Introduction
Basal cell carcinoma (BCC) is the most common type
of periocular cancer in human (1).
The epidermis originates from basal cell exchange, the infindibular
cells of hair follicles or from pluripotent stem cells and this may
explain why BCC does not develop from any precursor lesions
(2,3).
Squamous cell cancer (SCC) is the second most common tumor of the
eyelids (1,4). Although the exact mechanism in SCC
pathogenesis is unknown, environmental and internal stimulus and
interruption of control mechanisms that enable growth and
regulation are considered as the main causes of tumorigenesis
(5). Ocular surface squamous
neoplasia constitutes 14% of the eye tumors (6). Ocular surface squamous neoplasia
develops with the disruption in DNA repair mechanisms as a result
of somatic mutation caused by UV-B irradiation. Ocular surface
squamous neoplasia tends to start at the limbus because of the
preferential location of the stem cells (6). According to the stem cell theory,
external factors and tissue changes due to changing conditions in
this anatomical region disrupt the mechanism of stem cells and lead
to the development of abnormal epithelium giving rise to squamous
neoplasia (6).
Any event disrupting the self-renewal process of
stem cell may result in the initiation of carcinogenesis (7). Various signal transduction pathways take
part in self-renewal and repair of skin stem cells: These signaling
pathways are Wnt, Notch, TGFβ, EGF, FGF, IGF, hedgehog systems and
their secretory products (8). In the
misregulation of Hedgehog pathway, cell self-renewal cycle fails
and tumorigenesis is initiated (9).
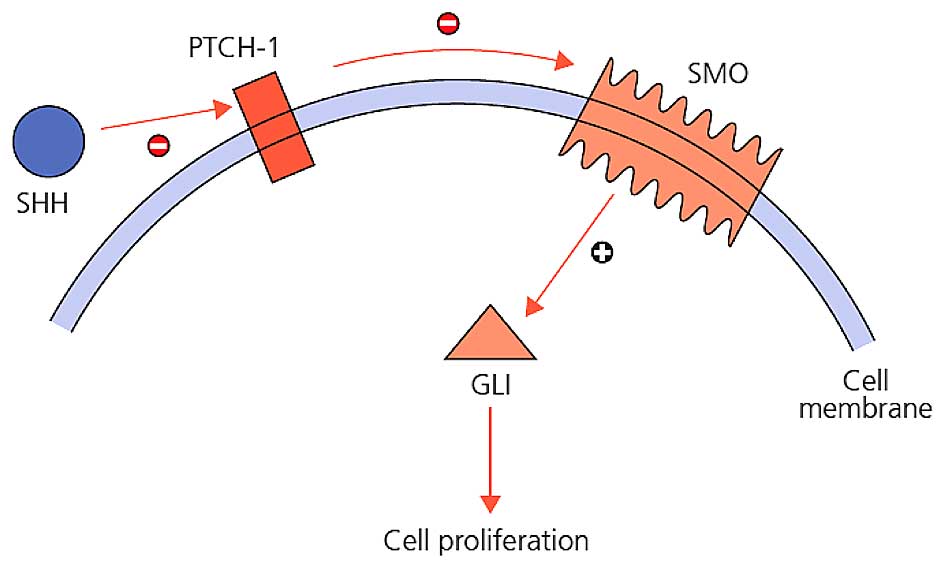
Sonic Hedgehog (Shh) is a crucial morphogen for proper cellular
proliferation in the development of mammals (10). Shh provides cell proliferation by cell
to cell communication, regulation of cell death, ensuring
continuity of stem cells, and plays role in stem cell self-renewal
process in hematopoietic system, skin, nervous system and breast
tissue (11). In this pathway, Shh,
Indian Hedgehog (Ihh), or Desert Hedgehog (Dhh) are secreted
ligands; and Patched-1 (PTCH1) and Patched-2 (PTCH2) are receptors
of those ligands. SHH is one of the most studied and well-known
protein ligands among the important proteins of hedgehog signaling
pathway. Disruptions in the Shh pathway has an effective role in
the abnormal development of stem cells. Shh signaling is increased
in epidermal tumors (12,13) and an increase in Shh signaling is
responsible for brain, skin, muscle, small cell lung,
gastrointestinal tract, prostate, breast and pancreatic cancer
(14). PTCH-1 is a protein product of
a tumor suppressor gene, directly connected to the early steps of
tumor formation process (15). Ptch-1
protein acts as a receptor for Shh family signaling molecules
(15). Ptch-1 inactivation is
responsible for basal cell nevus syndrome (16). The PTCH gene is mutated in sporadic
