Introduction
A large number of studies have demonstrated that
inflammatory process, mediated by the complex cytokine network, is
associated with a variety of tumors (1,2).
Hepatocellular carcinoma (HCC), a frequently occurring malignancy
with a high rate of mortality, is considered to be associated with
the development of chronic inflammation from hepatitis B and C
infection (3,4); however, the crucial molecular pathways
that permit communication between abnormally HCC and various
inflammatory cells are poorly understood. Interleukin-33 (IL-33), a
newly-discovered cytokine, belongs to the IL-1 family (5). By binding to the homolog of
sulfotransferase (ST2) receptor, IL-33 activates nuclear factor κB
(NFκB) and mitogen-activated protein kinase (MAPK) signaling
pathways, thereby regulating variety of inflammatory and immune
reactions (5,6). In addition, IL-33 also acts as a
chromatin-associated factor in the nucleus, thereby exhibiting
transcriptional repressor properties for the regulation of gene
transcription (7). The dual effects
of IL-33 has attracted attention in the study of tumor
pathogenesis. In vitro experiments have confirmed that
carcinoma-associated fibroblasts (CAFs), a major type of
tumor-surrounding stromal cell, promoted cancer invasiveness via
paracrine and autocrine effects on microenvironmental IL-33
signaling (8). Experiments on animal
models have demonstrated that the activation of IL-33/ST2 pathway
promoted breast cancer growth and metastases by facilitating
intratumoral accumulation of immunosuppressive and innate lymphoid
cells (9). Serum IL-33 levels have
been considered as a poor prognosis biomarker for a number of types
of tumor, including gastric cancer (10), nonsmall-cell lung cancer (11), and breast cancer (12). In contrast to these findings, other
studies have reported IL-33 as a potent inducer of anticarcinogenic
immunity, which results in enhanced activation of cytotoxic CD8+
cells (13). Thus, the association
between IL-33 expression and tumor development appears
controversial. In terms of liver disease, studies have demonstrated
that hepatocytes strongly expressed IL-33 in concanavalin A-induced
hepatitis model (14). Together with
upregulation of other proinflammatory factors, the increase of
serum IL-33 serves a role in the development of chronic viral
hepatitis (15), hepatic fibrosis
(16) and HCC (17). These findings indicated that IL-33 may
be important in promoting the oncogenesis and development of HCC.
However, other previous studies questioned the effect of IL-33 in
HCC patients (18) or even rendered
the hepatoprotective role of IL-33 in liver disease (19). The present study investigated the
expression and localization of IL-33 in HCC and non-cancerous liver
(NCL) tissues during different conditions, including normal liver,
chronic hepatitis, and liver cirrhosis by immunohistochemistry. In
addition, the present study also analyzed the correlation between
IL-33 and clinicopathological parameters of HCC. The objective of
the present study was to investigate the role of IL-33 in the
oncogenesis and progression of HCC, which may provide novel
histological data and theoretical basis for HCC inflammatory
pathogenesis.
Materials and methods
Samples
A total of 76 cases of HCC following surgical
resection were collected from the First Affiliated Hospital of
Bengbu Medical College (Bengbu, China) between January 2008 and
December 2013. The patients received no treatment preoperatively,
and completed clinical data was obtained. The pathological grading
was defined by Edmondson and Steiner classification (20): Grade I–II tumors accounted for 63% (48
samples), and grade III–IV tumors accounted for 37% (28 samples) of
the patient samples. The HCC study population included 61 males and
15 females. The age of participants ranged between 22–76 years,
with a median age of 50 years. For the 36 para-carcinoma controls,
tissues adjacent to carcinoma, which were diagnosed as normal by
the pathological methods, were taken from tissue ≥5 cm away from
the tumor in HCC patients.
During the same period, 33 cases (23 males and 10
females) of cirrhosis were also collected. The age of participants
ranged between 20–77 years, with a median age of 47 years. A total
of 30 cases (21 males and 9 females) of hepatitis were also
collected. The age ranged from 18–49 years, with a median age of
33.5 years. Chronic hepatitis and liver cirrhosis was
pathologically confirmed by needle biopsy. In addition, 20 cases
(11 males and 9 females) of normal liver tissue (specimens
following traumatic liver resection, or from healthy subjects
following accidental death) were used as control. The age of
participants ranged between 21–73 years, with a median age of 54.5
years. Approval was obtained from the medical ethics committee of
Bengbu Medical College (Bengbu, China), and written informed
consent was obtained from the patient or their immediate family
members.
Immunohistochemistry
All specimens were embedded in paraffin and were cut
into 4-µm sections by a microtome. Immunohistochemical staining was
performed according to previously described standard protocols
(21,22). More specifically, tissue sections were
baked at 62°C for 30 min, deparaffinized in xylene (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
and rehydrated in graded ethanol prior to pretreatment with 3%
hydrogen peroxide/methanol solution (Sinopharm Chemical Reagent
Co., Ltd., Beijing, China) for 15 min to block endogenous
peroxidase activity. The sections were then washed 3 times in PBS,
and heated in a microwave oven in the presence of 0.01 M citric
acid buffer pH 6.0 (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd.) for 15 min, and gradually cooled down to room
temperature. Sections were subsequently incubated with goat
anti-human IL-33 polyclonal antibody (1:200 dilution; catalog no.
