Introduction
Tongue carcinoma, which is a subtype of head and
neck cancer, is the most frequently occurring oral cancer, of
which, ~90% is tongue squamous cell carcinoma (TSCC) (1). Currently, TSCC is the tenth most common
solid neoplasm worldwide, with a comparatively low median survival
rate (5-year survival rate, ~50%) (2). Recent studies have reported that
chemotherapy decreases tumor size and reduces distant metastasis in
TSCC patients (3,4). Cisplatin (CDDP) is an efficacious
antineoplastic compound, which is used for the treatment of a wide
range of solid neoplasms, including non-small cell lung, ovarian,
bladder, and head and neck carcinoma (5–8). However,
the cytotoxicity caused by CDDP in TSCC chemotherapy remains
unsatisfactory (9), and the exact
mechanisms of CDDP-induced cytotoxicity have not been identified to
date. CDDP resistance is the major obstacle that prevents
successful treatment of TSCC (10).
Recently, it has been reported that not only DNA-damaging stress
but also endoplasmic reticulum (ER) and oxidative stress are
involved in the therapeutic effect of CDDP (11,12). In
particular, TSCC cells with high glutathione (GSH) levels exhibit
marked resistance to CDDP treatment (13). Thus, the association between CDDP
resistance and GSH-associated pathways requires investigation.
GSH is a triple peptide molecule consisting of
cysteine, glutamic acid and glycine residues that is critical for
the maintenance of the intracellular redox balance and
detoxification (14). As a result,
GSH levels exhibit a positive correlation with chemotherapy
resistance, including resistance to CDDP (14,15).
Numerous studies have revealed that CDDP resistance is associated
with increased GSH levels and decreased reactive oxygen species
(ROS) levels (15–17). However, the association between
increased GSH levels and CDDP treatment, as well as CDDP-induced
cytotoxicity in tongue carcinoma cells, remains unclear.
Cysteine is predominantly captured from the
extracellular environment by system xc− and
it functions as the essential raw material for intercellular GSH
synthesis (15). System
xc− consists of a light chain, xCT/solute
carrier family 7 A11, and its cell surface subunit 4F2hc/cluster of
differentiation (CD)98 (18). The
specific function of system xc− is determined
by xCT (18) and thus, intracellular
GSH levels are closely associated with the expression and function
of xCT (19). Increasing evidence has
revealed that xCT is expressed in various malignant tumors, and its
expression is associated with the development of preneoplastic
lesions and cancer, poor prognosis and drug resistance (20–23).
Notably, xCT is important in maintaining high levels of GSH and
contributes to CDDP resistance of ovarian cancer cell lines
(15). In addition, xCT is markedly
upregulated in resected tongue carcinoma specimens (24). Ye et al (19) demonstrated that proteasome
inhibitor-induced xCT expression is positively modulated by the
activation of nuclear factor erythroid 2-related factor 2 (Nrf2)
and activating transcription factor 4 (ATF4) via the antioxidant
response element (ARE) and amino acid response element (AARE) on
the promoter human xCT gene (19). In addition, Nrf2 and ATF4 are also
induced by CDDP (25,26), while the mechanism of CDDP-inducible
xCT expression and the association between that and CDDP resistance
of TSCC cells remains unclear.
A series of compounds exhibit xCT inhibition,
including the substrate inhibitor glutamine acid and the
non-substrate inhibitor sulfasalazine (SASP) (27). SASP, a sulfa immunosuppressant that is
widely used in the treatment of rheumatoid arthritis and
inflammatory bowel diseases, is also an effective pharmacological
inhibitor of xCT (28). Combined
treatment with SASP increases the efficacy of several
chemotherapeutic drugs, including gemcitabine, 5-fluouracil,
bortezomib and doxorubicin, to lung adenocarcinoma cells (19,29,30). SASP
has also been reported to decrease CDDP resistance in small cell
lung cancer cell lines (31).
However, further study regarding the combined effect of SASP and
CCDP on tongue carcinoma cell lines is required.
In the present study, the mechanism of
CDDP-inducible xCT expression and the function of xCT upregulation
in CDDP resistance in the Tca8113 tongue carcinoma cell line were
investigated. xCT expression was robustly induced by CDDP in an
Nrf2 and ATF4 activation-dependent manner. CDDP-induced cells death
was increased when xCT was suppressed by small interfering RNA
(siRNA) or its pharmacological inhibitor. These results indicate
that CDDP-prompted xCT activation increases the resistance of
tongue cancer cells to CDDP treatment, and the combination of CDDP
and xCT inhibitor could benefit tongue cancer chemotherapy.
Materials and methods
Cell line
The human TSCC cell line Tca8113 was obtained from
the China Center for Type Culture Collection (Wuhan, China).
Tca8113 cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St.
Louis, MO, USA) containing 10% fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 100
U/ml penicillin and 100 µg/ml streptomycin (Life Technologies;
Thermo Fisher Scientific, Inc.) at 37°C with in an atmosphere of 5%
CO2.
Reagents
CDDP and SASP (Sigma-Aldrich) were dissolved in 100%
dimethyl sulfoxide (DMSO; cat. no. 046-21981; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) and diluted to the appropriate
concentrations (CDDP, 20 mg/ml and SASP, 300 mM) with culture
medium prior to each experiment. The final DMSO concentration was
<0.1% for the cell experiments. Rabbit polyclonal anti-xCT
antibody (cat. no. ab37185) was purchased from Abcam (Cambridge,
MA, USA). Anti-4F2hc/CD98 (cat. no. sc-9160) and anti-β-actin
antibodies (cat. no. sc-47778) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rabbit polyclonal anti-CD44
variant (v) antibody (cat. no. 3578) was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from Tca8113 cells using the
Total RNA Purification kit (Norgen Biotek Corporation, Thorold, ON,
Canada), according to the manufacturer's protocol. Complementary
DNA was obtained from 1 µg total RNA using iScript cDNA Synthesis
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). RT-qPCR
analyses were performed using the iTaq Universal SYBR Green kit
(Bio-Rad Laboratories, Inc.) and CFX Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.), under the following cycling
conditions: 98°C for 1 min, followed by 45 cycles at 98°C for 5 sec
and 60°C for 30 sec. The designed RT-qPCR primers (Sigma-Aldrich)
were as follows: Forward (F), 5′-GCTGTGATATCCCTGGCATT-3′ and
reverse (R), 5′-GGCGTCTTTAAAGTTCTGCG-3′ for xCT; F,
5′-CCAGGTTCGGGACATAGAGA-3′ and R, 5′-GAGCCTTGCCTGAGACAAAC-3′ for
4F2hc/CD98; F, 5′-AGAAGGTGTGGGCAGAAGAA-3′ and R,
5′-AAATGCACCATTTCCTGAGA-3′ for CD44v; F, 5′-CGGTATGCAACAGGACATTG-3′
and R, 5′-ACTGGTTGGGGTCTTCTGTG-3′ for Nrf2; F,
5′-CTTACGTTGCCATGATCCCT-3′ and R, 5′-GAGAACACCTGGAGATGGGA-3′ for
ATF4; and F, 5′-TGAAGGTCGGAGTCAACGATTTGGT-3′ and R,
5′-GAAGATGGTGATGGGATTTC-3′ for glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). GAPDH was used as an internal control, and
the expression of the target gene was normalized relative to the
expression of GAPDH. Non-specific amplification and primer dimers
were examined by dissociation curves at the end of the PCRs. Data
was assessed according to the comparative Cq method
(2−ΔΔCq) (19).
