Introduction
Paraneoplastic Cushing's syndrome (CushingPS) has
been attributed to ectopic adrenocorticotropin (ACTH) secretion
(EAS), which may result in several types of tumors, including
carcinoid and pancreatic neuroendocrine tumors, small cell lung
cancer and thyroid medullary carcinoma (1). The bronchopulmonary carcinoid tumor
(BCT) accounts for 1–2% of all adult malignancies of the lung and
20–30% of all carcinoid tumors (2–5).
Based on the World Health Organization
classification, BCTs are divided into typical carcinoids, which are
low-grade tumors with a low mitotic rate, and atypical carcinoids,
which are intermediate-grade tumors with a higher mitotic rate
and/or necrosis. Typical carcinoids are approximately four times
more common than atypical carcinoids. The worldwide incidence of
BCT is 0.2–2 per 100,000 individuals annually, with the majority of
studies reporting a higher incidence in women compared with men,
and in Caucasian compared with African-American individuals
(2–4,6–8). Several reports have suggested that the
incidence of BCT is increasing (3,4,8); however, this may be partly due to the
increased use of advanced medical imaging techniques that are more
sensitive in detecting asymptomatic tumors.
In general, BCTs are considered to be low- to
moderate-grade malignant tumors; however, when associated with
CushingPS, they are clinically aggressive (9). The present study reports a rare case of
CushingPS in a teenager who was diagnosed with BCT and
lymphadenopathy.
Case report
In September 2014, an 18-year-old man visited the
Department of Endocrinology, The First Affiliated Hospital of China
Medical University, Liaoning, presenting with a 3-month history of
rapid weight gain (15 kg) characterized by central obesity
involving the trunk and face with sparing of the limbs. The patient
additionally complained of polyuria, polydipsia and progressive
muscle weakness. The patient's height was 167 cm and his weight was
83 kg. The patient's blood pressure was 160/100 mmHg at
presentation. Physical examination revealed typical Cushingoid
features, including fat pads along the collarbone, back of the neck
(buffalo hump) and face (moon face), along with generalized purple
striae and hair growth on the back and both arms.