AF3625; R&D Systems, Abingdon, UK) at 4°C overnight. The
sections were then washed 3 times with PBS, incubated for 20 min at
room temperature in a humidified chamber with reagent 1 (polymer
auxiliary agent; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.), washed again with PBS, and incubated for 30 min at 37°C with
horseradish peroxidase-conjugated anti-goat IgG (catalog no.
PV-9003; ready to use; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.). The slides were stained using a DAB
staining kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China),
counterstained with hematoxylin (Beyotime Institute of
Biotechnology, Shanghai, China) at 37°C for 3–5 min, and mounted.
The negative control slides were processed by omitting the primary
antibody, but including all other steps of the procedure. Protein
positive staining and cellular localization were observed and
images were captured by light microscope (Olympus BH-12, Tokyo,
Japan).
Evaluation of staining
Microscopic analysis of IL-33 was assessed
independently by two observers in a blinded manner. There was no
discrepancy between the two investigators. The cells with nuclear
and/or cytoplasmic marking were considered positive, and subjective
estimation was judged according to the criteria described by
Goncalves et al (23). Nuclear
IL-33 expression was scored by determining the percentage of nuclei
with IL-33 immunoreactivity, and was grouped as follows: Low
expression (<50% positive cells) and high expression (≥50% of
the cells showing nuclear immunoreactivity). For cytoplasmic IL-33
staining, the positive cells were also grouped as low expression
(weak pale brown staining) and high expression (strong dense brown
staining) cells. The result was defined as negative if neither the
nucleus or cytoplasm was stained. A total of 5 visual fields were
chosen randomly by high-power lens (×40 magnification) with 3
replicates, and the final evaluation was derived from the average
of staining results either in the nucleus or cytoplasm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RNA isolation and RT-PCR procedure was conducted as
previously described (24). Briefly,
total RNA was isolated from flash-frozen liver tissues using the
TriZOL reagent (Invitrogen; Thermofisher Scientific, Inc., Waltham,
MA, USA) and then converted to complementary DNA (cDNA) with avian
myeloblastosis virus reverse transcriptase (Promega Corporation,
Madison, WI, USA). A total of 2 µl of cDNA was amplified in a 20 µl
standard PCR reaction. The PCR was initiated at 94°C for 3 min
followed by 35 cycles consisting of 45 sec at 94°C, 45 sec at 55°C,
and 45 sec at 72°C, with the final cycle extended to 10 min at
72°C, followed by termination at 4°C. The following primers were
used: Human IL-33, F 5′-TCAGGTGACGGTGTTGATGG-3′ and R
5′-ACAAAGAAGGCCTGGTCTGG-3′, product size 140 bps; Human β-actin, F
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ and R 5′-TGGCACCCAGCACAATGAA-3′,
product size 186 bps. The detection of β-actin transcripts provided
an internal control for PCR, standardizing the quantity of input
cDNA. PCR products were analyzed on an ethidium bromide-stained 2%
agarose gel.
Statistical analysis