BCC (PTCH-1) and certain medulloblastomas (PTCH-2) with
rhabdomyomas and rhabdomyosarcomas (15,17,18). Gli-1
is one of the zinc finger proteins. Excessive activation of Gli-1
has been associated with the development of BCC (19,20). Smo
is the Gli activator under the suppression of Ptch. In the presence
of Shh ligands, suppression of Ptch on Smo ends. Smo, in this case
causes cytoplasmic Gli transcription factors to enter the nucleus
and these factors stimulate the increase in the expression of
proteins required for cell proliferation (11). The interactions between these
molecules are presented in Fig.
1.
In the present retrospective study, the levels of
Shh over-expression were investigated in human bulbar conjunctival
SCC, bulbar conjunctival in situ carcinoma, BCC of the
cutaneous eyelid paraffin tissue blocks by using
immunohistochemistry.
Materials and methods
Samples
The study protocol was approved by the Hacettepe
University Faculty of Medicine Medical Researches Local Ethics
Committee and was performed in accordance with the Declaration of
Helsinki. Each patient provided written informed consent for the
publication of the study. In this study, clinicopathological
records of patients with bulbar conjunctival invasive SCC, bulbar
conjunctival in-situ cancer and cutaneous eyelid BCC were reviewed
from the Ocular Oncology Unit, Department of Ophthalmology
(Hacettepe University School of Medicine, Ankara, Turkey). The 3
groups included: Bulbar conjunctival SCC group, 10 men and 12 women
aged between 15–83 years (mean: 61.27 years); a bulbar conjunctival
carcinoma in situ group, 9 men, 3 women, aged between 8–75 years
(mean: 58.9 years); and a cutaneous eyelid BCC group, 23 men, 18
women aged between 29–78 years (mean: 60.7 years) were included. No
differences were observed between groups regarding age and gender
(ANOVA, P>0.05).
Immunohistochemistry (IHC)
Indirect IHC was performed between January 1, 1998
and January 1, 2009 in the following samples: 22 cross-sections
were diagnosed with bulbar conjunctival invasive SCC, 12
cross-sections were diagnosed of bulbar conjunctival carcinoma
in situ and 41 cross-sections were diagnosed with cutaneous
eyelid BCC. Sections were incubated with SHH primary antibody
(AB73958; Abcam Biotechnology, Cambridge, Massachusetts, USA; 1:100
dilution) and cytoplasmic/membranous staining on human lung tissue
was regarded as a positive control. Sections were incubated with
PTCH-1 primary antibody (AB53715; Abcam Biotechnology; 1:100
dilution) and cytoplasmic/membranous staining on human brain tissue
was regarded as a positive control. Citrate pre-processing antibody
solution prepared in 1/100 concentration was used in preparations
[10X citrate buffer (pH 6.0); catalog no. AB64214; Abcam
Biotechnology). Sections were incubated with GLI-1 primary antibody
(SC20687; Santa Cruz Biotechnology Santa Cruz, California, USA).
