Introduction
Chronic lymphocytic thyroiditis (CLT), also termed
Hashimoto thyroiditis, is a type of autoimmune disease
characterized by thyroid antigen response and T and B lymphocytes
infiltration (1,2). Generally, 95% of CLT cases occur in
women, particularly among women between 30 and 50 years of age
(3). Thyroid nodule is a common
disease found in the thyroid, and CTL usually coexists with benign
and malignant thyroid nodules (4–6). In the
majority of cases, CTL combined with malignant thyroid nodules was
the reason for surgery and most patients underwent surgery
following an inconclusive diagnosis (7). Therefore, it may be of significant
clinical benefit to identify a method to distinguish between benign
and malign thyroid nodules.
Previously, cases of CLT with thyroid nodules were
typically diagnosed by ultrasonography (6,8,9) and fine needle aspiration biopsy (FNAB)
(10–12). However, there remains a lack of
specific sonographic features that provide a prediction value with
high sensitivity and high positivity in thyroid, which makes it
difficult to differentiate benign nodules from malignant nodules in
CLT patients. Yang et al (13)
conducted a clinical trial enrolling 1,100 patients and the authors
observed that only a specificity of 75% was confirmed by using
thyroid biopsy (13). Notably, a
study has recommended the application of multidetector computed
tomography (MDCT) in thyroid nodules diagnosis (14). However, MDCT has not previously been
used to differentiate the benign from malignant nodules thyroid
nodules in CLT patients.
In the present study, 137 thyroid nodules in 127 CLT
patients combined with benign and malignant thyroid nodules
underwent MDCT perfusion imaging. The present study retrospectively
analyzed the characteristics of nodules, including the size, solid
percentage, calcification, margin, capsule,
anteroposterior-transverse diameter ratio as well as the mode and
the degree of enhancement. In addition, the correlation between
pathological results and the computed tomography (CT) perfusion
imaging was also analyzed for assessing the differences between
benign and malignant nodules.
Subjects and methods
Subjects
A total of 127 CTL patients (10 males and 117
females; median age, 52 years, range 19–77 years) diagnosed with
thyroid nodules by palpation or B-mode ultrasound from January 2005
to December 2013 at the Tongren Hospital Affiliated to Shanghai
Jiaotong University School of Medicine (Shanghai, China) were
retrospectively reviewed in the study. The nodules were confirmed
as benign or malignant nodules by surgery. In addition, all the
patients received an MDCT scan on the thyroid less than a week
prior to surgery, and the patients did not receive any treatment
during the week.
The present study was approved by the hospital
medical ethics committees and written informed consent was obtained
from all participants.
MDCT technical parameters
The patients were placed in a supine position with
hyperextended neck. All CT perfusion images were obtained using a
MDCT scanner (Lightspeed Pro32 CT; GeneralElectric, Milwaukee, WI,
USA) with the following parameters: 2.5-mm section thickness and
section slice, and 30–35 sec scan delay at an arterial phase while
50–60 sec scan delay at a parenchymal phase. The scanning range was
initially performed in a craniocaudal direction from the superior
border of hyoid to the aorta arch, including the entire thyroid
mass (mean coverage, 250 mm). Each patient received 100 ml of
nonionic contrast material (iohexol, 300 mg/ml, GE Healthcare) via
a peripheral arm vein at a flow rate of 2.5–3 ml/s and a dose of
1.5 ml/kg. After 2 sec, axial images were reconstructed at 1.25-mm
increments at the end of the scan and then the coronal section was
reconstructed.
Image analysis
Hard copies were analyzed by two radiologists who
recorded every nodule with regard to diagnostic confidence and
nodule size to correct classification into one of the size classes.