All statistical analyses were performed using
Statistical Package for the Social Sciences (SPSS) version 17.0
statistical software (Chicago, IL, USA). The expression of IL-33 in
distinct tissue types and the association between the marker and
clinicopathological parameters were evaluated by χ2 test
and Fisher's exact test, wherever appropriate. Comparison of
numerical data was achieved with the unpaired Student's t-test.
P<0.05 indicates a statistically significant difference.
Results
IL-33 expression in HCC and NCL
tissues
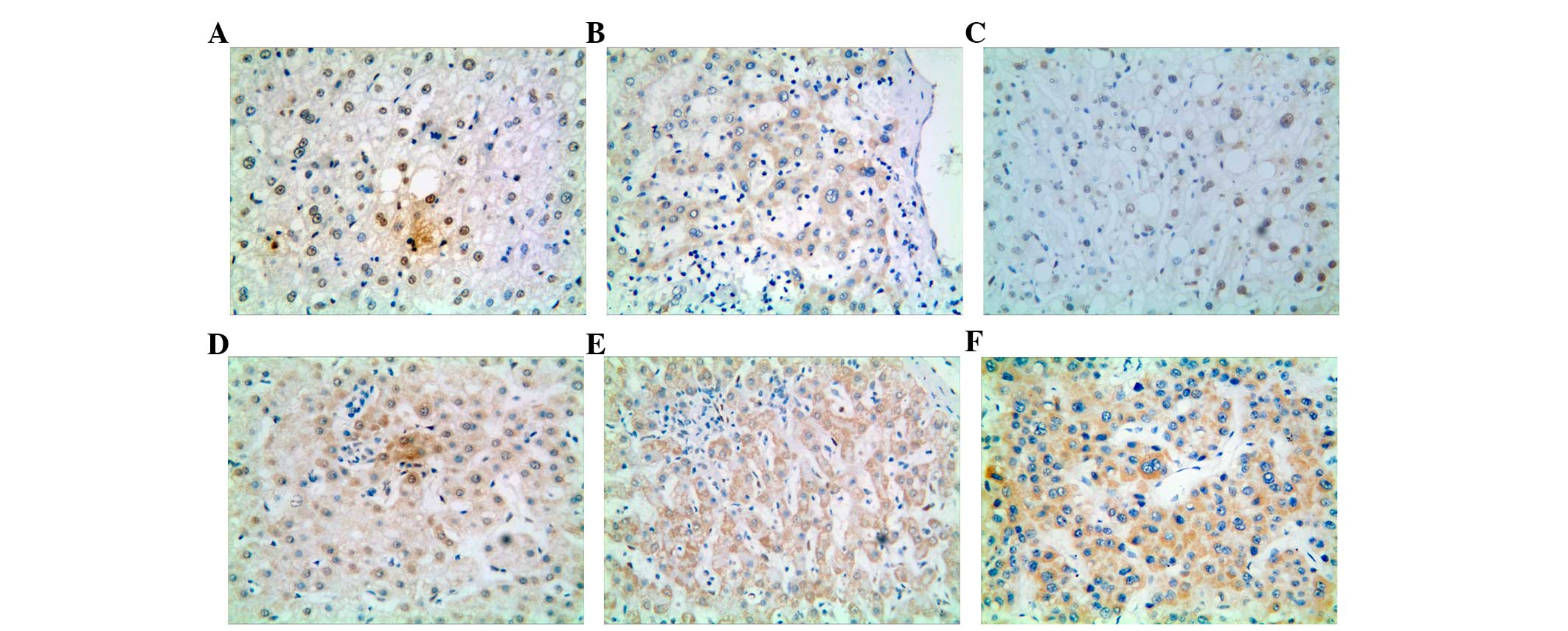
As presented in Fig.
1, IL-33 is visually located in the nucleus and cytoplasm of
hepatocytes. The positive rates in normal liver tissues, hepatitis
tissues, and cirrhosis tissues were 40.00% (8/20), 53.33% (16/30),
and 36.36% (12/33), respectively. Statistically significant
differences were not observed between these three groups
(χ2=1.965, P>0.05, Table
I). However, when compared to the total NCL tissues, the rate
of IL-33 protein expression in HCC tissues was markedly reduced to
22.37% (17/76; χ2=7.877, P=0.007, Table I).
 | Table I.Expression of IL-33 in NCL and HCC
tissues. |
Table I.
Expression of IL-33 in NCL and HCC
tissues.
|
|
| IL-33 |
|
|---|
|
|
|
|
|
|---|
| Group | n | − | + | Significance
(χ2 test) |
|---|
| HCC | 76 | 59 (77.63%) | 17 (22.37%) |
|
| NCL | 83 | 47 (56.63%) | 36 (43.37%) | P=0.007 |
| Normal liver | 20 | 12 (60.00%) | 8 (40.00%) |
|
| Hepatitis | 30 | 14 (46.67%) | 16
(53.33%)a | NS |
| Cirrhosis | 33 | 21 (63.64%) | 12 (36.36%) |
|
IL-33 localization in HCC and NCL
tissues
Nucleic and cytoplasmic staining of IL-33 was
observed in the normal liver tissue (Fig.
1A and B); whereas in hepatitic liver tissue, IL-33 expression
was only observed in the nucleus, but not in the cell membrane or
cytoplasm (Fig. 1C). However in
chronic cirrhosis liver, nucleus staining of IL-33 was observed in
only 1 case, both cytoplasmic and nuclear staining in 3 cases
(Fig. 1D), whereas 29 cases
demonstrated rich cytoplasmic expression of IL-33 (Fig. 1E). In HCC tissues, all the
IL-33-positive HCCs showed cytoplasmic staining (Fig. 1F), with only 3 cases of concurrent
nuclear staining. Statistical analysis indicated that with the
progression of liver disease from normal to hepatitis, cirrhotic,
and HCC, the localization of IL-33 gradually changes from the
nucleus to cytoplasm, with the difference in expression of
cytoplasmic IL-33 between NCL and HCC being statistically
significant (χ2=19.188, P<0.0001, Table II). In NCL, the cytoplasmic IL-33 was
expressed at a low level, whereas in HCC, the expression was
comparatively higher (χ2=7.938, P=0.010, Table III).
 | Table II.Intracytoplasmic positive staining of
IL-33 in NCL and HCC tissues. |
Table II.
Intracytoplasmic positive staining of
IL-33 in NCL and HCC tissues.
|
|
| IL-33 localized in
cytoplasm |
|
|---|
|
|
|
|
|
|---|
| Group | All positive cases
(n) | Cases
(n) | Rate (%) | Significance
(χ2 test) |
|---|
| HCC | 17 | 17 | 100.00a,b |
| NCL | 36 | 13 | 36.11 | P=0.000 |
| Normal liver | 8 | 4 | 50.00 |
|
| Hepatitis | 16 | 0 | 0.00a |
|
| Cirrhosis | 12 | 9 |
75.00b |
|
 | Table III.IL-33 expression level in NCL and HCC
tissues. |
Table III.