Western blot analysis
Tca8113 cells were seeded at a density of
1.5×105 cells/well in 12-well plates (cat. no. CLS3513;
Sigma-Aldrich), and 24 h later, cells were lysed with CelLytic M
Cell Lysis Reagent (Sigma-Aldrich) for whole-cell protein
extraction. Genomic DNA was sonicated for 15 sec with an output
frequency of 20 kHz and 50% amplitude, using a 150VT sonicator
(Biologics, Inc, Cary, NC, USA). Protein concentration was measured
using a Bicinchoninic Acid Protein Assay kit (Thermo Fisher
Scientific, Inc.). Sample buffer (4X) and 1% β-mercaptoethanol
(β-ME; Wako Pure Chemical Industries, Ltd.) were added to
equivalent amounts of protein (10 µg), and the samples were
incubated at 95°C for 5 min. The samples were next subjected to
electrophoresis on an 8% (v/v) sodium dodecyl
sulfate-polyacrylamide gel (cat. no. WT0081BOX; Thermo Fisher
Scientific, Inc.), and transferred to a polyvinylidene difluoride
hybridization transfer membrane (EMD Millipore, Billerica, MA,
USA). The membranes were blocked at room temperature with Blocker
BLOTTO Blocking Buffer (Thermo Fisher Scientific, Inc.), washed 3
times with Tris-buffered saline containing 0.05% Tween (TBST; cat.
no. 206-19131; Wako Pure Chemical Industries, Ltd.), and incubated
with anti-xCT, anti-4F2hc, anti-CD44v and anti-β-actin antibodies
overnight at 4°C. All the above primary antibodies were diluted in
1:1,000 with 1X TBST with 5% bovine serum albumin (cat. no. A9418;
Sigma-Aldrich). Following 3 washes with TBST, the membranes were
incubated for 1 h at room temperature with goat anti-rabbit
immunoglobulin (Ig)G-horseradish peroxidase-(HRP) (cat. no.
sc-2054; Santa Cruz Biotechnology, Inc.) and goat anti-mouse
IgG-HRP (cat. no. sc-2005; Santa Cruz Biotechnology, Inc.)
secondary antibodies diluted 1:10,000 with 1X TBST, and visualized
in a ChemiDoc MP system (Bio-Rad Laboratories, Inc.) using the
ImmunoStar LD chemiluminescence system (Wako Pure Chemical
Industries, Ltd.).
Plasmid construction and luciferase
activity assay
Human xCT gene promoter-luciferase wild-type and
mutant reporter plasmids were constructed as reported previously
(19). For the luciferase activity
assay, Tca8113 cells were seeded in 12-well plates at a density of
0.5×105 cells/well for 24 h prior to transfection. The
cells were co-transfected with 0.9 µg luciferase reporter plasmids
and 0.1 µg pRL-TK (internal control; cat. no. E2241; Promega
Corporation, Madison, WI, USA) using Lipofectamine LTX Reagent
(Life Technologies; Thermo Fisher Scientific, Inc.). The luciferase
activity assay was performed 24 h subsequent to transfection using
the Dual-Luciferase® Reporter Assay System (Promega
Corporation).
Intracellular GSH level
determination
GSH was derivatized using monobromobimane (mBBr;
Life Technologies; Thermo Fisher Scientific, Inc.) and separated by
reverse-phase high-performance liquid chromatography as previously
described (32). Briefly, Tca8113
cells were collected with phosphate-buffered saline (cat. no.
1662403; Bio-Rad Laboratories, Inc.) and lysed in 0.2 M
5-sulfosalicylic acid (cat. no. 197-04582; Wako Pure Chemical
Industries, Ltd.) on ice for 10 min. Samples were separated by
centrifugation at 8,000 × g for 5 min. Next, mBBr was added to the
supernatant fraction and allowed to react in the dark at room
temperature for 30 min, and then the absorbance at 490 nm was
detected with an iMark™ Microplate Absorbance Reader (Bio-Rad
Laboratories, Inc.). The precipitate was diluted in 0.1 N NaOH
(cat. no. 1310-73-2; Wako Pure Chemical Industries, Ltd.), and
protein determination was performed using the Pierce Coomassie
(Bradford) Protein Assay kit (Thermo Fisher Scientific, Inc.).
Bovine gamma globulin (cat. no. 5000208; Bio-Rad Laboratories,
Inc.) was used for the standard curve.
siRNA transfection of Tca8113
cells
Human Nrf2 siRNA#1 (cat. no. 107966) and #2 (cat.
no. 115762); human ATF4 siRNA #1 (cat. no. 122168) and #2 (cat. no.
122372); human xCT siRNA (cat. no. 108518); and negative control
siRNA (cat. no. AM4611) were purchased from Life Technologies
(Thermo Fisher Scientific, Inc.). Tca8113 cells were seeded in
12-well plates at a density of 1.5×105 cells/well.. The
following day, the cells were transfected with siRNA using
Lipofectamine RNAiMax (Life Technologies; Thermo Fisher Scientific,
Inc.). Upon 24 h incubation, the transfected cells were treated
with CDDP for the indicated concentrations and times.
