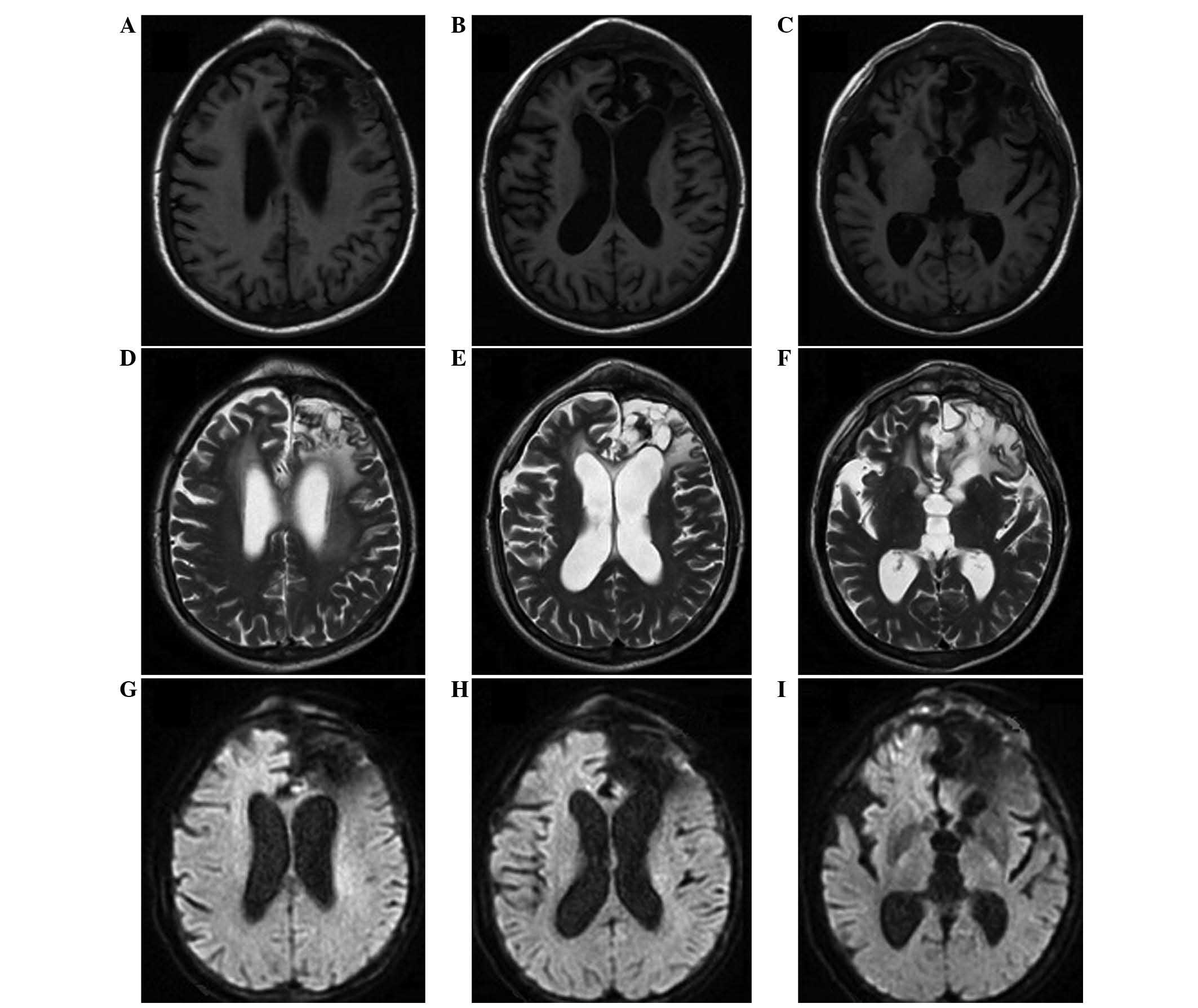
These results were consistent with the brain biopsy
results of 5 years prior, and the brain MRI scan showed no primary
malignant tumors (Fig. 5); therefore,
the subcutaneous masses were finally diagnosed as a metastases from
oligodendroglioma. The patient was prescribed 2 cycles of
temozolomide chemotherapy (200 mg on day 1 and 250 mg on days 2–5
of a 28-day cycle). Despite the slight shrinking of the
subcutaneous masses, the patient developed epilepsy and pneumonia
and eventually succumbed to multiple organ failure on September 13,
2014.
Discussion
In cases like the present one, making a correct
diagnosis is particularly challenging; the symptoms, signs and
laboratory test results of this patient met the 2001 World Health
Organization diagnostic criteria for MM (13); however, there were certain reasons to
question this diagnosis. Firstly, the abnormal cells found in the
BM had not been confirmed to be malignant monoclonal plasma cells;
their morphology was the only indication. Secondly, the patient had
no response to the multiple therapies administered, which were
specific to MM and extramedullary plasmocytoma. Thirdly, this
patient had different bone imaging characteristics from the typical
characteristics of MM bone lesions, which usually demonstrate
osteoporosis instead of diffusely increased bone density. Finally,
the patient's levels of ALP were found to be markedly elevated,
which usually indicates osteogenic activity and is rarely observed
in MM.