IL-33 expression level in NCL and HCC
tissues.
|
| Nuclear IL-33
expression | Cytoplasmic IL-33
expression |
|---|
|
|
|
|
|---|
| Group | n | High | Low | P-value | n | High | Low | P-value |
|---|
| HCC | 3 | 0 | 3 | 1.000 | 17 | 14 | 3 |
|
| NCL | 24 | 5 | 19 |
| 15 | 5 | 10 | 0.010a |
| Normal liver | 4 | 0 | 4 | 0.018a | 4 | 0 | 4 |
|
| Hepatitis | 16 | 2 | 14 |
| 0 | 0 | 0 | 0.231 |
| Cirrhosis | 4 | 3 | 1 |
| 11 | 5 | 6 |
|
IL-33 expression and localization in
carcinoma and para-carcinoma tissues
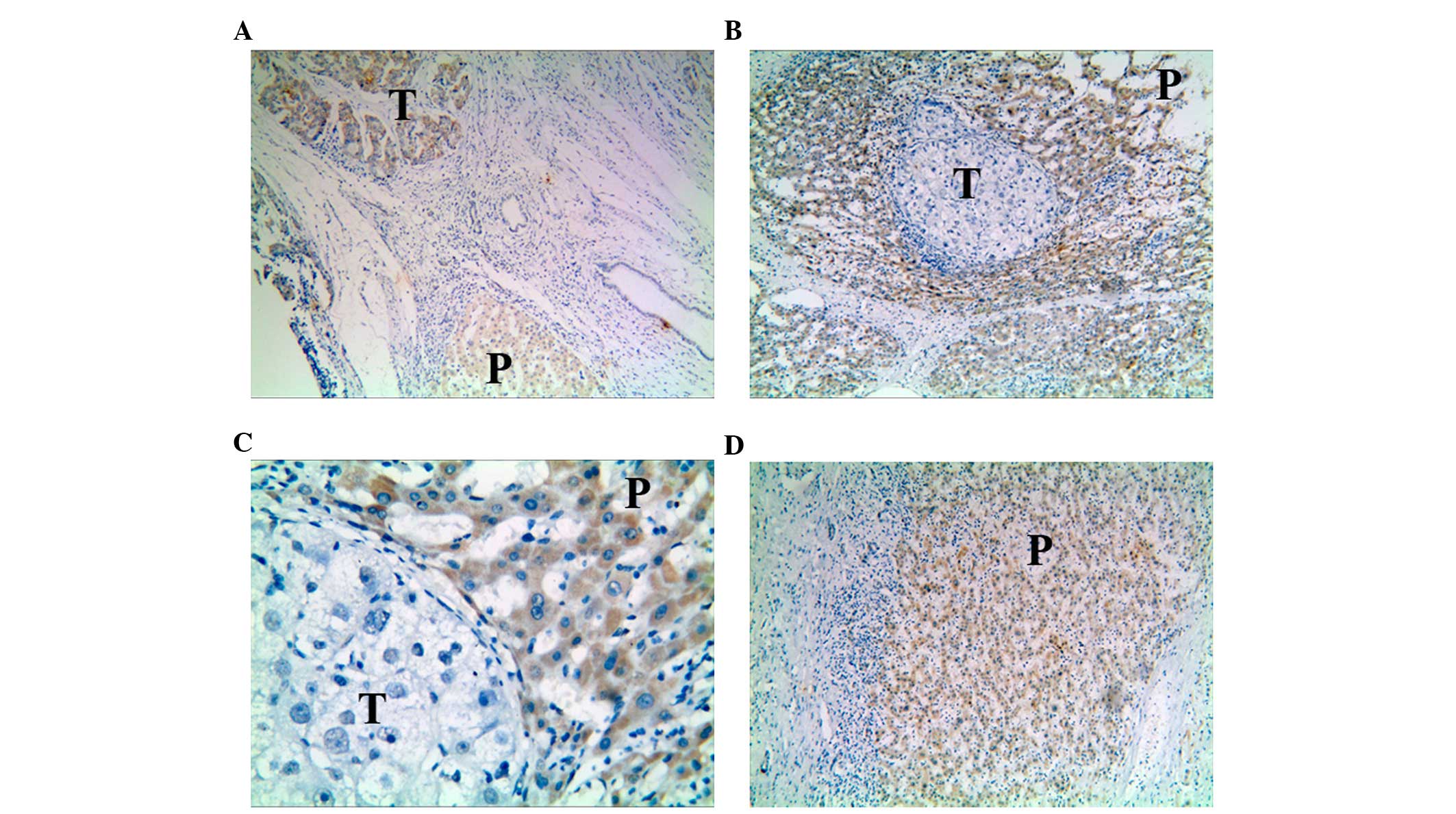
When comparing the expression of IL-33 in carcinoma
and para-carcinoma tissues, it was observed that when cancer cells
were stained positive for IL-33 protein, positive staining was also
detected in para-carcinoma tissues (Fig.