Cell viability assay
Tca8113 cells were seeded in 96-well plates
(1.5×104 cells/well) prior to be subjected to
transfection with xCT, Nrf2 or ATF4 siRNA, or to treatment with
SASP/β-ME for 24 h, followed by incubation with 5, 10, 20, 30 or 40
µg/ml CDDP for an additional 48 h. Cell viability was measured
using Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich). Briefly, the
culture medium was replaced following CDDP treatment, and 10 µl
CCK-8 was added. Following incubation for 45 min at 37°C, the
absorbance was measured at a wavelength of 450 nm using a
microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was conducted using one-way analysis
of variance. All statistical analyses were performed using SPSS
18.0 statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
CDDP induces xCT expression in Tca8113
cells
To elucidate the function of xCT in CDDP resistance,
CDDP-inducible messenger RNA (mRNA) and protein xCT expression
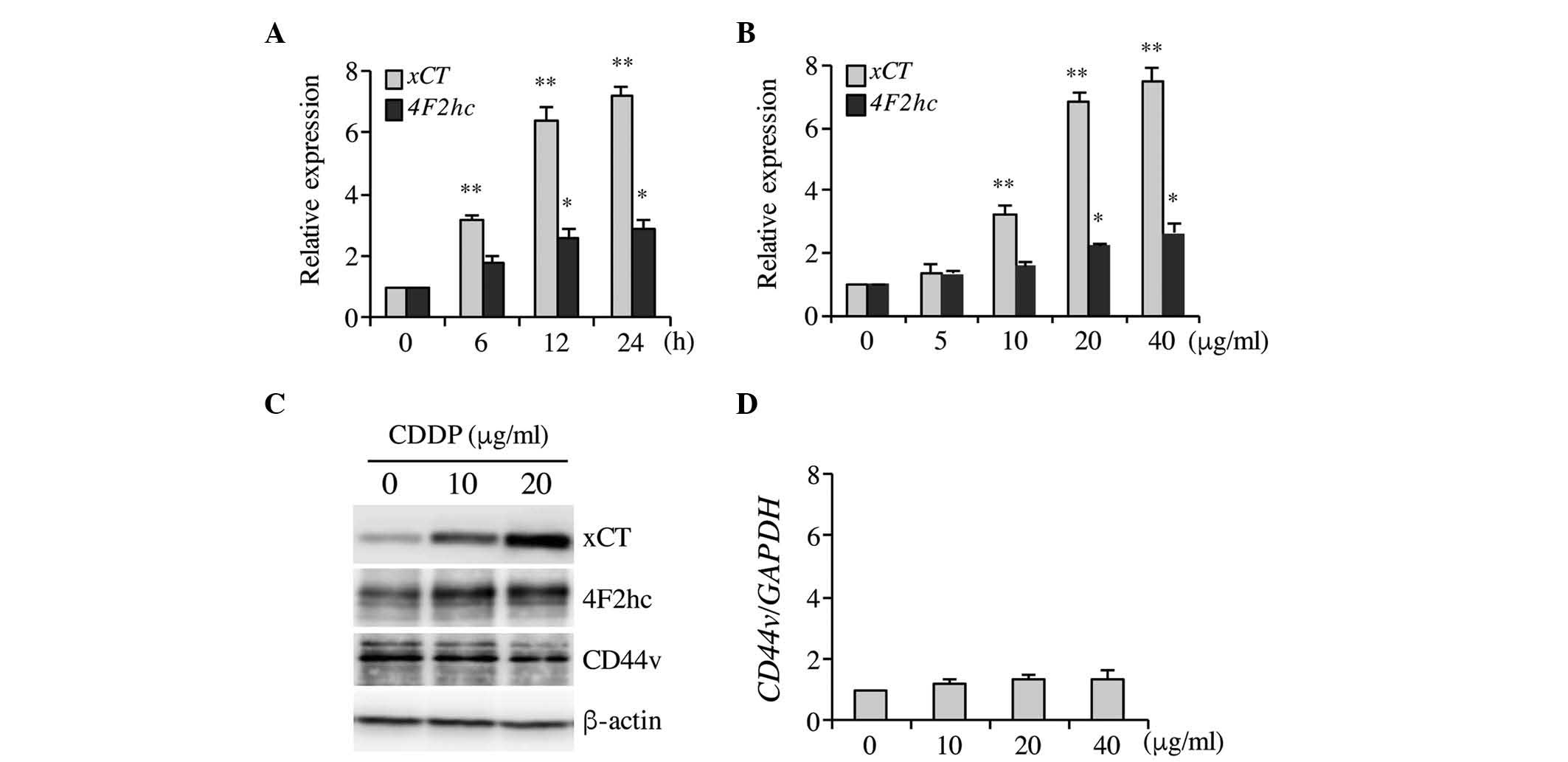
levels were investigated. As indicated in Fig. 1A–C, CDDP significantly increased xCT
mRNA and protein expression levels in a time- and
concentration-dependent manner, and 20 µg/ml CDDP induced maximal
xCT expression following 6-h treatment. Therefore, this
concentration of CDDP and treatment time was selected for
subsequent experiments. CDDP induced xCT upregulation and a
marginal increase in the expression of the heavy chain of system
xc− (4F2hc/CD98) 4F2hc, which may be due to a
positive feedback as a consequence of xCT induction (Fig. 1A–C). Ishimoto et al (33) indicated that CD44v interacts with and
stabilizes xCT, thus regulating the redox status and promoting
tumor growth, however no significant differences in CD44v
expression were observed in the present study following CDDP
treatment in Tca8113 cells (Fig. 1C and
D). These results demonstrated that CDDP treatment
significantly increased xCT expression in Tca8113 cells.
CDDP enhances xCT expression in an
Nrf2 and ATF4-dependent manner
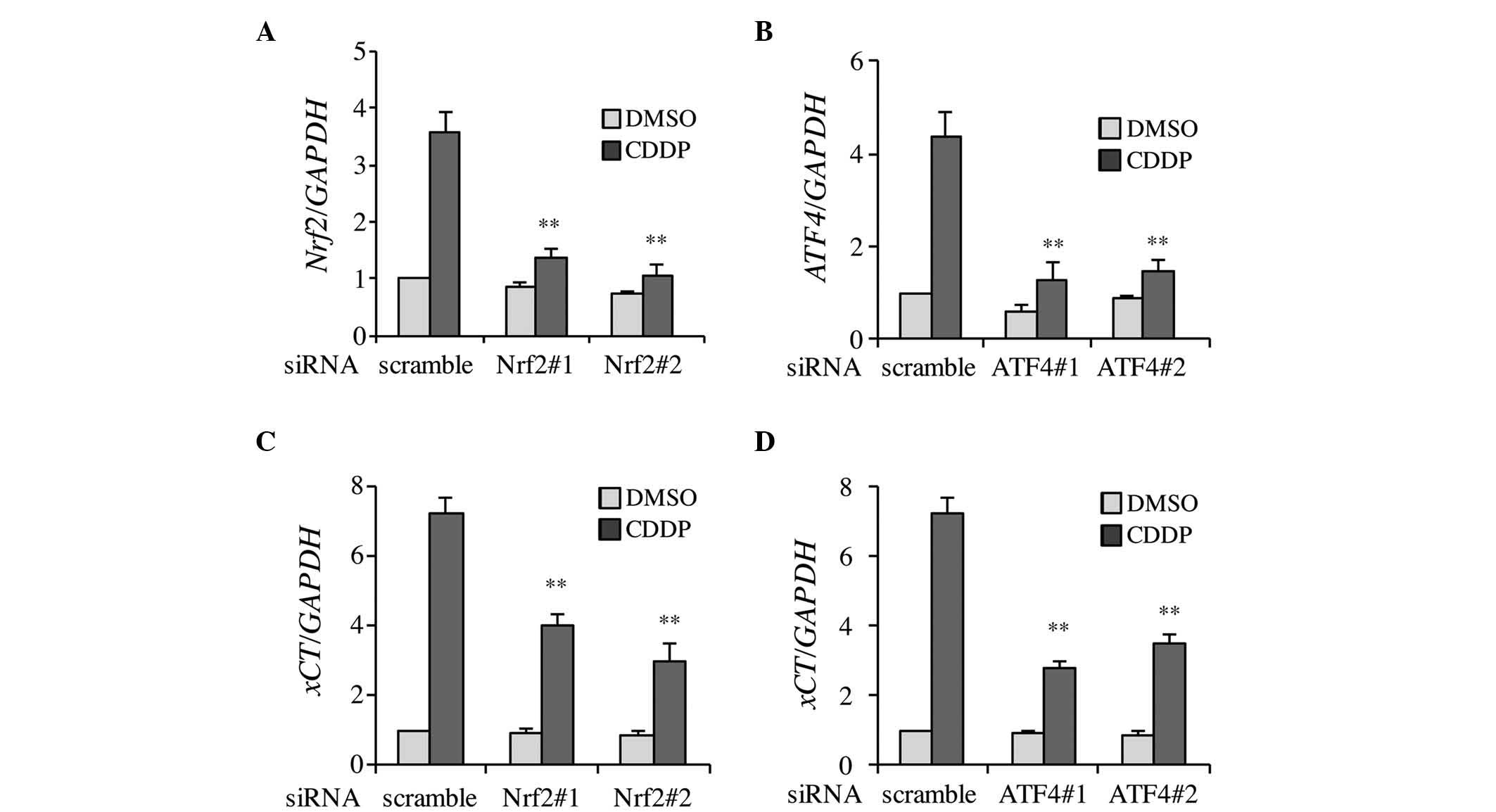
Firstly, to clarify whether CDDP-induced xCT
expression was dependent on Nrf2 and ATF4, the knockdown efficiency
of siRNA transfection was confirmed. As shown in Fig. 2A and B, Nrf2 and ATF4 expression was