Nuclear staining on seminiferous canal and cytoplasmic staining on
Leydig cell were regarded as a positive control. EDTA
pre-processing antibody solution prepared in 1/100 concentration
was used in preparations. Subsequent to being washed twice in
phosphate-buffered saline (PBS; 20X PBS Buffer with Tween 20;
catalog no. AB64028; Abcam Biotechnology), for 5 min each, the
sections were incubated with the secondary antibody, anti-rabbit
IgG VHH Single Domain-HRP, at a 0.1 µg/ml concentration (catalog
no. AB191866; Abcam Biotechnology) for 30 min at room temperature.
Subsequent to being washed twice in PBS, for 5 min each, color
development was assessed in the sections using DAB chromogen (DAB
Substrate kit; catalog no. AB64238; Abcam Biotechnology) and a
hematoxylin counterstain.
Scoring
Bulbar conjunctival invasive SCC, bulbar
conjunctival in-situ cancer and BCC of the eyelid have been
reviewed by a professional neuro-ophthalmic pathology professor.
The scores obtained from multiplying tumor percentage and dye
intensity were used in statistical evaluation. The percentage of
tumor dyed by primary antibody was 100% in all of tumor area. This
process was performed by separating the specimen slides into three
groups (<10%, 10–50%, >50%) under 4x or 10x magnification of
microscope. The preparations with <10% received 1, those that
scored between 10–50% were designated 2 and those with >50%
received 3 points, respectively. Dye intensity was expressed as
weak, medium and strong. Weak staining was used for preparation
with only selectable staining pattern of primary antibody under 40x
magnification of microscope and got 1 point. Moderately stained
preparations were used for preparations with selectable staining
pattern of primary antibody under 20x magnification of microscope
and got 2 points. Strong staining was only used for preparation
with selectable staining pattern of primary antibody under 10x
magnification of microscope and got 3 points (Fig. 2).
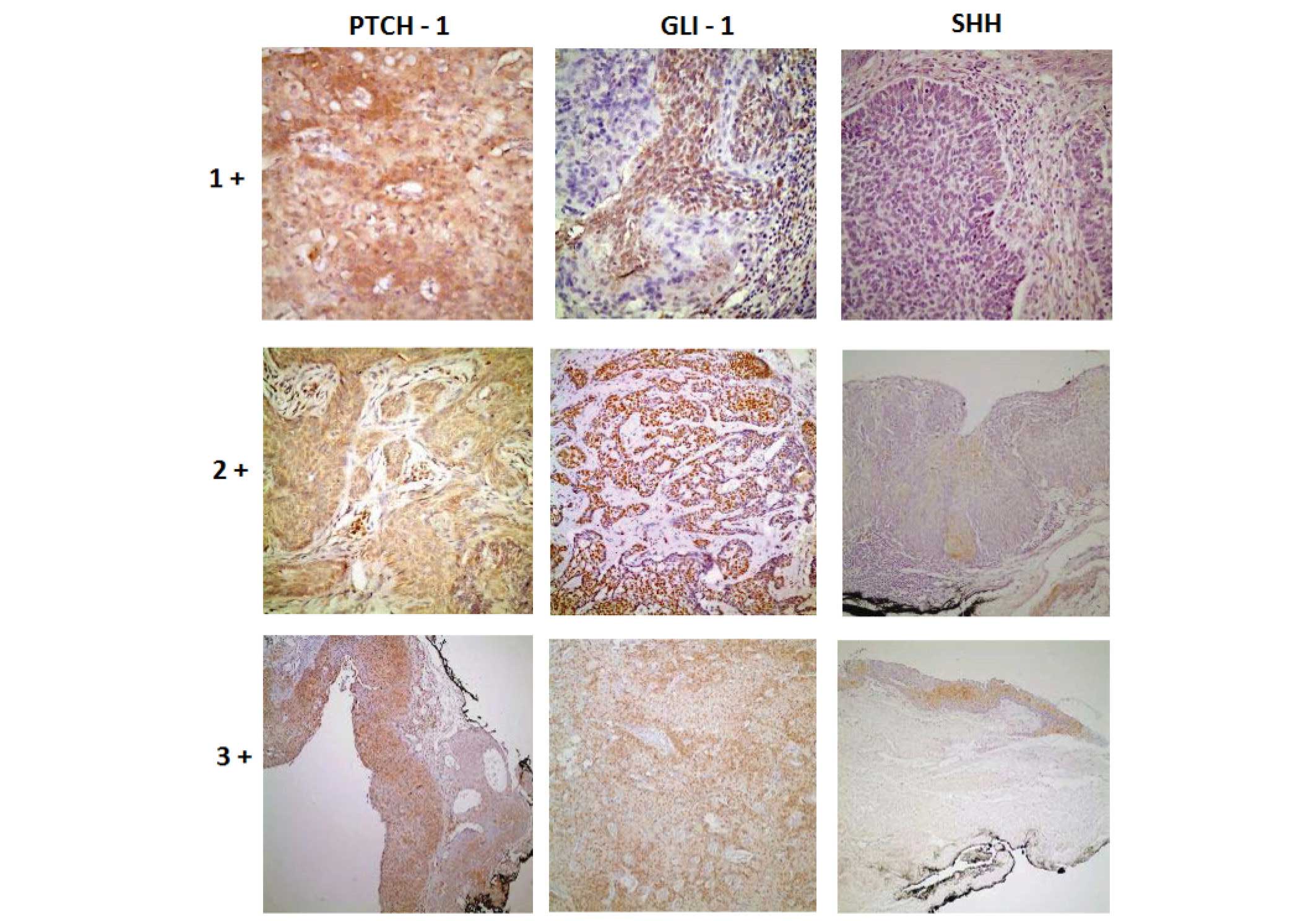 | Figure 2.Histopathological view of samples
demonstrating how antibodies dye intensities were assessed (stain,