The main reason for the misdiagnosis of the present
case is that the key qualitative diagnosis was based only on
morphology, as BM aspiration failed to obtain a sufficient sample
quantity for flow cytometric, chromosome and FISH analyses. As the
morphology of the oligodendroglioma cells in the BM of the present
patient resembled that of MM cells to a considerable extent, and
the symptoms and imaging characteristics of the bone lesions were
identical to those of MM bone lesions, the aforementioned evidence
indicating a diagnosis other than MM, which could have prevented
the misdiagnosis, were ignored upon the patient's first admission.
In addition, despite the patient's history of oligodendroglioma,
the primary tumor did not show any signs of recurrence, which
contributed to the misdiagnosis.
Distant metastasis from brain oligodendroglioma is
considered rare (14–16). The presence of the blood-brain
barrier, the absence of lymphatics within the CNS, the short
survival time of these patients, the inaccessibility of the venous
system to the neoplastic cells, the host's immune response and
possibly certain biological features of the transformed cells are
believed to be the main barriers to the spread of gliomas outside
the CNS (15).
The present case exhibited certain considerably rare
features, such as the long disease-free interval, the length of
time that the patient survived, and the presence of BM metastasis
without any primary tumor recurrence in the brain. This metastasis
may have been caused by cellular diffusion through the meningeal
venous system following surgery, and tumor cell dormancy may
explain why the patient remained asymptomatic for such a long
period of time without a residual tumor (11).
According to the literature and to the best of our
knowledge, this the first report of metastatic oligodendroglioma
presenting with myeloma-like symptoms without any tumor recurrence
in the brain. In summary, extracranial metastases of
oligodendroglioma can occur; however, they are extremely rare. In
the present and previously reported cases (7–11), bone
metastases occurred following prior craniotomy. In such cases, the
BM should be carefully evaluated for glial immunohistochemical
markers and using FISH analysis. In addition, the current case
highlights the importance of confirming the monoclonality of plasma
cells prior to making a final diagnosis of MM, in order to
facilitate differentiation between BM metastatic tumors and MM.
References
|
1
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roodman GD: Mechanisms of bone metastasis.
New Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leonardi MA and Lumenta CB:
Oligodendrogliomas in the CT/MR-era. Acta Neurochir (Wien).
143:1195–1203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van den Bent MJ, Reni M, Gatta G and Vecht
C: Oligodendroglioma. Crit Rev Oncol Hematol. 66:262–272. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith DR, Hardman JM and Earle KM:
Metastasizing neuroectodermal tumors of the central nervous system.
J Neurosurg. 31:50–58. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sha SJ, Wu HP, Lu K, Chen HJ, Huang PH,
Huang SH and Hsu CT: Extraneural metastases of anaplastic
oligodendroglioma. APMIS. 122:660–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Ali F, Hendon AJ, Liepman MK,
Wisniewski JL, Krinock MJ and Beckman K: Oligodendroglioma
metastatic to bone marrow. AJNR Am J Neuroradiol. 26:2410–2414.
2005.PubMed/NCBI
|
|
8
|
Gru AA, Fulling K and Perry A: A 39
year-old man with a cerebellar mass and pancytopenia. Brain Pathol.
22:251–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li G, Zhang Z, Zhang J, Jin T, Liang H,
Gong L, Cui G, Yang H, He S, Zhang Y and Gao G: Occipital
anaplastic oligodendroglioma with multiple organ metastases after a
short clinical course: A case report and literature review. Diagn
Pathol. 9:172014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cordiano V, Miserocchi F and Storti M:
Bone marrow metastases from anaplastic oligodendroglioma presenting
with pancytopenia and hypogammaglobulinemia: A case report. Tumori.
97:808–811. 2011.PubMed/NCBI
|
|
11
|
Tanaka Y, Nobusawa S, Ikota H, Yokoo H,
Hirato J, Ito H, Saito T, Ogura H and Nakazato Y: Leukemia-like
onset of bone marrow metastasis from anaplastic oligodendroglioma
after 17 years of dormancy: An autopsy case report. Brain Tumor
Pathol. 31:131–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
International Myeloma Working Group:
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: A report of the
International Myeloma Working Group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jaffe ES, Harris NL and Stein H: World
Health Organization Classification of Tumors. IARC Press. Lyon,
France: 142–145. 2001.
|
|
14
|
Maiuri F, Del Basso De Caro ML, Iaconetta
G, Peca C, Esposito M and de Divitiis E: Prognostic and
survival-related factors in patients with well-differentiated
oligodendrogliomas. Zentralbl Neurochir. 67:204–209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma A, Agarwal A, Sharma MC, Anand M,
Agarwal S and Raina V: Bone marrow metastasis in anaplastic
oligodendroglioma. Int J Clin Pract. 57:351–352. 2003.PubMed/NCBI
|
|
16
|
Zustovich F, Della Puppa A, Scienza R,
Anselmi P, Furlan C and Cartei G: Metastatic oligodendrogliomas: A
review of the literature and case report. Acta Neurochir (Wien).
150:699–703. 2008. View Article : Google Scholar : PubMed/NCBI
|