effectively inhibited by individual siRNAs in the DMSO- and
CDDP-treated Tca8113 cells. Two types of siRNA, which targeted Nrf2
or ATF4, were used to prevent potential off-target effects
(silencing of genes other than Nrf2 and ATF4) arising from
non-specific binding of the siRNAs to unrelated genes. The results
demonstrated that CDDP-induced xCT expression was significantly
decreased in Nrf2 and ATF4 knockdown cells (Fig. 2C and D). These results indicate that
CDDP-induced xCT expression occurs in a Nrf2 and ATF4-dependent
manner.
CDDP induces xCT activation via ARE
and AARE elements on the promoter of the human xCT gene
The function of the cis elements on the
promoter of human xCT in CDDP-induced xCT expression was
investigated using a series of xCT gene promoter luciferase
reporter plasmids. As represented in Fig.
3, CDDP induced reporter activity in the wild-type construct.
Notably, constitutive and CDDP-inducible reporter activities were
decreased in ARE, AARE-F and AARE-R mutant reporter genes, and this
effect was decreased further in the AARE-F&R mutant reporter
construct. CDDP-inducible reporter activity was almost eliminated
in the triple mutant reporter gene. These results indicate that ARE
and AARE are required for CDDP-triggered xCT induction.
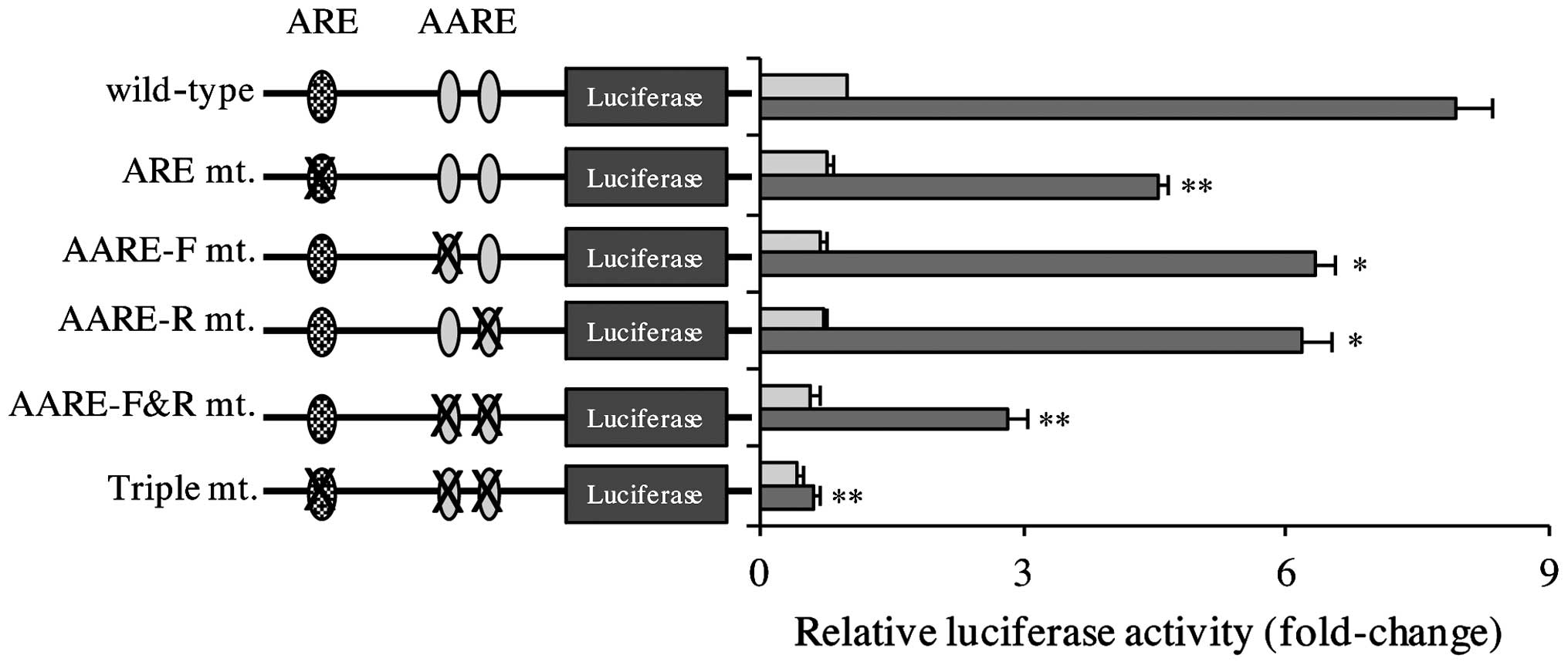 | Figure 3.CDDP-inducible xCT activation was
dependent on the ARE and AARE on the promoter of the human
xCT gene. Tca8113 cells were transfected with wild-type or
mutant xCT promoter luciferase reporter plasmids, and 24 h later,
the culture medium was replaced with 20 µg/ml CDDP. Following 24 h,
the reporter activity was determined. Data are presented as the
mean ±standard error of the mean. *P<0.05, **P<0.01 vs.
control. ARE, antioxidant response element; AARE, amino acid
response element; CDDP, cisplatin; F, forward; R, reverse; mt.,
mutant. |
xCT knockdown or inhibition increases
Tca8113 cell sensitivity to CDDP
Firstly, the siRNA knockdown efficiency for
targeting human xCT was confirmed. xCT expression levels were
effectively decreased in both DMSO- and CDDP-treated cells
(Fig. 4A). Notably, CDDP cytotoxicity
was markedly increased in xCT knockdown cells compared with
scramble siRNA-transfected cells (Fig.