Disagreement would be resolved through discussing with another
experienced radiologist. The CT features were defined as follows
through combining the studies by Kim et al (12) and Kang et al (15): i) Solid vs. cystic: Completely solid
(>95% solid), predominantly solid (50–95% solid), predominantly
cystic (1–49% solid); ii) calcification: Micro-calcification
(diameter ≤2 mm), macro-calcification (diameter >2 mm), eggshell
(arc-shaped or semi arc-shaped) or mixed (coexist with multiple
calcification) in morphology; internal or peripheral calcification
in position; iii) margins: Well-defined or ill-defined at
enhancement stage; iv) capsule: Intact, incomplete, or unclear and
none; v) enhancement mode: Completely cystic, not enhanced,
homogeneous enhancement or heterogeneous enhancement; vi) lesion
size: Maximum diameter of the lesion; vii) the ratio of
anteroposterior to transverse diameter; viii) enhancement degree:
The net enhancement value at arterial or venous phase was
calculated by enhanced CT scan value subtracting plain CT scan
value. The measurement of the CT values should avoid regions
presented with cystic change, necrosis, calcification and
vessels.
Hematoxylin and eosin (HE)
staining
The nodule tissues of the CLT patients were fixed in
10% formalin and embedded in paraffin. Next, the tissues were cut
into 5-µm consecutive sections and stained with HE. The images were