Routine laboratory test results revealed the
following: Leukocytosis (11.4×109/l) with neutrophilia
(81.2%); hyperglycemia (11.2 mmol/l); hypokalemia (2.54 mmol/l);
metabolic alkalosis (pH 7.56); base excess, 6.8 mmol/l; and
standard HCO3−, 31.7 mmol/l. A marked elevation in serum
cortisol (00:00 h, 1,397.0 nmol/l; 08:00 h, 1,750.0 nmol/l; 15:00
h, 1,566.0 nmol/l) and ACTH (00:00 h, 149.8 pg/ml; 08:00 h, 211.0
pg/ml; 15:00 h, 198.6 pg/ml) levels was also observed, without
diurnal rhythm. Additional examinations revealed no response to
either low- or high-dose dexamethasone suppression. Based on the
aforementioned findings, CushingPS due to EAS was initially
suspected. Abdominal computed tomography (CT) and brain magnetic
resonance imaging (MRI) scans revealed no abnormalities in the
adrenal and pituitary glands. A chest CT scan revealed a mass in
the right middle lung measuring 1.2 cm in diameter, which was
evidenced by bilateral and diffusely scattered micronodular
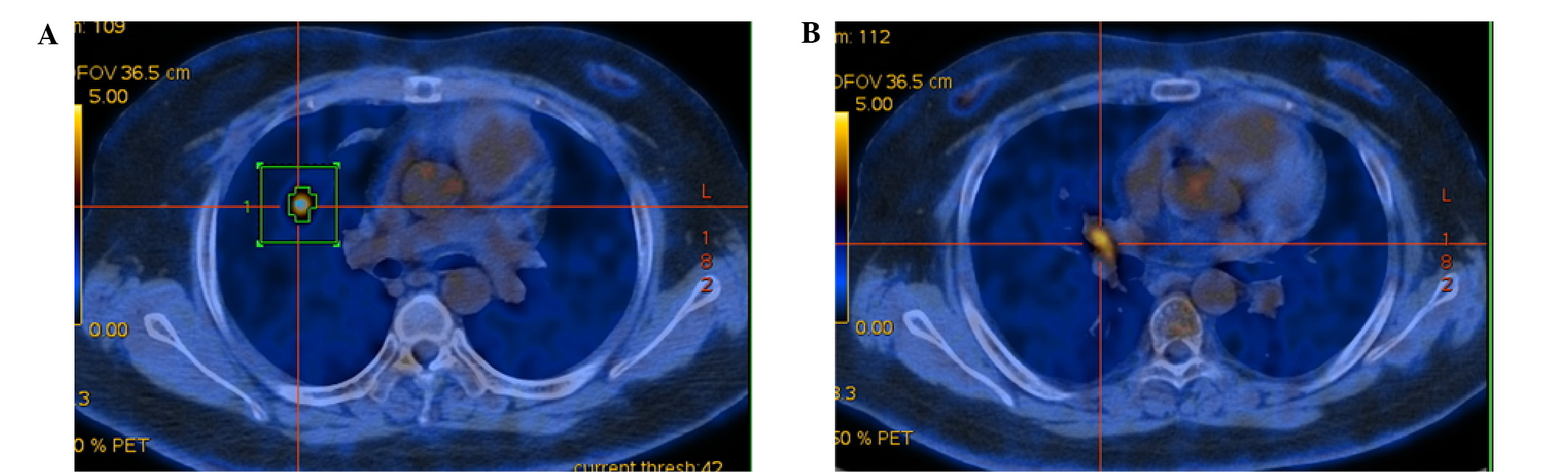
shadows. Whole-body 18fluorine-fluorodeoxyglucose
positron-emission tomography/computed tomography
(18F-FDG PET/CT) confirmed a high 18F-FDG
uptake in the right middle lung [maximum standard uptake value,
(SUVmax), 5.68] and lobar lymph node (SUVmax,
4.89; Fig. 1), which supported the
clinical diagnosis of CushingPS associated with right middle lung
cancer.
The patient was subsequently treated with
ketoconazole (1,000 mg/day) and intravenous potassium
supplementation for 1 week. Following the decrease of the plasma
cortisol levels, the middle lobe was resected with hilar and
mediastinal lymph node dissection.
Tissue specimens were fixed with 10% neutral
formalin and embedded in paraffin, and 4-µm-thick sections were
prepared. Immunostaining was performed using the
avidin–biotin–peroxidase complex method (Ultrasensitive™, MaiXin,
Fuzhou, China). Paraffin sections were dewaxed in xylene and
rehydrated in graded alcohols. Antigen retrieval was performed by
heating the sections for 1.5 min in 0.01 mol/L citrate buffer, pH
6.0. Non-specific staining was reduced by incubation in blocking
buffer containing goat serum (SP KIT-B1; Maixin-Bio, Fuzhou, China)
for 30 min. Then, the sections were incubated with ACTH antibody