4B). Similarly, following co-treatment with DMSO or CDDP and
the xCT pharmacological inhibitor SASP, the levels of GSH were
downregulated in both DMSO- and CDDP-treated cells (Fig. 4C). Furthermore, co-treatment with SASP
and CDDP markedly sensitized Tca8113 cells to CDDP (Fig. 4D). The inhibitory effect of SASP was
suppressed following the addition of β-ME, which could bypass xCT
to allow cysteine uptake via natural amino acid transporters
(19). Previously, it has been
demonstrated that Nrf2 and ATF4 regulate xCT following CDDP
treatment (15,18). Thus, the effect of Nrf2 and ATF4
knockdown on the sensitivity of Tca8113 cells to CDDP was also
assessed in the present study. As shown in Fig. 4E, CDDP inducible cytotoxicity was
markedly increased in Nrf2 knockdown cells compared with the
control. By contrast, following ATF4 knockdown, cell viability
increased in both high and low CDDP concentration-treatment groups
compared with the control (Fig.
4F).
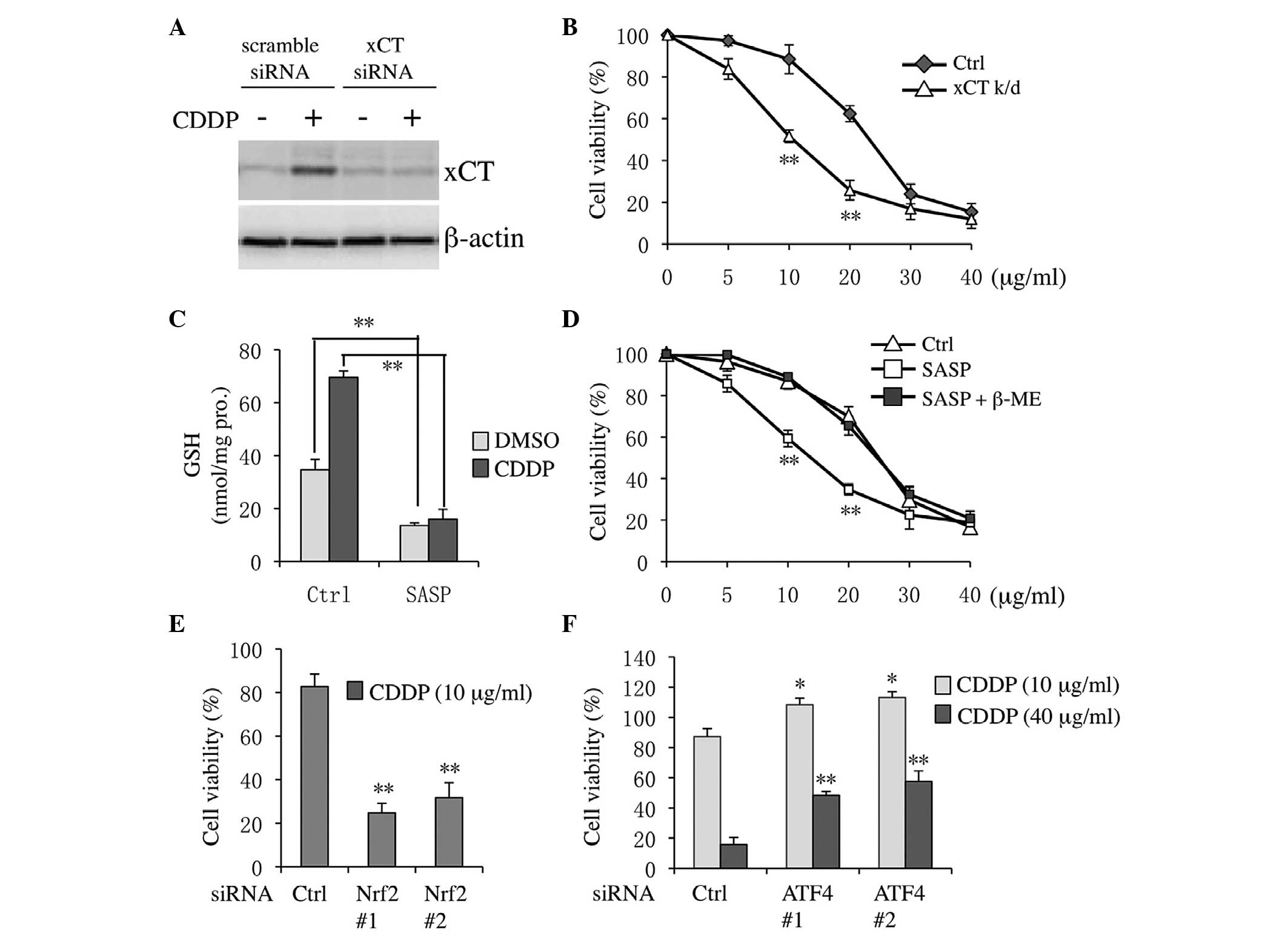 | Figure 4.xCT inhibition sensitized Tca8113
cells to CDDP. (A) Tca8113 cells were treated with 20 µg/ml CDDP
for 12 h following transfection with xCT or scramble siRNA. (B)
Tca8113 cells were transfected with xCT or scramble siRNA for 24 h,
and cells were next incubated with 0, 5, 10, 20, 30 or 40 µg/ml
CDDP for an additional 48 h. Cell viability was measured with the
Cell Counting Kit-8 assay. (C) Tca8113 cells were pre-treated with
0.3 mM SASP for 30 min, and then cultured with 20 µg/ml CDDP for 24
h. Subsequently, intercellular glutathione levels were estimated.