observed under a Leica DM2500 fluorescence microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
All the statistical analyses were performed using
SPSS 17.0 software (SPSS, Chicago, IL, USA). The Chi-square test
was used to analyze the differences among enumeration data.
Student's t-test was used for data measurement after
homogeneity test of variance. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Pathological features of the
lesions
Among the CTL patients, 40 cases coexisted with
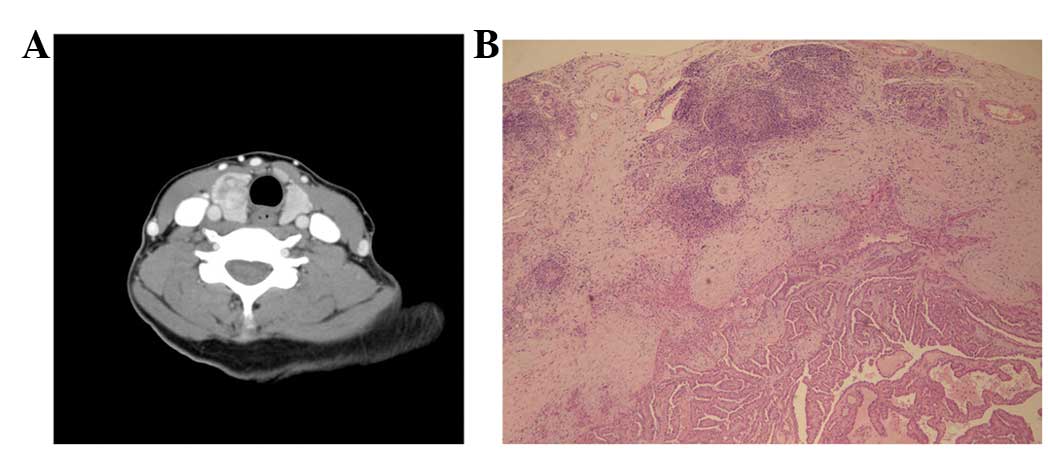
malignant nodules, including 26 cases of papillary cancer (Fig. 1A and B), 10 cases of microcarcinoma, 1
case of papillary cancer combined with microcarcinoma, 2 cases of
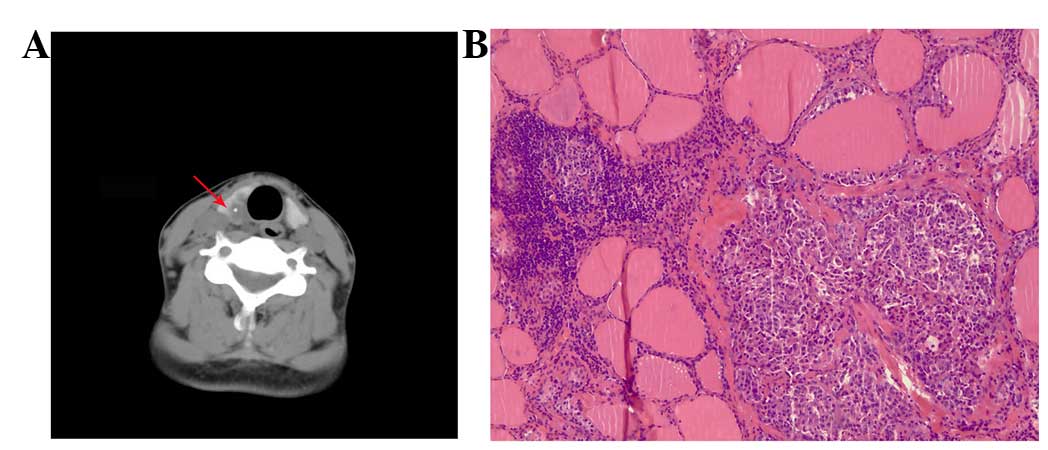
medullary cancer (Fig. 2A and B), and
1 case of lymphoma. Totally, 87 CLT patients coexisted with benign
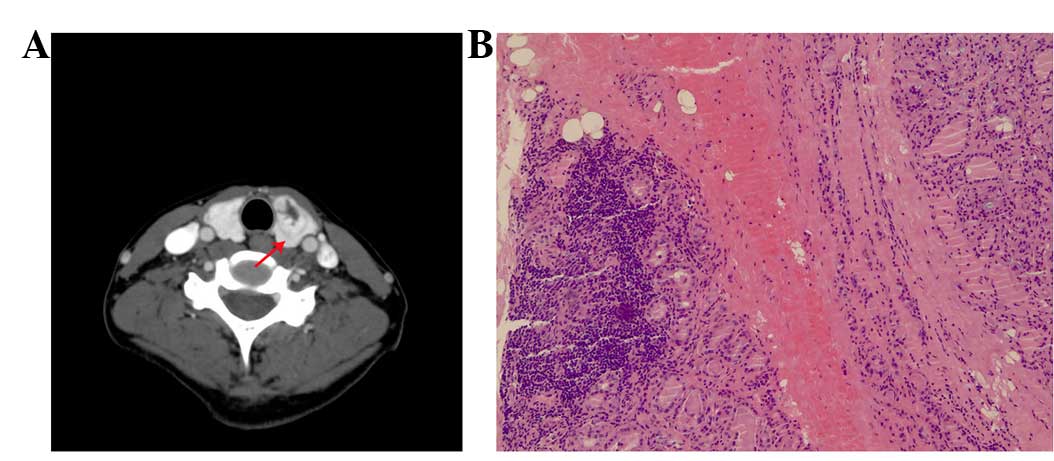
nodules, including 63 cases of follicular adenoma (Fig. 3A and B), 7 cases of nodules goiter and
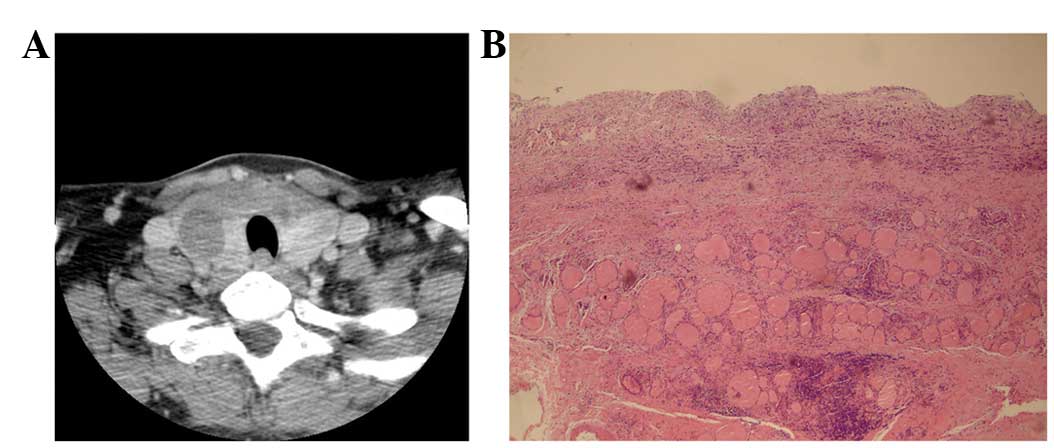
17 cases of nodules hyperplasia (Fig. 4A
and B). In addition, among the 40 patients with malignant
nodules, including 3 papillary cancer patients, 1 medullary cancer
patient and 5 microcarcinoma patients combined with benign
follicular adenoma at contralateral side, were also divided into
benign group. Finally, 137 nodes from 127 cases were enrolled in
this study, including 97 nodules from benign group and 40 nodules
from malignant group.