2A). IL-33 in para-carcinoma tissues was also noted in a
proportion of the specimens for which the carcinoma cells exhibited
negative IL-33 expression (Fig. 2B and
C). The positive rate of IL-33 expression in para-carcinoma
tissues was as high as 58.33% (21/36; χ2=14.095,
P<0.0001, Table IV). The staining
of IL-33 in the two types of liver tissues was mostly observed in
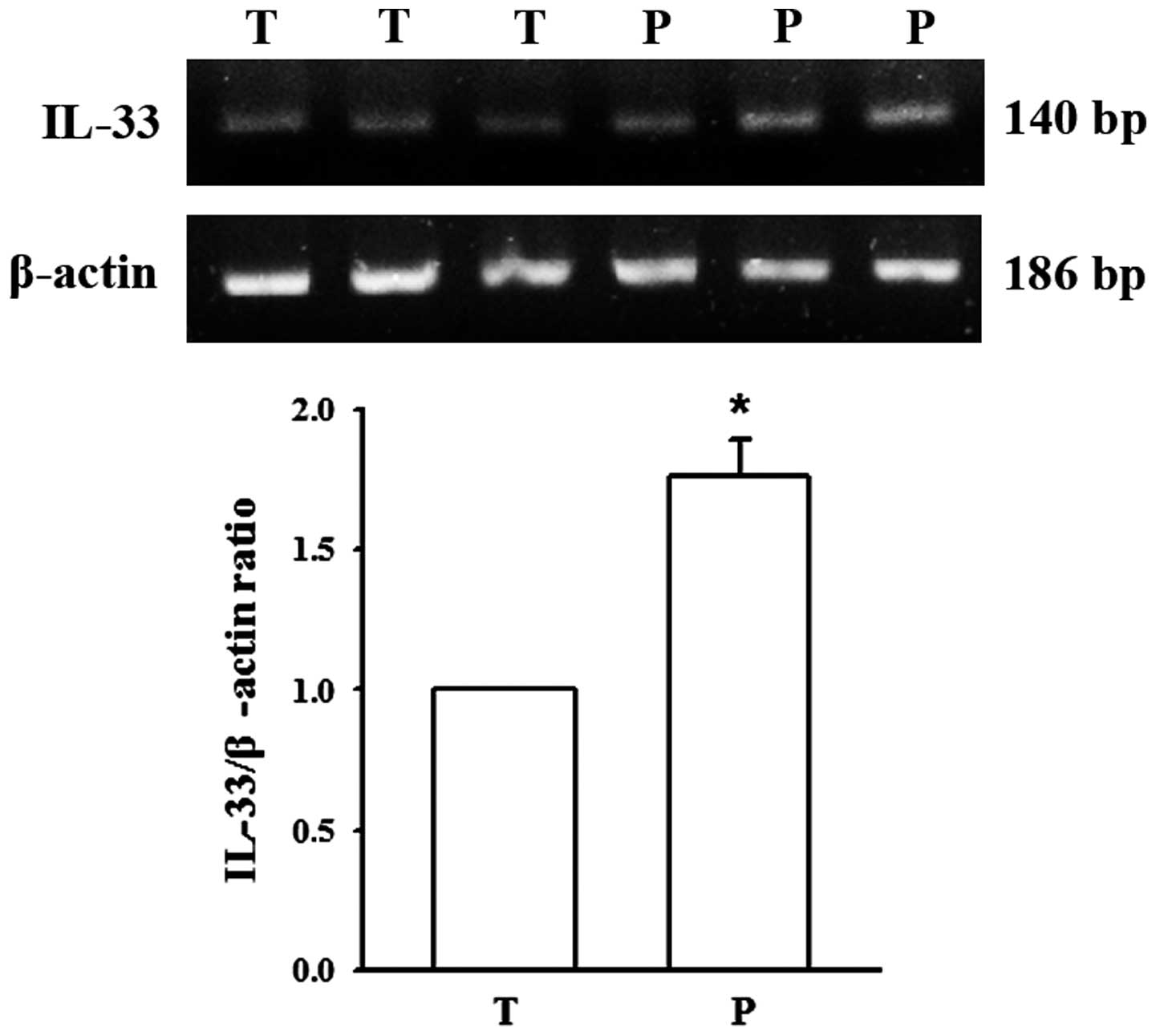
the cytoplasm (Fig. 2). To verify the
results of immunohistochemistry, IL-33 mRNA expression was further
assessed by RT-PCR. The results indicated that IL-33 mRNA levels
were significantly higher in adjacent para-carcinoma tissues
compared with primary liver carcinoma tissues (P<0.01, Fig. 3).
 | Table IV.Expression of IL-33 in carcinoma and
para-carcinoma liver tissues. |
Table IV.
Expression of IL-33 in carcinoma and
para-carcinoma liver tissues.
|
|
| IL-33 |
|
|
|---|
|
|
|
|
|
|
|---|
| Group | n | − | + | χ2
value | P-value |
|---|
| Carcinoma | 76 | 59 (77.63%) | 17 (22.37%) | 14.095 | 0.000a |
| Para-carcinoma | 36 | 15 (41.67%) | 21 (58.33%) |
Association between IL-33 expression
and HCC clinical pathological characteristics
The expression of IL-33 in different subgroups was
compared and is summarized in Table
V. From the results, it was inferred that IL-33 status was not
associated with patient age, gender, tumor size, TNM stage,
cirrhosis or hepatitis background, lymph node metastasis, or
intrahepatic vascular embolism (P>0.05); but it was associated
with histological grade (χ2=5.918, P=0.021). In
addition, it was observed that histological grade and IL-33
positive expression were negatively correlated (r=−0.279, P=0.015).