(D) Tca8113 cells were treated with SASP and 0–40 μg/ml CDDP for 48
h, and cell viability was analyzed. Cells were transfected with (E)
Nrf2 or (F) ATF4 siRNAs for 24 h, and then subjected to 10 or 40
µg/ml CDDP treatment for an additional 48 h. Data are presented as
the mean ±standard error of the mean. *P<0.05, **P<0.01 vs.
control. CDDP, cisplatin; siRNA, small interfering RNA; SASP,
sulfasalazine; Nrf2, nuclear factor erythroid 2-related-factor 2;
ATF4, activating transcription factor 4; β-ME, β-mercaptoethanol;
Ctrl, control; GSH, glutathione; DMSO, dimethyl sulfoxide; k/d,
knockdown. |
Discussion
Chemotherapy is a widely used cancer treatment, in
addition to surgery and radiotherapy (3). CDDP is the standard chemotherapeutic
drug administered to patients with tongue carcinoma (3). However, drug resistance is common and
leads to the failure of CDDP therapy (10). Thus, the mechanisms underlying tongue
carcinoma cell resistance to CDDP require investigation. An
increasing number of studies have indicated that CDDP resistance is
associated with various factors, including various microRNAs, which
modulate CDDP chemosensitivity by targeting certain genes (34,35); cell
protective autophagy, which diminishes CDDP-induced apoptotic cell
death (36); upregulated GSH levels;
and downregulated ROS levels (15,37). In
the present study, the mechanism of CDDP-triggered xCT induction
and the function of xCT in tongue carcinoma cell CDDP resistance
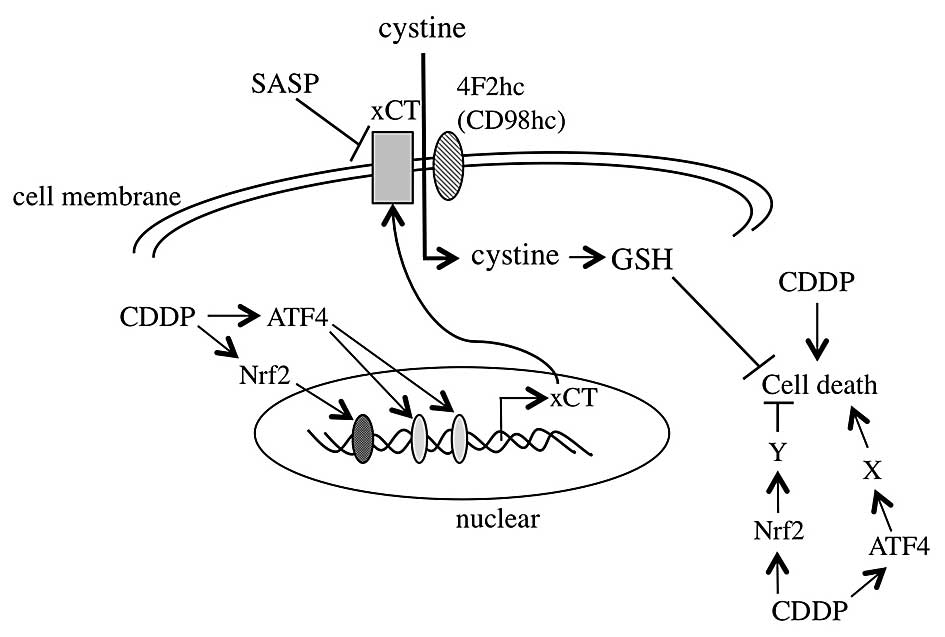
was investigated, and the conclusion is summarized in Fig. 5.
xCT is essential for GSH synthesis and maintenance,
which is a critical regulator of the cellular redox state (38). Thus, xCT has been considered as a
potential target for cancer treatment, including tongue carcinoma
(24). Previous studies have
demonstrated that SASP enhances CDDP-inducible cytotoxicity by
preventing electrophiles from conjugating with GSH, indicating that
combined treatment with CDDP and SASP may be beneficial (31). SASP is a well-established
pharmacological inhibitor of xCT, however, the effect of SASP
treatment on xCT and GSH levels has not yet been investigated
(31,39). In the present study, xCT suppression
increased CDDP cytotoxicity. In addition, CDDP treatment markedly
increased GSH levels in Tca8113 tongue cancer cells, which was
attenuated by SASP-induced xCT suppression (Fig. 4C). Furthermore, the sensitivity of
Tca8113 cells to CDDP treatment increased under conditions of low
GSH levels as a result of xCT inhibition caused by SASP or siRNA
(Fig. 4B and D). However, the present
study did not provide immediate evidence that decreased GSH levels
trigger ROS accumulation and subsequent activation of the cell
death signaling pathway in CDDP and SASP co-treated Tca8113 cells,
although previous studies have suggested such association in
various experimental conditions (40–42).
It has been reported that xCT is modulated in an
Nrf2 and ATF4-dependent manner following proteasome inhibitor
treatment (19). In the present
study, Nrf2 and ATF4 knockdown by siRNAs attenuated CDDP-triggered
xCT induction (Fig. 2). Furthermore,
the binding sites ARE and AARE of Nrf2 and ATF4 are critical for
CDDP-inducible xCT activation (Fig.
3). Additionally, no significant differences in the mRNA or
protein expression levels of CD44v were identified following CDDP
treatment in Tca8113 cells, although previous studies have
indicated that CD44v may promote tumor growth by stabilizing xCT
(33) and increasing CDDP resistance
in head and neck squamous cells (43). It is hypothesized that CD44v may
stabilize xCT; however, the association between CDDP treatment and
CD44v induction remains unclear. The present authors hypothesize
that CDDP-inducible xCT activation predominantly occurs via binding
of Nrf2 and ATF4 to the ARE and AARE elements on the promoter of
the xCT gene, rather than as a result of increased stabilization of
the xCT-CD44v complex. Although the present study demonstrated that
xCT is modulated by Nrf2 and ATF4 and contributes to the resistance
of CDDP treatment in Tca8113 cells, the effect of Nrf2 and ATF4
suppression by siRNA was not consistent with the effects of xCT
knockdown (Fig. 4E and F).
Previously, Tanabe et al (26)
reported that CDDP-inducible upregulation of ATF4 increased CDDP
resistance in human KB epidermoid and prostate cancer cell lines.
However, in the present study, Tca8113 cells treated with 10 or 40
µg/ml CDDP exhibited lower sensitivity to CDDP compared with the
control following ATF4 knockdown (Fig.
4F). It is possible to hypothesize that an alternative gene, in
addition to xCT, is downregulated in the downstream ATF4 signaling
cascade, such as the C/EBP homologous protein (CHOP) (44). CHOP induction is the key factor in ER
stress-induced apoptosis (45,46), and
is also a repressor of Wnt/T-cell factor signaling (47), which is associated with angiogenesis,
migration and survival of cancer cells (48–50).