DAB; +1, magnification, 40; +2, magnification, ×20; +3,
magnification, ×10). Shh, Sonic hedgehog protein; Gli-1,
Glioma-associated oncogene-1 protein: Ptch-1, Patched-1
protein. |
Statistical analysis
Data entry was performed using SPSS version 11.5
(SPSS, Inc., Chicago, IL, USA), and an analysis of variance and
Kruskall-Wallis test were used for significance testing of the
differences between the three groups, as the data showed an ordered
sequence. P<0.05 was used to indicate a statistically
significant difference.
Results
SHH dye intensity distribution of the 3 cancer
groups (bulbar conjunctival SCC, bulbar conjunctival carcinoma
in situ and cutaneous eyelid BCC) are summarized in Table I and differences in the negative
staining between the 3 cancer groups were statistically significant
(P=0.043). PTCH dye intensity distribution of every 3 cancer groups
are summarized in Table I and no
significant difference was observed for the negative staining
between the 3 cancer groups (P=0.170). However, weak staining in
BCC group is noteworthy. Distribution of GLI-1 dye intensity in the
3 cancer groups are summarized in Table
I and the negative staining betwen the 3 cancer groups did not
show any statistically significant differences (P=0.135). The
percentage of SHH staining in cancer groups are presented in
Table II. Staining >50% was not
observed in any groups. The difference between negative and total
number of positive cases in terms of the SHH scores did not
demonstrate any significant differences between the tumor groups
(P=0.182), but in general, SHH scores were generally low in all
groups. Differences in negative and total number of positive cases
in terms of PTCH-1 score between the tumor groups demonstrated
statistically significant differences (P=0.030). The difference
between negative and total number of positive cases in terms of
GLI-1 scores did not change significantly among tumor groups
(P=0.064), but GLI-1 scores in BCC group had higher scores compared
with the other tumor types (Table
III).
 | Table I.Shh, Ptch-1 and Gli-1 dye intensity in
cancer groups. |
Table I.