Notably, among all the IL-33-positive HCCs, the only 3 cases that
exhibited both cytoplasmic and nuclear staining, belonged to I–II
histological grade.
 | Table V.Association between IL-33 expression
and clinicopathological parameters. |
Table V.
Association between IL-33 expression
and clinicopathological parameters.
|
|
| IL-33 |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | − | + | χ2
value | P-value |
|---|
| Age (years) |
|
|
| 0.079 | 0.746 |
|
<60 | 60 | 47 | 13 |
|
|
|
≥60 | 16 | 12 | 4 |
|
|
| Gender |
|
|
| 0.962 | 0.498 |
|
Male | 61 | 46 | 15 |
|
|
|
Female | 15 | 13 | 2 |
|
|
| Tumor size
(cm) |
|
|
| 0.045 | 1.000 |
| ≤5 | 43 | 33 | 10 |
|
|
|
>5 | 33 | 26 | 7 |
|
|
| Edmondson type |
|
|
| 5.918 | 0.021a |
|
I–II | 48 | 33 | 15 |
|
|
|
III–IV | 28 | 26 | 2 |
|
|
| TNM stage |
|
|
| 1.461 | 0.365 |
|
I–II | 54 | 40 | 14 |
|
|
|
III–IV | 22 | 19 | 3 |
|
|
| Cirrhosis or
hepatitis background |
|
|
| 1.256 | 0.500 |
|
Present | 60 | 45 | 15 |
|
|
|
Absent | 16 | 14 | 2 |
|
|
| Lymph node
metastasis |
|
|
| 0.700 | 0.723 |
|
Negative | 62 | 47 | 15 |
|
|
|
Positive | 14 | 12 | 2 |
|
|
| Intrahepatic
vascular embolism |
|
|
| 0.089 | 1.000 |
|
Present | 20 | 16 | 4 |
|
|
|
Absent | 56 | 43 | 13 |
|
|
Discussion
Chronic inflammation serves a key role in the
development of liver tumor, particularly for HCC (3,4). HCC
develops as a result of various chronic liver injuries, such as
viral hepatitis and alcoholic hepatitis, which is vital mechanism
of liver injury repair (25).
However, during the repair of liver injury by inflammation,
additional reactions develop simultaneously, including hepatic
fibrosis and cirrhosis. These reactions contribute to the growth
and metastasis of tumor, where inflammatory cytokine-mediated
abnormal signal transduction serves a major role. IL-33, which was
first separated from endothelial cells by Baekkevold, was
originally called ‘nuclear factor derived of endothelial cell’
(26). It was further discovered as a
novel cytokine belonging to the IL-1 family, when comparing the
homology of the IL-33 with that of IL-1 (5). Therefore, IL-33 is a dual-function
protein that acts as an intracellular nuclear factor and a secreted
cytokine. IL-33 has been demonstrated as an abundant
chromatin-associated factor in the nucleus of endothelial cells,
where it exhibits transcriptional repressor properties (7). Notably, IL-33 has also been identified
as the natural ligand for ST2 receptor (5,27), thereby
activating NF-κB and other downstream molecules, which also
explains why IL-33 and IL-1 share similar receptor signaling
pathways (28). Currently, it is
proposed that IL-33 is released upon cellular injury as a 30-kDa
molecule (full-length IL-33) and is processed into less active, but
more mature forms of 20–22 kDa units by caspase cleavage (29,30).
Research has indicated that the full-length IL-33 precursor,
located in the nucleus, functions as a nuclear factor with
transcriptional regulatory activity, while the mature IL-33 in
cytoplasm may be involved in inflammatory reaction (31). In cells which express ST2, IL-33
interacts with ST2 to activate NF-κB and MAPK signaling pathway,
thereby leading to the induction of cytokines and subsequent
modulation of T helper type 2 (Th2) cells' regulatory functions
(32). Considering the dual function
of IL-33, IL-33 may also serve significant roles in carcinogenesis
and tumor progression.