However, in the present study, the suppression of Nrf2 by siRNA
exhibited the opposite effect to ATF4 suppression. It was
demonstrated that Nrf2 knockdown sensitized Tca8113 cells to CDDP
and exceeded the effects of xCT knockdown (Fig. 4E). Nrf2 exhibits a critical function
in the induction of phase II detoxifying enzymes, including
glutamate-L-cysteine ligase catalytic subunit, nicotinamide adenine
dinucleotide (P) H: quinone oxidoreductase and heme oxygenase-1,
via the Kelch-like ECH-associated protein 1-Nrf2-ARE pathway, which
has been reported to induce CDDP resistance in tumor cells
(51,52). Although Nrf2 and its target gene
contribute to CDDP resistance, the present authors postulate that
it is not advisable to increase CDDP cytotoxicity via Nrf2
suppression. In various animal models (53–55),
Nrf2-ARE activation has been demonstrated to prevent CDDP-induced
nephrotoxicity, which is the most severe adverse effect that limits
high-dose CDDP therapy (56).
In conclusion, the present study demonstrated that
Nrf2/ATF4-dependent xCT induction is involved in CDDP resistance of
Tca8113 tongue carcinoma cells. These results suggest that it may
be beneficial to combine CDDP and pharmacological xCT inhibitors
for the treatment of tongue carcinoma.
Glossary
Abbreviations
Abbreviations:
|
TSCC
|
tongue squamous cell carcinoma
|
|
CDDP
|
cisplatin
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
ATF4
|
activating transcription factor 4
|
|
SASP
|
sulfasalazine
|
|
GSH
|
glutathione
|
|
ER
|
endoplasmic reticulum
|
|
ARE
|
antioxidant response element
|
|
AARE
|
amino acid response element
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Neill VJ and Twelves CJ: Oral cancer
treatment: Developments in chemotherapy and beyond. Br J Cancer.
87:933–937. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gibson MK and Forastiere AA: Reassessment
of the role of induction chemotherapy for head and neck cancer.
Lancet Oncol. 7:565–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukada H, Yokoyama A, Goto K, Shinkai T,
Harada M, Ando M, Shibata T, Ohe Y, Tamura T and Saijo N: Lung
Cancer Study Group of the Japan Clinical Oncology Group (JCOG):
Randomized controlled trial comparing docetaxel-cisplatin
combination with weekly docetaxel alone in elderly patients with
advanced non-small-cell lung cancer: Japan Clinical Oncology Group
(JCOG) 0207. Jpn J Clin Oncol. 45:88–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Wan L, Zhai LY and Wang J: Effects
of SC-560 in combination with cisplatin or taxol on angiogenesis in
human ovarian cancer xenografts. Int J Mol Sci. 15:19265–19280.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu MH, Wang LW, Lu HJ, Chu PY, Tai SK, Lee
TL, Chen MH, Yang MH and Chang PM: Cisplatin-based chemotherapy
versus cetuximab in concurrent chemoradiotherapy for locally
advanced head and neck cancer treatment. BioMed Res Int.
2014:9043412014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Zhang Y, Zhao J, Xiao E, Lu J, Fu
S and Wang Z: Combination of bladder cancer-specific oncolytic
adenovirus gene therapy with cisplatin on bladder cancer in vitro.
Tumour Biol. 35:10879–10890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang B, Zhang S, Yue K and Wang XD: The
recurrence and survival of oral squamous cell carcinoma: A report
of 275 cases. Chin J Cancer. 32:614–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandic A, Hansson J, Linder S and Shoshan
MC: Cisplatin induces endoplasmic reticulum stress and
nucleus-independent apoptotic signaling. J Biol Chem.
278:9100–9106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou XJ, Chen WT, Li Q and He RG:
Establishment and biological characteristics of cisplatin resistant
cell line from human tongue squamous cell carcinoma Tca8113.
Shanghai Kou Qiang Yi Xue. 10:31–34. 2001.(In Chinese). PubMed/NCBI
|
|
14
|
Lu SC: Glutathione synthesis. Biochim
Biophys Acta. 1830:3143–3153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okuno S, Sato H, Kuriyama-Matsumura K,
Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T and Bannai
S: Role of cystine transport in intracellular glutathione level and
cisplatin resistance in human ovarian cancer cell lines. Br J
Cancer. 88:951–956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wangpaichitr M, Sullivan EJ,
Theodoropoulos G, Wu C, You M, Feun LG, Lampidis TJ, Kuo MT and
Savaraj N: The relationship of thioredoxin-1 and cisplatin
resistance: Its impact on ROS and oxidative metabolism in lung
cancer cells. Mol Cancer Ther. 11:604–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wangpaichitr M, Wu C, You M, Maher JC,
Dinh V, Feun LG and Savaraj N:
N',N'-Dimethyl-N',N'-bis(phenylcarbonothioyl) propanedihydrazide
(elesclomol) selectively kills cisplatin resistant lung cancer
cells through reactive oxygen species (ROS). Cancers (Basel).
1:23–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye P, Mimura J, Okada T, Sato H, Liu T,
Maruyama A, Ohyama C and Itoh K: Nrf2- and ATF4-dependent
upregulation of xCT modulates the sensitivity of T24 bladder
carcinoma cells to proteasome inhibition. Mol Cell Biol.
34:3421–3434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wada T, Ishimoto T, Seishima R,
Tsuchihashi K, Yoshikawa M, Oshima H, Oshima M, Masuko T, Wright
NA, Furuhashi S, et al: Functional role of CD44v-xCT system in the
development of spasmolytic polypeptide-expressing metaplasia.
Cancer Sci. 104:1323–1329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi S, Wada K, Toyooka T, Shinomiya
N, Shimazaki H, Nakanishi K, Nagatani K, Otani N, Osada H, Uozumi
Y, et al: Increased xCT expression correlates with tumor invasion
and outcome in patients with glioblastomas. Neurosurgery. 72:33–41.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Dai Z, Barbacioru C and Sadée W:
Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity
and chemoresistance. Cancer Res. 65:7446–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savaskan NE, Heckel A, Hahnen E, Engelhorn
T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M and Eyüpoglu IY:
Small interfering RNA-mediated xCT silencing in gliomas inhibits
neurodegeneration and alleviates brain edema. Nat Med. 14:629–632.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toyoda M, Kaira K, Ohshima Y, Ishioka NS,
Shino M, Sakakura K, Takayasu Y, Takahashi K, Tominaga H, Oriuchi
N, et al: Prognostic significance of amino-acid transporter
expression (LAT1, ASCT2, and xCT) in surgically resected tongue
cancer. Br J Cancer. 110:2506–2513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
El-Sawalhi MM and Ahmed LA: Exploring the
protective role of apocynin, a specific NADPH oxidase inhibitor, in
cisplatin-induced cardiotoxicity in rats. Chem Biol Interact.
207:58–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanabe M, Izumi H, Ise T, Higuchi S,
Yamori T, Yasumoto K and Kohno K: Activating transcription factor 4
increases the cisplatin resistance of human cancer cell lines.