Shh, Ptch-1 and Gli-1 dye intensity in
cancer groups.
|
| Shh | Ptch-1 | Gli-1 |
|---|
| Antibody type |
|
|
|
|---|
| Intensity/Tumor
group | 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ |
| Lid+conjunctiva
SCC | 8 | 8 | 5 | 1 | 6 | 12 | 3 | 1 | 7 | 5 | 8 | 2 |
| Conjunctival in
situ cancer | 3 | 3 | 5 | 1 | 4 | 3 | 5 | 0 | 4 | 7 | 1 | 0 |
| Lid BCC | 21 | 16 | 2 | 2 | 18 | 19 | 4 | 0 | 8 | 15 | 13 | 5 |
 | Table II.Shh, Ptch-1, Gli-1 staining percentage
in cancer groups. |
Table II.
Shh, Ptch-1, Gli-1 staining percentage
in cancer groups.
|
| Shh | Ptch-1 | Gli-1 |
|---|
| Antibody type |
|
|
|
|---|
| Tumor
percentage/Tumor group | <10 | 10–50 | >50 | <10 | 10–50 | >50 | <10 | 10–50 | >50 |
| Lid+conjunctival
SCC | 14 | 8 | 0 | 9 | 11 | 2 | 10 | 9 | 3 |
| Conjunctival in
situ cancer | 6 | 6 | 0 | 4 | 6 | 2 | 7 | 5 | 0 |
| Lid BCC | 33 | 8 | 0 | 25 | 15 | 1 | 13 | 18 | 10 |
 | Table III.Shh, Ptch-1 and Gli-1 score
distribution in cancer groups. |
Table III.
Shh, Ptch-1 and Gli-1 score
distribution in cancer groups.
| Score/Tumor
group | 0 | 1 | 2 | 4 | 6 | 9 | Total |
|---|
| Shh SCORES |
|
|
Lid+conjunctival SCC | 8 | 5 | 4 | 4 | 1 | 0 | 22 |
|
Conjunctival in situ
cancer | 3 | 3 | 0 | 5 | 1 | 0 | 12 |
| Lid
BCC | 21 | 11 | 6 | 1 | 2 | 0 | 41 |
| Ptch-1 SCORES |
|
|
Lid+conjunctival SCC | 6 | 3 | 9 | 2 | 2 | 0 | 22 |
|
Conjunctival in situ
cancer | 4 | 0 | 3 | 3 | 2 | 0 | 12 |
| Lid
BCC | 18 | 7 | 12 | 3 | 1 | 0 | 41 |
|
| Gli-1 SCORES |
|
|
Lid+conjunctival SCC | 7 | 3 | 2 | 6 | 3 | 1 | 22 |
|
Conjunctival in situ
cancer | 4 | 3 | 4 | 1 | 0 | 0 | 12 |
| Lid
BCC | 8 | 5 | 10 | 7 | 7 | 4 | 41 |
Discussion
When the hedgehog signaling pathway is
over-activated, the self-renewal cell cycle fails and the tumor
formation process is initiated (9,21–24). An increase in Shh signaling is
responsible for the development of brain, skin, muscle, small cell
lung, gastrointestinal tract, prostate, breast and pancreatic
cancers (14). Tojo et al
(24) studied 19 nodular and 6
superficial sporadic BCC, and demonstrated that in superficial BCC
Ptch mRNA expression was in a level that could not be identified
with in situ hybridization method and they also predicted
that it was associated with invasion of dermis. In the present
study, the low Ptch-1 expression score in the BCC group was found
to be statistically significant and this evidence also supports the
evidence that Ptch-1 mutations may serve a role in the development
of sporadic BCC, hypothesized by Kim et al (25). In the present study, Ptch-1 score
regarded as the final protein product of mutated Ptch-1 gene, was
zero (0) in 18/41 (~40%) patients as assessed by IHC. This may mean
that mutation occurred in these cases. De Grujil et al
(26) suggested that UV radiation may
result in Ptch-1 mutations and long-term effects of UV radiation on
the development of BCC may also be effective through Ptch-1
mutation. In the present study, UV radiation may be responsible for
the 18/41 (~40%) patients in which the Ptch-1 score was 0. In 5 BCC
cases where exenteration was performed, the Ptch-1 score was ≤2 and
this also supports the idea that Ptch-1 mutation may contribute to
BCC development. However, this mutation needs to be verified using
RNA in situ hybridization methods in further studies. In a
previous study on sporadic BCC conducted by Holikova et al
(27), Ptch-1 mutations were
identified with increased inactive Ptch-1 mRNA in cytoplasm by
in situ hybridization method and this was detected in
particular in the outer tumor layers with palisade formation;