(rabbit polyclonal to ACTH, dilution, 1/200, cat no. ab74976,
Abcam) overnight at 4°C. The following day, the sections were
incubated with appropriate secondary antibodies for 30 min. The
reaction was visualized using DAB (DAB-0031; Maixin-Bio) plus
chromogen. Specimens were examined using a BX50 microscope (Olympus
Corp. Tokyo, Japan). For serum controls, 1% BSA in PBS was used in
place of the primary antibody as a negative control. Pathology
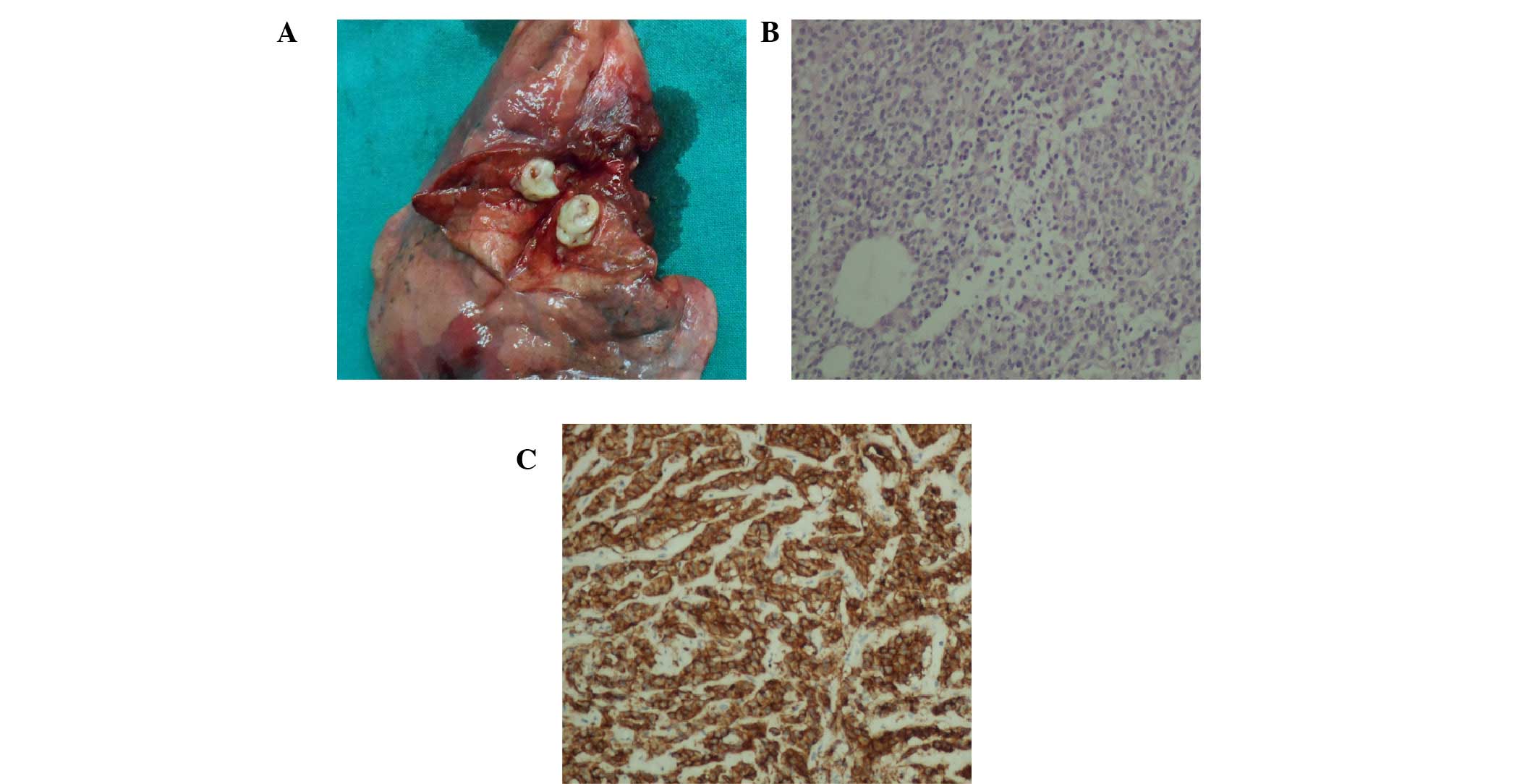
indicated a typical carcinoid tumor with lobar lymph node
metastasis, staged as IIA (T1aN1M0). Immunohistochemistry
additionally revealed that the tumor was positive for ACTH
(Fig. 2). Symptoms were alleviated
following surgery. Blood pressure, plasma glucose, potassium,
cortisol, ACTH and 24-h urinary cortisol were normalized within a
week. The postoperative recovery was uneventful, and the patient
was discharged 14 days after the surgery. No CushingPS or tumor
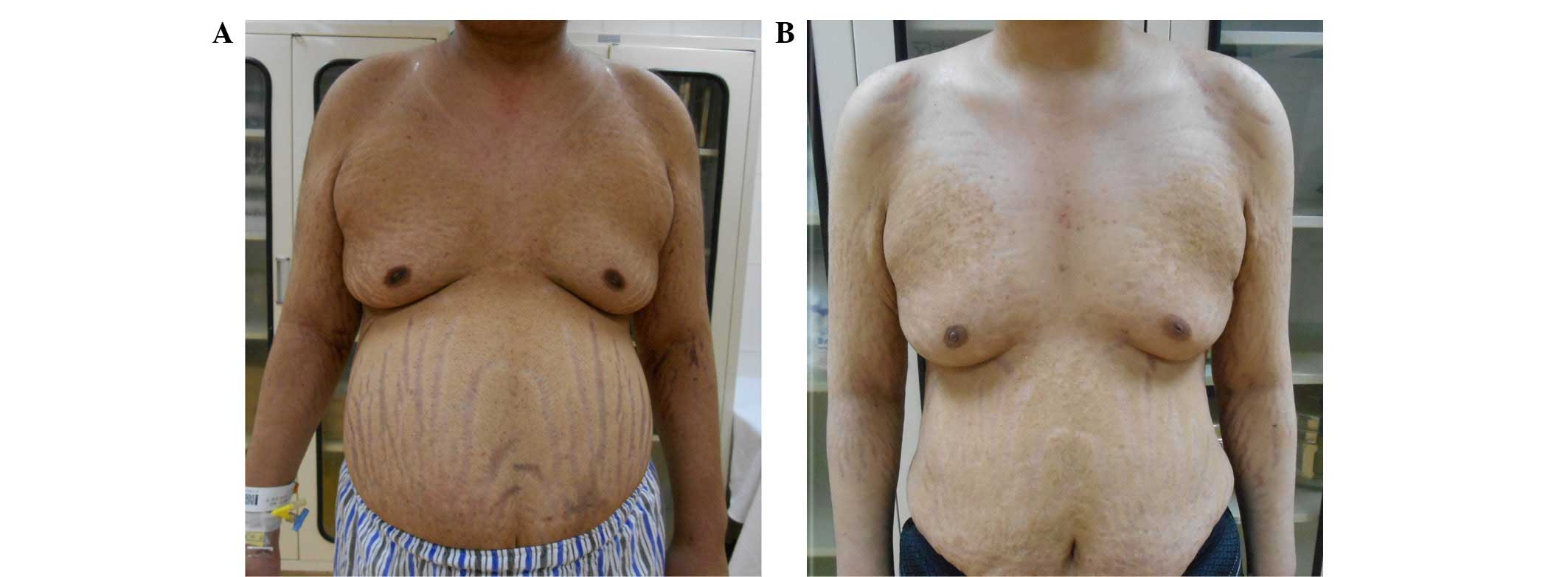
recurrence was noted at the 3-month postoperative follow-up. In
addition, the patient had lost 13 kg (Fig. 3). Postoperative chemotherapy was
administrated for four courses (Docetaxel 75mg/m2 and
Cisplatin 75mg/m2), and the patient reached 15-months
follow-up with no CushingPS or tumor recurrence.
Discussion
CushingPS due to BCT is a rare clinical occurrence,
which has been reported in only a few case reports and case series.
In fact, to the best of our knowledge, only four published surgical
studies have reported data from >5 patients (10–13).
According to a report of 19 BCT-induced CushingPS cases, the mean
age of the patients was observed to be 43 years (11). Terzolo et al (12) reported 14 cases of EAS associated with
a variety of tumor types of patients aged 26–76 years (mean age 53
years), with CushingPS being the predominant diagnosis in patients
of advanced age, suggesting an increased occurrence of CushingPS in
elderly compared with young individuals. However, sporadic cases of
CushingPS in young patients have additionally been reported.
Arioglu et al (13) reported a
case of CushingPS in a 9-year-old girl, which was attributed to
ectopic hormone secretion. The present study reported a case of
CushingPS secondary to BCT in a teenager, which suggested an early