Cancer Res. 63:8592–8595. 2003.PubMed/NCBI
|
|
27
|
Lo M, Wang YZ and Gout PW: The
xc− cystine/glutamate antiporter: A potential target for
therapy of cancer and other diseases. J Cell Physiol. 215:593–602.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo W, Zhao Y, Zhang Z, Tan N, Zhao F, Ge
C, Liang L, Jia D, Chen T, Yao M, et al: Disruption of xCT inhibits
cell growth via the ROS/autophagy pathway in hepatocellular
carcinoma. Cancer Lett. 312:55–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Z, Guo KJ, Guo RX and He SG: Effects
of 5-fluouracil combined with sulfasalazine on human pancreatic
carcinoma cell line BxPC-3 proliferation and apoptosis in vitro.
Hepatobiliary Pancreat Dis Int. 6:312–320. 2007.PubMed/NCBI
|
|
30
|
Lay JD, Hong CC, Huang JS, Yang YY, Pao
CY, Liu CH, Lai YP, Lai GM, Cheng AL, Su IJ and Chuang SE:
Sulfasalazine suppresses drug resistance and invasiveness of lung
adenocarcinoma cells expressing AXL. Cancer Res. 67:3878–3887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Awasthi S, Sharma R, Singhal SS, Herzog
NK, Chaubey M and Awasthi YC: Modulation of cisplatin cytotoxicity
by sulphasalazine. Br J Cancer. 70:190–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Newton GL, Dorian R and Fahey RC: Analysis
of biological thiols: Derivatization with monobromobimane and
separation by reverse-phase high-performance liquid chromatography.
Anal Biochem. 114:383–387. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc(−) and thereby promotes
tumor growth. Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren W, Wang X, Gao L, Li S, Yan X, Zhang
J, Huang C, Zhang Y and Zhi K: MiR-21 modulates chemosensitivity of
tongue squamous cell carcinoma cells to cisplatin by targeting
PDCD4. Mol Cell Biochem. 390:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin J, Luo M, Qian H and Chen W:
Upregulated miR-182 increases drug resistance in cisplatin-treated
HCC cell by regulating TP53INP1. Gene. 538:342–347. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang N, Dai L, Qi Y, Di W and Xia P:
Combination of FTY720 with cisplatin exhibits antagonistic effects
in ovarian cancer cells: Role of autophagy. Int J Oncol.
42:2053–2059. 2013.PubMed/NCBI
|
|
37
|
Li Y, Li X, Wong YS, Chen T, Zhang H, Liu
C and Zheng W: The reversal of cisplatin-induced nephrotoxicity by
selenium nanoparticles functionalized with 11-mercapto-1-undecanol
by inhibition of ROS-mediated apoptosis. Biomaterials.
32:9068–9076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barros MP, Marin DP, Bolin AP, de Cássia
Santos Macedo R, Campoio TR, Fineto C Jr, Guerra BA, Polotow TG,
Vardaris C, Mattei R and Otton R: Combined astaxanthin and fish oil
supplementation improves glutathione-based redox balance in rat
plasma and neutrophils. Chem Biol Interact. 197:58–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gout PW, Buckley AR, Simms CR and
Bruchovsky N: Sulfasalazine, a potent suppressor of lymphoma growth
by inhibition of the x(c)− cystine transporter: A new
action for an old drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
You BR and Park WH: Arsenic trioxide
induces human pulmonary fibroblast cell death via increasing ROS
levels and GSH depletion. Oncol Rep. 28:749–757. 2012.PubMed/NCBI
|
|
41
|
Park WH and Kim SH: MG132, a proteasome
inhibitor, induces human pulmonary fibroblast cell death via
increasing ROS levels and GSH depletion. Oncol Rep. 27:1284–1291.
2012.PubMed/NCBI
|
|
42
|
You BR and Park WH: Suberoyl bishydroxamic
acid-induced apoptosis in HeLa cells via ROS-independent,
GSH-dependent manner. Mol Biol Rep. 40:3807–3816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Torre C, Wang SJ, Xia W and Bourguignon
LY: Reduction of hyaluronan-CD44-mediated growth, migration, and
cisplatin resistance in head and neck cancer due to inhibition of
Rho kinase and PI-3 kinase signaling. Arch Otolaryngol Head Neck
Surg. 136:493–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao J, Dai DL, Yao L, Yu HH, Ning B, Zhang
Q, Chen J, Cheng WH, Shen W and Yang ZX: Saturated fatty acid
induction of endoplasmic reticulum stress and apoptosis in human
liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell
Biochem. 364:115–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Horndasch M, Lienkamp S, Springer E,
Schmitt A, Pavenstädt H, Walz G and Gloy J: The C/EBP homologous
protein CHOP (GADD153) is an inhibitor of Wnt/TCF signals.
Oncogene. 25:3397–3407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang X, Gaspard JP and Chung DC:
Regulation of vascular endothelial growth factor by the Wnt and
K-ras pathways in colonic neoplasia. Cancer Res. 61:6050–6054.
2001.PubMed/NCBI
|
|
50
|
Fujita M, Furukawa Y, Tsunoda T, Tanaka T,
Ogawa M and Nakamura Y: Up-regulation of the ectodermal-neural
cortex 1 (ENC1) gene, a downstream target of the
beta-catenin/T-cell factor complex, in colorectal carcinomas.
Cancer Res. 61:7722–7726. 2001.PubMed/NCBI
|
|
51
|
Bao LJ, Jaramillo MC, Zhang ZB, Zheng YX,
Yao M, Zhang DD and Yi XF: Nrf2 induces cisplatin resistance
through activation of autophagy in ovarian carcinoma. Int J Clin
Exp Pathol. 7:1502–1513. 2014.PubMed/NCBI
|
|
52
|
Hayden A, Douglas J, Sommerlad M, Andrews
L, Gould K, Hussain S, Thomas GJ, Packham G and Crabb SJ: The Nrf2
transcription factor contributes to resistance to cisplatin in
bladder cancer. Urol Oncol. 32:806–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li M, Jin J, Li J, Guan CW, Wang WW, Qiu
YW and Huang ZY: Schisandrin B protects against nephrotoxicity
induced by cisplatin in HK-2 cells via Nrf2-ARE activation. Yao Xue
Xue Bao. 47:1434–1439. 2012.(In Chinese). PubMed/NCBI
|
|
54
|
Sahin K, Tuzcu M, Gencoglu H, Dogukan A,
Timurkan M, Sahin N, Aslan A and Kucuk O:
Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in
cisplatin-induced nephrotoxicity in rats. Life Sci. 87:240–245.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aleksunes LM, Goedken MJ, Rockwell CE,
Thomale J, Manautou JE and Klaassen CD: Transcriptional regulation
of renal cytoprotective genes by Nrf2 and its potential use as a
therapeutic target to mitigate cisplatin-induced nephrotoxicity. J
Pharmacol Exp Ther. 335:2–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Luke DR, Vadiei K and Lopez-Berestein G:
Role of vascular congestion in cisplatin-induced acute renal
failure in the rat. Nephrol Dial Transplant. 7:1–7. 1992.PubMed/NCBI
|