Comparison of benign and malignant
nodules within CLT
As demonstrated in Table
I, there were significant differences in the CT features,
including completely solid, predominantly cystic, calcification
rate, micro-calcification, peripheral calcification, internal
calcification, well or ill-defined margins, intact or incomplete
capsule and homogenous enhancement between benign and malignant
group (P<0.05). Most of the malignant nodules showed completely
solid composition (31/40), and none of samples among malignant was
predominantly cystic. However, only 33% (32/97) of the
predominantly cystic composition were found in benign nodules.
Compared with the benign nodules, calcification was more frequently
observed in malignant nodules (37.5 vs. 21.6%), mainly exhibiting
as micro-calcification (22.5 vs. 5.2%) and internal calcification
(88.2 vs. 19.0%). Well-defined (76.3%) and intact capsule (63.9%)
were present in most of benign nodules. There was a trend towards
ill-defined margin (75.0%), unclear (65.0%) or incomplete (27.5%)
capsule in most of malignant nodules. Among malignant nodules, 7
presented with incomplete enhanced ring. Among the heterogeneous
benign nodules, 16 were peninsula-like enhancement which has
irregular low density area in the periphery region or significantly
enhanced nodules in the central region, while only 7 patients in
the malignant group presented peninsula-like enhancement. In
addition, the incidence of malignant nodules was significantly
higher than the benign nodules (55.0 vs. 25.8%, P<0.05) among
the homogeneous nodules.
 | Table I.Comparison of multi-slice computer
tomography features of benign and malignant nodules. |
Table I.
Comparison of multi-slice computer
tomography features of benign and malignant nodules.
| Nodule
characteristic | Benign (%) | Malignant (%) | χ2 value | P-value |
|---|
| Solid vs. cystic |
|
|
|
|
|
Completely solid | 32.0 (31/97) | 77.5 (31/40) | 23.709 | <0.001 |
|
Predominantly solid | 35.0 (34/97) | 22.5 (9/40) | 2.072 | 0.150 |
|
Predominantly cystic | 33.0 (32/97) | 0 (0/40) | 17.217 | <0.001 |
| Calcifications |
|
|
|
|
|
Incidence | 21.6 (21/97) | 37.5 (17/40) | 6.143 | 0.013 |
|
Micro | 5.2 (5/97) | 22.5 (11/40) | 11.629 | 0.001 |
|
Macro | 8.2 (8/97) | 7.5 (3/40) | 0.000 | 1.000 |
|
Eggshell | 2.1 (2/97) | 2.5 (1/40) | 0.000 | 1.000 |
|
Mixed | 6.2 (6/97) | 5 (2/40) | 0.000 | 1.000 |
|
Internal | 19.0 (4/21) | 88.2 (15/17) | 17.989 | <0.001 |
|
Peripheral | 81.0 (17/21) | 11.8 (2/17) | 17.989 | <0.001 |
| Margins |
|
|
|
|
|
Well-defined | 76.3 (74/97) | 25.0 (10/40) | 31.408 | <0.001 |
|
Ill-defined | 23.7 (23/97) | 75.0 (30/40) | 31.408 | <0.001 |
| Capsule |
|
|
|
|
|
Intact | 63.9 (62/97) | 7.5 (3/40) | 36.152 | <0.001 |
| Unclear
or none | 36.1 (35/97) | 65.0 (26/40) | 9.588 | 0.002 |
|
Incomplete | 0 (0/97) | 27.5 (11/40) | 25.399 | <0.001 |
| Enhancement |
|
|
|
|
|
Completely cystic | 21.6 (21/97) | 0 (0/40) | 10.228 | 0.001 |
|
Homogenous | 25.8 (25/97) | 55 (22/40) | 10.734 | 0.001 |
|
Heterogeneous | 52.8 (51/97) | 37.5 (15/40) | 2.579 | 0.108 |
As presented in Table
II, the size of the benign nodules was significantly larger
than the malignant nodules, but the ratio of anteroposterior to
transverse diameter, anteroposterior to net enhancement degree at
arterial or venous phase were not significantly different between
the two groups (P>0.05).
 | Table II.Comparison of morphological
characteristics of benign and malignant nodules (mean ± standard
deviation). |
Table II.