onset of the syndrome.
EAS is difficult to diagnose and accurately
localize. Symptoms typically manifest 6–84 months ahead of the
diagnosis, with the average time to diagnosis ranging up to 24
months (14). In 12–19% of patients,
the source of the tumor is unknown (15,16).
Imaging modalities, including CT and MRI, lack the sensitivity and
specificity required to identify an ectopic ACTH lesion (16). 111Indium-labeled octreotide
scintigraphy (111In-OCT) has been reported as an
effective diagnostic modality, as 80% of BCTs express receptors for
somatostatin (17).
111In-OCT is considered helpful in the localization of
EAS tumors. In addition, it may enable rapid, non-invasive
differential diagnosis between ACTH-secreting pituitary adenomas
and malignancies caused by EAS, followed by radioguided surgery in
the treatment of bronchial carcinoid (18). 18FDG-PET and
18F-Fluorine-18-l-dihydroxyphenylalanine
(18F-DOPA)-PET scans should also be considered as a
secondary diagnostic tool for early diagnosis of EAS when
conventional imagery fails to detect the presence of tumors
(19). FDG-PET has a sensitivity of
64% and a positive predictive value of 53% in the identification of
EAS (20). In the present case,
18F-FDG PET indicated an EAS tumor and a metastatic
lymph node; the patient underwent radical resection, which led to
the amelioration of the clinical symptoms.
Surgery is considered to be one of the preferred
treatment options for localized ectopic lesions, and leads to a
reduction in cortisol levels (17).
In the present case, a right middle lobectomy with mediastinal
lymph node resection was selected based on the pathological
diagnosis of a typical carcinoid and lobar lymph node metastasis
(stage N1). As reported, 87% of typical carcinoids do not present
with lymph node involvement. Combined with CushingPS, a higher rate
of lymph node metastasis and postoperative local recurrence rate
was found (10,21). These findings indicated that BCT with
CushingPS is an aggressive clinical entity. Lobectomy combined with
complete lymphadenectomy is recommended for carcinoid tumors
associated with CushingPS (10,14,22);
otherwise, an occult residual metastatic lymph node may present,
leading to persistent or recurrent CushingPS (11).