The present study demonstrated that IL-33 is
moderately expressed in normal liver tissues and is located in both
liver nucleus and cytoplasm. The results indicated that IL-33 may
have dual functions both as nuclear factor and inflammatory
mediators in normal hepatocytes at physiological state. In
hepatitis patients, the expression rate and level of IL-33 were
similar to that of normal liver, yet all positive IL-33 expression
was solely located in nucleus. This indicated the active
transcription inhibition properties of IL-33 may serve a role
during early inflammatory response. This property may inhibit the
expression of a number of associated cytokines and proteins, and
therefore avoids excessive inflammatory reaction. The protective
role of IL-33 for liver injury was demonstrated previously. Sakai
et al (19) reported that in
the hepatic response to ischemia/reperfusion, IL-33 appeared to
have direct protective effects on hepatocytes that limits liver
injury and reduces the stimulus for inflammation. However, the
continuous inflammatory reaction keeps the repair mechanism of the
liver active and leads to the aggravation of hepatic fibrosis and
eventually results in the development of cirrhosis. The present
study confirmed this hypothesis; IL-33 tended to be located in
cytoplasm in cirrhosis tissues, which is consistent with previous
report (16). Therefore, the
localization of IL-33 in cells will change with the associated
biological function of IL-33 during different stimuli. One
hypothesis is that when the balance of the dual functions of IL-33
is altered, the activated IL-33 is released from the nucleus and is
synthesized largely in the cytoplasm. The precursor IL-33 is
cleaved into the mature protein by caspase-1, which acts together
with the transmembrane receptor ST2 to regulate the inflammatory
reaction. When liver injury progresses to carcinoma, the positive
expression of IL-33 in HCC is markedly decreased when compared to
NCL, thereby indicating the diminish effect of IL-33 as protective
factor in the development and progression of HCC. However, this
hypothesis needs further study to prove. Additionally, it was
observed in the present study that the small amount of positive
IL-33 in HCC was mostly recognized as cytoplasmic accumulation.
This finding was in accordance with the localization of IL-33 in
HCC tissue as described by Zhang et al (17), thereby rendering the role of IL-33 as
an inflammatory mediator in HCC cells. While considering the
expression of IL-33 in para-carcinoma tissues, the rate of protein
expression was highly positive and located predominantly in the
cytoplasm of liver cells. Subsequent RT-PCR analysis further
confirmed an increase in IL-33 mRNA expression in para-carcinoma
tissues compared to that in primary liver carcinoma tissues. The
present authors speculate that in response to hepatocarcinogenic
factors, IL-33 may be recruited in the tumor microenvironment,
cytoplasmic IL-33 accumulation activates its downstream signalling
pathways and induces subsequent inflammatory regulatory biological
functions. Thus, IL-33 in para-tumor hepatocytes may be an
important endogenous chemotactic factor, and its expression level
may determine the biological behaviors and outcomes in pathological
liver.
On further analyzing the correlation between the
expression of IL-33 and HCC clinical pathological characteristics,
it was observed that the level of IL-33 expression was not
associated with patient age, gender, tumor size, TNM stage,
cirrhosis or hepatitis background, lymph node metastasis or
intrahepatic vascular embolism, but was associated with the
histological grade. The expression of IL-33 in the highly
differentiated group (I–II) was higher than the low differentiation
group (III–IV). To some extent, the differentiation level reflects
the malignant grade of the cancer cells. The low-differentiated
cells have higher malignancy and exhibit more chances of recurrence
and metastasis. Thus the association between IL-33 and histological
grade further highlights the protective effect of IL-33 in liver
under pathophysiological conditions (19).
In conclusion, during the progression of liver
disease from normal tissue to hepatitis, cirrhotic, and HCC, the
expression and localization of IL-33 are altered, which leads to a
reduction in its protective effect. Although further studies are
warranted to explore the mechanisms of downregulation and
cytoplasmic retention of IL-33, the elucidation of the important
dual role of IL-33 and its mediated signaling pathways may result
in novel directions and strategies for the diagnosis and treatment
of HCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402514), a grant
from the Natural Science Foundation of Anhui Province, China (grant
no. 1408085QH166), and an internal grant from Bengbu Medical
College (grant no. Bykf13A12).
References
|
1
|
Marx J: Cancer research. Inflammation and
cancer: The link grows stronger. Science. 306:966–968. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hofseth LJ and Ying L: Identifying and
defusing weapons of mass inflammation in carcinogenesis. Biochim
Biophys Acta. 1765:74–84. 2006.PubMed/NCBI
|
|
3
|
Chew V, Tow C, Teo M, et al: Inflammatory
tumour microenvironment is associated with superior survival in
hepatocellular carcinoma patients. J Hepatol. 52:370–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong CM and Ng IO: Molecular pathogenesis
of hepatocellular carcinoma. Liver Int. 28:160–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milovanovic M, Volarevic V, Radosavljevic
G, Jovanovic I, Pejnovic N, Arsenijevic N and Lukic ML: IL-33/ST2
axis in inflammation and immunopathology. Immunol Res. 52:89–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carriere V, Roussel L, Ortega N, Lacorre
DA, Americh L, Aguilar L, Bouche G and Girard JP: IL-33, the
IL-1-like cytokine ligand for ST2 receptor, is a
chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci
USA. 104:282–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen SF, Nieh S, Jao SW, Wu MZ, Liu CL,
Chang YC and Lin YS: The paracrine effect of cancer-associated
fibroblast-induced interleukin-33 regulates the invasiveness of
head and neck squamous cell carcinoma. J Pathol. 231:180–189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Pantic JM, Milovanovic MZ, Arsenijevic NN and Lukic ML:
Interleukin-33/ST2 axis promotes breast cancer growth and
metastases by facilitating intratumoral accumulation of
immunosuppressive and innate lymphoid cells. Int J Cancer.