Comparison of morphological
characteristics of benign and malignant nodules (mean ± standard
deviation).
| Classification of
nodules | Nodule
sizea | Anteroposterior and
transverse diameter ratio | Plain
scanb | Net enhancement
degree at arterial phaseb | Net enhancement
degree at venous phaseb |
|---|
| Benign | 15.5±8.1 | 1.10±0.25 | 71.1±22.1 | 86.8±40.5 | 48.6±27.6 |
| Malignancy | 11.8±6.1 | 1.03±0.14 | 66.5±23.1 | 88.3±51.4 | 54.1±27.5 |
| t value | −2.544 | 1.618 | −1.024 | 0.165 | 1.01 |
| P-value | 0.012 | 0.112 | 0.308 | 0.8691 | 0.315 |
Discussion
Although the association between CLT and thyroid
cancer remains controversial (16),
emerging evidence has demonstrated that there is an increased risk
of papillary thyroid carcinoma for patients with CLT (17–19). In
the present study, among patients with CLT, the incidence of
malignancy was 31.5% (40/127), including 26 papillary cancer cases
and 11 microcarcinoma cases. Notably, malignant nodules were
normally characterized by having a completely solid composition,
internal calcification, ill-defined margin, an unclear or
incomplete capsule, while benign nodules were more likely to have
peripheral calcification, well-defined margins and an intact
capsule. In addition, the size of the benign nodules was
significantly larger than the malignant nodules.
In accordance with the findings by MDCT, sonographic
analysis of benign and malignant nodules in diffuse hashimoto
thyroiditis patients has demonstrated that malignant nodules were
more likely to be solid and hypoechoic (1). In addition, Hashimoto's thyroiditis
cases associated with thyroid nodular disease were also represented
by solid composition, hypoechogenicity and micro-calcifications
(6). A previous study by Kim et
al (12) reported the sonographic
characteristics of micro-calcifications, an irregular or
microlobulated margin, marked hypoechogenicity, and a shape that
was more tall than it was wide as the criteria for malignant
nodules and obtained 93.8% sensitivity and 66% specificity. A
significant difference was not observed on length-to-width ratio
using MDCT, which might attribute to technological disparity. Thus,
it may be inferred that the MDCT features on solid composition and
margin shape would be helpful for preoperative diagnosis for CTL
patients.
The present findings differed from that of a
previous study, which concluded that on diameter comparison, no
significant association was found between malignancy and a nodule
size of >15 mm using FNAB (20).
The higher incidence of microcarcinoma in the present study and
timely surgery on suspected carcinomas may contribute to the
difference in conclusions. Generally, the density is homogenous for
small nodules, but the density is gradually heterogeneous and
cystic changed as the size enlarged, which was also confirmed by
the enhancement results in the present study. However, no
significant differences were observed in the CT value of plain
scan, enhancement degree at arterial or venous phase in CTL
patients with benign or malignant nodules, indicating that the
enhancement degree on the parenchyma demonstrated no significant
value in differentiating benign from malignant nodules.