Patients with CushingPS are at increased risk of
life-threatening infection and venous thromboembolism (23); however, due to their resistance to
cytotoxic agents and radiation (10),
such patients generally have a poor prognosis. In a clinical study
conducted by the MD Anderson Cancer Center, 82% of patients with
CushingPS caused by small-cell lung cancer succumbed to their
condition within 2 weeks of the initiation of chemotherapy.
Rectifying the patients' biochemical profile over a period of a few
weeks prior to chemotherapy markedly improved the prognosis
(24). Regulating the production of
cortisol drastically alleviated the complications. Ketoconazole
(200–1,200 mg/day) has been shown to inhibit the enzymes
11-hydroxylase and 17-hydroxylase in the cortisol biosynthesis
pathway, and has been widely used due to its rapid mechanism of
action and low frequency of side-effects (25). The present study demonstrated that
ketoconazole combined with potassium supplementation was able to
effectively control the preoperative hormone and ion levels of the
patient. Ketoconazole treatment resulted in the biochemical and
hormonal improvement of the patient with EAS; however, this drug
may impair the cortisol response to stress; therefore, replacement
corticosteroids should be considered for patients that exhibit a
hormonal response, and use of moderate- to high-dose
corticosteroids is recommended for any potential stress situations.
Successful treatment of the underlying tumor is key to controlling
the syndrome (26). A diminished
objective response and sensitivity to first-line therapy and lack
of a response to second-line therapy are indicators of the
necessity to initiate palliative care early at first line and
exclusively at relapse (27).
In conclusion, the present study reported a rare
case of CushingPS in a teenager with BCT and N1 lymph node
metastasis. The present findings suggested that BCT associated with
CushingPS may be associated with a young age of onset, high rate of
lymphadenopathy and poor prognosis. Radical resection of the lung
and systemic lymph nodes may provide complete relief from
symptoms.
Acknowledgements
The current study was supported by the Natural
Science Foundation of Liaoning Province (grant no. 2015020561) and
the Fund for Scientific Research of The First Hospital of China
Medical University (grant no. fsfh1514).
References
|
1
|
Wajchenberg BL, Mendonca BB, Liberman B,
Pereira MA, Carneiro PC, Wakamatsu A and Kirschner MA: Ectopic
adrenocorticotropic hormone syndrome. Endocr Rev. 15:752–787. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quaedvlieg PF, Visser O, Lamers CB,
Janssen-Heijen ML and Taal BG: Epidemiology and survival in
patients with carcinoid disease in the Netherlands. An
epidemiological study with 2391 patients. Ann Oncol. 12:1295–1300.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hemminki K and Li X: Incidence trends and
risk factors of carcinoid tumors: A nationwide epidemiologic study
from Sweden. Cancer. 92:2204–2210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fink G, Krelbaum T, Yellin A, Bendayan D,
Saute M, Glazer M and Kramer MR: Pulmonary carcinoid: Presentation,
diagnosis, and outcome in 142 cases in Israel and review of 640
cases from the literature. Chest. 119:1647–1651. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gatta G, Ciccolallo L, Kunkler I,
Capocaccia R, Berrino F, Coleman MP, De Angelis R, Faivre J, Lutz
JM, Martinez C, et al: EUROCARE Working Group: Survival from rare
cancer in adults: A population-based study. Lancet Oncol.
7:132–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuetenhorst JM and Taal BG: Metastatic
carcinoid tumors: A clinical review. Oncologist. 10:123–131. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beasley MB, Brambilla E and Travis WD: The
2004 World Health Organization classification of lung tumors. Semin
Roentgenol. 40:90–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boddaert G, Grand B, Le Pimpec-Barthes F,
Cazes A, Bertagna X and Riquet M: Bronchial carcinoid tumors
causing Cushing's syndrome: More aggressive behavior and the need
for early diagnosis. Ann Thorac Surg. 94:1823–1829. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amer KM, Ibrahim NB, Forrester-Wood CP,
Saad RA and Scanlon M: Lung carcinoid related Cushing's syndrome:
Report of three cases and review of the literature. Postgrad Med J.