increase of Gli-1 activity was also observed in tumor areas. The
authors pointed out that Ptch-1 mutation increases Shh signaling,
initiating cell proliferation through the Gli family of
transcriptional factors (25). In the
present study, 18 (7+7+4) /41 (~%45) patients were detected with
Gli-1 score ≥4, and a nuclear increase was shown using the IHC
method. In a study conducted by Green et al (28), an increase in Gli-1 nuclear
transcription factors was shown to have an important role in the
development of BCC. Lupi showed BCC development after just a single
somatic mutation through the Shh pathway and suggested that Ptch-1
mutation was responsible for the development of sporadic and
hereditary BCC (29). Decreased Shh
intensity in the BCC group was found to be statistically
significant in the present study. SHH staining was not observed in
21 of our 41 patients.
In the present study, 6/22 (~25%) of patients in SCC
group had Ptch-1 score as (0), and were not found to be supportive
of SCC development on SHH pathway. However, Xuan et al
(30) have demonstrated that hedgehog
signaling increases together with HPV-16 infection in uterine
cervical squamous carcinoma. The authors highlighted that 95% of
cervical squamous carcinomas demonstrated an increase in Shh
expression. The authors also emphasized that the level of Shh
expression increased in parallel with frequency of lymph node
metastasis (30). In the present
study, the SCC group expressed lower levels of Shh and these
patients did not have lymph node metastases, suggesting that an
increase in Shh expression may be a more effective clue for
advanced and metastatic SCC. In another study carried out by Xuan
et al (31), approximately
twofold increase in Shh expression was observed in transition from
intraepithelial lesion to invasive uterine cervical squamous cell
carcinoma. In a recent study by Cavicchioli Buim et al
(32), low levels of GLI-1 were
observed in non-neoplastic oral mucosal squamous epithelial line
that was adjacent to the tumor. All oral squamous cell carcinoma
cases in this study expressed high levels of Gli-1. Another study
by Nishimaki et al (33)
demonstrated overexpression of Shh in 5 cell lines among 14 human
oral squamous cell carcinoma cell lines. In the present study, the
Shh score of 6/12 (50%) patients suffering from in situ
squamous cell carcinoma was ≥4. Again, Gli-1 score of 11 patients
in the in situ squamous cell group was ≤2 and Shh increase
was not enough on its own in the process of transition from in
situ cancer to invasion; and increase was necessary in Gli-1
transcription factor, which is the final effector of hedgehog
signaling pathway. In 22 patients in the SCC group, the Ptch-1
score was 0 (zero) in 6 (~%25) patients, the Gli-1 score was ≥4 in
10 (66%) patients. In 12 patients in the in-situ cancer
group, Ptch-1 score was ≥2 in 8 (66%) patients. Of all the patients
in the in-situ cancer group Gli-1 score was ≤4 or fewer.
This suggests that in the conversion process from in situ
cancer to invasive SCC, hedgehog signaling may take an active
role.
The present study present evidence that Ptch-1
mutations contribute to the development of cutaneous eyelid basal
cell carcinoma. Alterations in hedgehog signaling pathways may lead
to transformation of the conjunctival intraepithelial neoplasia
into invasive squamous cell carcinoma. The use of more
comprehensive in situ hybridization techniques in addition
to IHC method may aid in demonstrating the effect of hedgehog
signaling in the pathogenesis of BCC and SCC.