Traditionally, FANB has been recognized as the
standard test to determine whether surgical removal of a detected
nodule. Given the low risk for thyroid cancer, the technology of
FNAB performed on all nodules detected by imaging was not feasible
or advisable (21,22). Thereafter, a serious of imaging
modalities was developed, including ultrasound, carotid duplex
scan, CT, magnetic resonance imaging (MRI) or positron emission
tomography (PET). However, mixed results were reported using these
imaging modalities. For example, Mitchell et al (23) reported that 18FDG-PET with sensitivity
of 60% and a specificity of 91% based on 48 malignant lesions and 33
benign lesions, which performed similarly with sonographic
diagnosis (91.4–92.5% specificity) when the ratio of
anteroposterior to transverse diameter ratio was >1. Basharat
et al (24) has compared
thyroid scan with FNAB and suggests fine needle aspiration cytology
is more specific than sensitive whereas thyroid scan is more
sensitive than specific in detecting thyroid malignancy. In 2011, a
study reported the highest values for sensitivity and specificity
based on (nodule/cord SI)/nodule apparent diffusion coefficient
ratio using DW-MRI limited on 44 patients with nodules (25). Despite the high values for sensitivity
and specificity, a missed malignancy would cause huge damage for
the patient. However, to establish general criteria for
differentiating malignant nodules from benign nodules according to
the different backgrounds of CTL patients is difficult. Therefore,
CT features that can differentiate between benign and malignant
nodules are required.
Certain limitations of the present study should be
discussed. Firstly, no gold standard for detecting nodules was
designed in the study. Although we have defined the criteria for
each feature through combining the previous studies (12,15), the
criteria have limited the rigorous of the result. Secondly,
malignant and benign thyroid nodules were just confirmed by
surgery, and no follow-up was performed. Therefore, the possibility
that the benign nodule may develop into the malignant nodule could
not be excluded. Moreover, the conclusion from the small sample
size of the enrolled cases may limit the reliability of the
conclusion, and MDCT characterization for benign and malign nodules
on a larger sample size is needed.
In conclusion, the findings suggest that CLT
patients with malignant nodules mainly present with features of
solid composition, ill-defined margin, unclear or no capsule, or
micro-calcifications, which could be used as the diagnosis method
in advance for FANB. However, further study based on a larger
sample size and cases with different backgrounds is required in
order to confirm the above method is suitable to differentiate
benign and malign nodules.
References
|
1
|
Anderson L, Middleton WD, Teefey SA,
Reading CC, Langer JE, Desser T, Szabunio MM, Mandel SJ, Hildebolt
CF and Cronan JJ: Hashimoto thyroiditis: Part 2, sonographic
analysis of benign and malignant nodules in patients with diffuse
Hashimoto thyroiditis. AJR Am J Roentgenol. 195:216–222. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson L, Middleton WD, Teefey SA,
Reading CC, Langer JE, Desser T, Szabunio MM, Hildebolt CF, Mandel
SJ and Cronan JJ: Hashimoto thyroiditis: Part 1, sonographic
analysis of the nodular form of Hashimoto thyroiditis. AJR Am J
Roentgenol. 195:208–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakiyama R: Thyroiditis: A clinical
review. Am Fam Physician. 48:615–621. 1993.PubMed/NCBI
|
|
4
|
Mazokopakis EE, Tzortzinis AA,
Dalieraki-Ott EI, Tsartsalis AN, Syros PK, Karefilakis CM,
Papadomanolaki MG and Starakis IK: Coexistence of Hashimoto's
thyroiditis with papillary thyroid carcinoma. A retrospective
study. Hormones (Athens). 9:312–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Dai J, Wu T, Yang N and Yin Z:
The study of the coexistence of Hashimoto's thyroiditis with
papillary thyroid carcinoma. J Cancer Res Clin Oncol.
140:1021–1026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zosin I and Balaş M: Clinical,
ultrasonographical and histopathological aspects in Hashimoto's
thyroiditis associated with malignant and benign thyroid nodules.
Endokrynol Pol. 64:255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reading CC, Charboneau JW, Hay ID and Sebo
TJ: Sonography of thyroid nodules: A ‘classic pattern’ diagnostic
approach. Ultrasound Q. 21:157–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Xia Y, Jiang YX, Dai Q and Li XY:
Likelihood ratio-based differentiation of nodular Hashimoto
thyroiditis and papillary thyroid carcinoma in patients with
sonographically evident diffuse hashimoto thyroiditis: Preliminary
study. J Ultrasound Med. 31:1767–1775. 2012.PubMed/NCBI
|
|
9
|
Gul K, Dirikoc A, Kiyak G, Ersoy PE, Ugras
NS, Ersoy R and Cakir B: The association between thyroid carcinoma
and Hashimoto's thyroiditis: The ultrasonographic and
histopathologic characteristics of malignant nodules. Thyroid.