134:1669–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun P, Ben Q, Tu S, Dong W, Qi X and Wu Y:
Serum interleukin-33 levels in patients with gastric cancer. Dig
Dis Sci. 56:3596–3601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu LA, Fu Y, Zhang DN and Zhang J: Serum
IL-33 as a diagnostic and prognostic marker in non-small cell lung
cancer. Asian Pac J Cancer Prev. 14:2563–2566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Shen JX, Hu JL, Huang WH and Zhang
GJ: Significance of interleukin-33 and its related cytokines in
patients with breast cancers. Front Immunol. 5:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonilla WV, Fröhlich A, Senn K, Kallert S,
Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon
PG, et al: The alarmin interleukin-33 drives protective antiviral
CD8+ T cell responses. Science. 335:984–989. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arshad MI, Rauch M, L'Helgoualc'h A, Julia
V, Leite-de-Moraes MC, Lucas-Clerc C, Piquet-Pellorce C and Samson
M: NKT cells are required to induce high IL-33 expression in
hepatocytes during ConA-induced acute hepatitis. Eur J Immunol.
41:2341–2348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu
J and Jiang Y: Serum IL-33 levels are associated with liver damage
in patients with chronic hepatitis B. J Interferon Cytokine Res.
32:248–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marvie P, Lisbonne M, L'Helgoualc'h A,
Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Théret N, Gascan H,
Piquet-Pellorce C and Samson M: Interleukin-33 overexpression is
associated with liver fibrosis in mice and humans. J Cell Mol Med.
14:1726–1739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang P, Liu XK, Chu Z, Ye JC, Li KL,
Zhuang WL, Yang DJ and Jiang YF: Detection of interleukin-33 in
serum and carcinoma tissue from patients with hepatocellular
carcinoma and its clinical implications. J Int Med Res.
40:1654–1661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bergis D, Kassis V, Ranglack A, Koeberle
V, Piiper A, Kronenberger B, Zeuzem S, Waidmann O and Radeke HH:
High serum levels of the interleukin-33 receptor soluble ST2 as a
negative prognostic factor in hepatocellular carcinoma. Transl
Oncol. 6:311–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakai N, Van Sweringen HL, Quillin RC,
Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ and Lentsch
AB: Interleukin-33 is hepatoprotective during liver
ischemia/reperfusion in mice. Hepatology. 56:1468–1478. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng R, Wang J, Wu Q, Wang Z, Ou Y, Ma L,
Wang M, Wang J and Yang Y: Expression of ALDH1 and TGFβ2 in benign
and malignant breast tumors and their prognostic implications. Int
J Clin Exp Pathol. 7:4173–4183. 2014.PubMed/NCBI
|
|
22
|
He XD, Wang Y, Wu Q, Wang HX, Chen ZD,
Zheng RS, Wang ZS, Wang JB and Yang Y: Xuebijing protects rats from
sepsis challenged with acinetobacter baumannii by promoting annexin
A1 expression and inhibiting proinflammatory cytokines secretion.
Evid Based Complement Alternat Med. 2013:8049402013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goncalves CK, Fregnani ER, Leon JE,
Silva-Sousa YT and Perez DE: Immunohistochemical expression of p63,
epidermal growth factor receptor (EGFR) and notch-1 in radicular
cysts, dentigerous cysts and keratocystic odontogenic tumors. Braz
Dent J. 23:337–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Qin SK, Wu Q, Wang ZS, Zheng RS,
Tong XH, Liu H, Tao L and He XD: Connexin-dependent gap junction
enhancement is involved in the synergistic effect of sorafenib and
all-trans retinoic acid on HCC growth inhibition. Oncol Rep.
31:540–550. 2014.PubMed/NCBI
|
|
25
|
Zhang DY and Friedman SL:
Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology.
56:769–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baekkevold ES, Roussigné M, Yamanaka T,
Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M,
Haraldsen G and Girard JP: Molecular characterization of NF-HEV, a
nuclear factor preferentially expressed in human high endothelial
venules. Am J Pathol. 163:69–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayakawa H, Hayakawa M, Kume A and
Tominaga S: Soluble ST2 blocks interleukin-33 signaling in allergic
airway inflammation. J Biol Chem. 282:26369–26380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arend WP, Palmer G and Gabay C: IL-1,
IL-18, and IL-33 families of cytokines. Immunol Rev. 223:20–38.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lüthi AU, Cullen SP, McNeela EA, Duriez
PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K,
Vandenabeele P, et al: Suppression of interleukin-33 bioactivity
through proteolysis by apoptotic caspases. Immunity. 31:84–98.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cayrol C and Girard JP: The IL-1-like
cytokine IL-33 is inactivated after maturation by caspase-1. Proc
Natl Acad Sci USA. 106:9021–9026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kunes P, Holubcová Z, Kolácková M and
Krejsek J: The counter-regulation of atherogenesis: A role for
interleukin-33. Acta Medica (Hradec Kralove). 53:125–129.
2010.PubMed/NCBI
|
|
32
|
Miller AM: Role of IL-33 in inflammation
and disease. J Inflamm (Lond). 8:222011. View Article : Google Scholar : PubMed/NCBI
|