Acknowledgements
The present study was supported by Hacettepe
University The Scientific Research and Development Office. In
addition, this study was presented at the 15th biannual
meeting of the International Society of Ocular Oncology (ISOO),
November 14-17, 2011, Buenos Aires, Argentina.
References
|
1
|
Rosner M: Section 2 eyelid tumors basal
cell carcinoma. Clinical Ophthalmic Oncology. Singh AD, Damato BE,
Murphree AL and Perry JD: Saunders, Elsevier. (Philadelphia, USA).
76–80. 2007. View Article : Google Scholar
|
|
2
|
Margo CE and Waltz K: Basal cell carcinoma
of the eyelid and periocular skin. Surv Ophthalmol. 38:169–192.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allali J, D'Hermies F and Renard G: Basal
cell carcinomas of the eyelids. Ophthalmologica. 219:57–71. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Green A: Changing patterns in incidence of
non-melanoma skin cancer. Epithelial Cell Biol. 1:47–51. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donaldson MJ, Sullivan TJ, Whitehead KJ
and Williamson RM: Squamous cell carcinoma of the eyelids. Br J
Ophthalmol. 86:1161–1165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee GA and Hirst LW: Ocular surface
squamous neoplasia. Surv Ophthalmol. 39:429–450. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tumbar T, Guasch G, Greco V, Blanpain C,
Lowry WE, Rendl M and Fuchs E: Defining the epithelial stem cell
niche in skin. Science. 303:359–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molofsky AV, Pardal R and Morrison SJ:
Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell
Biol1. 6:700–707. 2004. View Article : Google Scholar
|
|
9
|
Evangelista M, Tian H and de Sauvage FJ:
The hedgehog signaling pathway in cancer. Clin Cancer Res.
12:5924–5928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guerrero I and Ruizi Altaba A:
Development: Longing for ligand: Hedgehog, patched, and cell death.
Science. 301:774–776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toftgård R: Hedgehog signalling in cancer.
Cell Mol Life Sci. 57:1720–1731. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hutchin ME, Kariapper MS, Grachtchouk M,
Wang A, Wei L, Cummings D, Liu J, Michael LE, Glick A and Dlugosz
AA: Sustained Hedgehog signaling is required for basal cell
carcinoma proliferation and survival: Conditional skin
tumorigenesis recapitulates the hair growth cycle. Genes Dev.
19:214–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Owens DM and Watt FM: Contribution of stem
cells and differentiated cells to epidermal tumors. Nat Rev Cancer.
3:444–451. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruiz A, Altaba I, Stecca B and Sánchez P:
Hedgehog-Gli signaling in brain tumors: Stem cells and
paradevelopmental programs in cancer. Cancer Lett. 204:145–157.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolter M, Reifenberger J, Sommer C,
Ruzicka T and Reifenberger G: Mutations in the human homologue of
the Drosophila segment polarity gene patched (PTCH) in sporadic
basal cell carcinomas of the skin and primitive neuroectodermal
tumors of the central nervous system. Cancer Res. 57:2581–2585.
1997.PubMed/NCBI
|
|
16
|
Chidambaram A, Goldstein AM, Gailani MR,
Gerrard B, Bale SJ, DiGiovanna JJ, Bale AE and Dean M: Mutations in
the human homologue of the Drosophila patched gene in Caucasian and
African-American nevoid basal cell carcinoma syndrome patients.
Cancer Res. 56:4599–4601. 1996.PubMed/NCBI
|
|
17
|
Tostar U, Malm CJ, Meis-Kindblom JM,
Toftgård R and Undén AB: Deregulation of the hedgehog signalling
pathway: A possible role for the PTCH and SUFU genes in human
rhabdomyoma and rhabdomyosarcoma development. J Pathol. 208:17–25.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie J, Johnson RL, Zhang X, Bare JW,
Waldman FM, Cogen PH, Menon AG, Warren RS, Chen LC, Scott MP and
Epstein EH Jr: Mutations of the PATCHED gene in several types of
sporadic extracutaneous tumors. Cancer Res. 57:2369–2372.