20:873–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cronan JJ: Thyroid nodules: Is it time to
turn off the US machines? Radiology. 247:602–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frates MC, Benson CB, Charboneau JW, Cibas
ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB,
Goellner JR, et al: Management of thyroid nodules detected at US:
Society of radiologists in ultrasound consensus conference
statement. Radiology. 237:794–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim EK, Park CS, Chung WY, Oh KK, Kim DI,
Lee JT and Yoo HS: New sonographic criteria for recommending
fine-needle aspiration biopsy of nonpalpable solid nodules of the
thyroid. AJR Am J Roentgenol. 178:687–691. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang GC, Liebeskind D and Messina AV:
Ultrasound-guided fine-needle aspiration of the thyroid assessed by
Ultrafast Papanicolaou stain: Data from 1135 biopsies with a two-to
six-year follow-up. Thyroid. 11:581–589. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishigaki S, Shimamoto K, Satake H, Sawaki
A, Itoh S, Ikeda M, Ishigaki T and Imai T: Multi-slice CT of
thyroid nodules: Comparison with ultrasonography. Radiat Med.
22:346–353. 2004.PubMed/NCBI
|
|
15
|
Kang HW, No JH, Chung JH, Min YK, Lee MS,
Lee MK, Yang JH and Kim KW: Prevalence, clinical and
ultrasonographic characteristics of thyroid incidentalomas.
Thyroid. 14:29–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anil C, Goksel S and Gursoy A: Hashimoto's
thyroiditis is not associated with increased risk of thyroid cancer
in patients with thyroid nodules: A single-center prospective
study. Thyroid. 20:601–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim KW, Park YJ, Kim EH, Park SY, Park Do
J, Ahn SH, Park Do J, Jang HC and Cho BY: Elevated risk of
papillary thyroid cancer in Korean patients with Hashimoto's
thyroiditis. Head Neck. 33:691–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Repplinger D, Bargren A, Zhang YW, Adler
JT, Haymart M and Chen H: Is Hashimoto's thyroiditis a risk factor
for papillary thyroid cancer? J Surg Res. 150:49–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiore E, Rago T, Latrofa F, Provenzale MA,
Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L,
et al: Hashimoto's thyroiditis is associated with papillary thyroid
carcinoma: Role of TSH and of treatment with L-thyroxine. Endocr
Relat Cancer. 18:429–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahimi M, Farshchian N, Rezaee E,
Shahebrahimi K and Madani H: To differentiate benign from malignant
thyroid nodule comparison of sonography with FNAC findings. Pak J
Med Sci. 29:77–80. 2013.PubMed/NCBI
|
|
21
|
Lawrence W and Kaplan BJ: Diagnosis and
management of patients with thyroid nodules. J Surg Oncol.
80:157–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khalid AN, Hollenbeak CS, Quraishi SA, Fan
CY and Stack BC: The cost-effectiveness of iodine 131 scintigraphy,
ultrasonography, and fine-needle aspiration biopsy in the initial
diagnosis of solitary thyroid nodules. Arch Otolaryngol Head Neck
Surg. 132:244–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitchell JC, Grant F, Evenson AR, Parker
JA, Hasselgren PO and Parangi S: Preoperative evaluation of thyroid
nodules with 18FDG-PET/CT. Surgery. 138:1166–1175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Basharat R, Bukhari MH, Saeed S and Hamid
T: Comparison of fine needle aspiration cytology and thyroid scan
in solitary thyroid nodule. Patholog Res Int.
2011:7540412011.PubMed/NCBI
|
|
25
|
Mutlu H, Sivrioglu AK, Sonmez G, Velioglu
M, Sildiroglu HO, Basekim CC and Kizilkaya E: Role of apparent
diffusion coefficient values and diffusion-weighted magnetic
resonance imaging in differentiation between benign and malignant
thyroid nodules. Clin Imaging. 36:1–7. 2012.
|