77:464–467. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terzolo M, Reimondo G, Ali A, Bovio S,
Daffara F, Paccotti P and Angeli A: Ectopic ACTH syndrome:
Molecular bases and clinical heterogeneity. Ann Oncol. 12(Suppl 2):
S83–S87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arioglu E, Doppman J, Gomes M, Kleiner D,
Mauro D, Barlow C and Papanicolaou DA: Cushing's syndrome caused by
corticotropin secretion by pulmonary tumorlets. N Engl J Med.
339:883–886. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shrager JB, Wright CD, Wain JC, Torchiana
DF, Grillo HC and Mathisen DJ: Bronchopulmonary carcinoid tumors
associated with Cushing's syndrome: A more aggressive variant of
typical carcinoid. J Thorac Cardiovasc Surg. 114:367–375. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aniszewski JP, Young WF Jr, Thompson GB,
Grant CS and van Heerden JA: Cushing syndrome due to ectopic
adrenocorticotropic hormone secretion. World J Surg. 25:934–940.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ilias I, Torpy DJ, Pacak K, Mullen N,
Wesley RA and Nieman LK: Cushing's syndrome due to ectopic
corticotropin secretion: Twenty years' experience at the National
Institutes of Health. J Clin Endocrinol Metab. 90:4955–4962. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Matos LL, Trufelli DC, das
Neves-Pereira JC, Danel C and Riquet M: Cushing's syndrome
secondary to bronchopulmonary carcinoid tumor: Report of two cases
and literature review. Lung Cancer. 53:381–386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mansi L, Rambaldi PF, Panza N, Esposito D,
Esposito V and Pastore V: Diagnosis and radioguided surgery with
111In-pentetreotide in a patient with paraneoplastic Cushing's
syndrome due to a bronchial carcinoid. Eur J Endocrinol.
137:688–690. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Markou A, Manning P, Kaya B, Datta SN,
Bomanji JB and Conway GS: [18F]fluoro-2-deoxy-D-glucose ([18F]FDG)
positron emission tomography imaging of thymic carcinoid tumor
presenting with recurrent Cushing's syndrome. Eur J Endocrinol:.
152:521–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zemskova MS, Gundabolu B, Sinaii N, Chen
CC, Carrasquillo JA, Whatley M, Chowdhury I, Gharib AM and Nieman
LK: Utility of various functional and anatomic imaging modalities
for detection of ectopic adrenocorticotropin-secreting tumors. J
Clin Endocrinol Metab. 95:1207–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gustafsson BI, Kidd M, Chan A,
Malfertheiner MV and Modlin IM: Bronchopulmonary neuroendocrine
tumors. Cancer. 113:5–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kara M, Ucar I, Yazicioglu A and Firat P:
An overlooked tumor of the lung in Cushing's syndrome:
Adrenocorticotropic hormone-secreting carcinoid tumor. Thorac
Cardiovasc Surg. 55:527–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ejaz S, Vassilopoulou-Sellin R, Busaidy
NL, Hu MI, Waguespack SG, Jimenez C, Ying AK, Cabanillas M, Abbara
M and Habra MA: Cushing syndrome secondary to ectopic
adrenocorticotropic hormone secretion: The University of Texas MD
Anderson Cancer Center Experience. Cancer. 117:4381–4389. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dimopoulos MA, Fernandez JF, Samaan NA,
Holoye PY and Vassilopoulou-Sellin R: Paraneoplastic Cushing's
syndrome as an adverse prognostic factor in patients who die early
with small cell lung cancer. Cancer. 69:66–71. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Izzedine H, Besse B, Lazareth A, Bourry EF
and Soria JC: Hypokalemia, metabolic alkalosis, and hypertension in
a lung cancer patient. Kidney Int. 76:115–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Winquist EW, Laskey J, Crump M, Khamsi F
and Shepherd FA: Ketoconazole in the management of paraneoplastic
Cushing's syndrome secondary to ectopic adrenocorticotropin
production. J Clin Oncol. 13:157–164. 1995.PubMed/NCBI
|
|
27
|
Nagy-Mignotte H, Shestaeva O, Vignoud L,
Guillem P, Ruckly S, Chabre O, Sakhri L, Duruisseaux M, Mousseau M,
Timsit JF and Moro-Sibilot D: Multidisciplinary Thoracic Oncology
Group at Grenoble University Hospital, France: Prognostic impact of
paraneoplastic cushing's syndrome in small-cell lung cancer. J
Thorac Oncol. 9:497–505. 2014. View Article : Google Scholar : PubMed/NCBI
|