1997.PubMed/NCBI
|
|
19
|
Ruizi Altaba A: Gli proteins encode
context-dependent positive and negative functions: Implications for
development and disease. Development. 126:3205–3216.
1999.PubMed/NCBI
|
|
20
|
Dahmane N, Lee J, Robins P, Heller P and
Ruizi Altaba A: Activation of the transcription factor Gli1 and the
Sonic hedgehog signalling pathway in skin tumours. Nature.
389:876–881. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mori Y, Okumura T, Tsunoda S, Sakai Y and
Shimada Y: Gli-1 expression is associated with lymph node
metastasis and tumor progression in esophageal squamous cell
carcinoma. Oncology. 70:378–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schneider S, Thurnher D, Kloimstein P,
Leitner V, Petzelbauer P, Pammer J, Brunner M and Erovic BM:
Expression of the Sonic hedgehog pathway in squamous cell carcinoma
of the skin and the mucosa of the head and neck. Head Neck.
33:244–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wicking C and McGlinn E: The role of
hedgehog signalling in tumorigenesis. Cancer Lett. 173:1–7. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tojo M, Mori T, Kiyosawa H, Honma Y, Tanno
Y, Kanazawa KY, Yokoya S, Kaneko F and Wanaka A: Expression of
sonic hedgehog signal transducers, patched and smoothened, in human
basal cell carcinoma. Pathol Int. 49:687–694. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim MY, Park HJ, Baek SC, Byun DG and Houh
D: Mutations of the p53 and PTCH gene in basal cell carcinomas: UV
mutation signature and strand bias. J Dermatol Sci. 29:1–9. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Gruijl FR, Van Kranen HJ and Mullenders
LH: UV-induces DNA damage, repair, mutations and oncogenic pathways
in skin cancer. J Photochem Photobiol B. 63:19–27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holikova Z, Massi D, Lotti T and Hercogová
J: Insight into the pathogenesis of sporadic basal cell carcinoma.
Int J Dermatol. 43:865–869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Green J, Leigh IM, Poulsom R and Quinn AG:
Basal cell carcinoma development is associated with induction of
the expression of the transcription factor Gli-1. Br J Dermatol.
139:911–915. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lupi O: Correlations between the sonic
hedgehog pathway and basal cell carcinoma. Int J Dermatol.
46:1113–1117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xuan YH, Li GL, Jiang HY and Lin ZH:
Relationship between hedgehog signaling pathway molecules and HPV16
infection in uterine cervical cancers. Zhonghua Bing Li Xue Za Zhi.
38:178–182. 2009.(In Chinese). PubMed/NCBI
|
|
31
|
Xuan YH, Jung HS, Choi YL, Shin YK, Kim
HJ, Kim KH, Kim WJ, Lee YJ and Kim SH: Enhanced expression of
hedgehog signaling molecules in squamous cell carcinoma of uterine
cervix and its precursor lesions. Mod Pathol. 19:1139–1147.
2006.PubMed/NCBI
|
|
32
|
Cavicchioli Buim ME, Gurgel CA, Gonçalves
Ramos EA, Lourenço SV and Soares FA: Activation of sonic hedgehog
signaling in oral squamous cell carcinomas: A preliminary study.
Hum Pathol. 42:1484–1490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishimaki H, Kasai K, Kozaki Ki, Takeo T,
Ikeda H, Saga S, Nitta M and Itoh G: A role of activated Sonic
hedgehog signaling for the cellular proliferation of oral squamous
cell carcinoma cell line. Biochem Biophys Res Commun. 314:313–320.
2004. View Article : Google Scholar : PubMed/NCBI
|