Introduction
Pancreatic cancer (PC; OMIM, 260350) is a highly
lethal disease; it is one of the deadliest cancers worldwide, with
a mortality rate of 99% and 5-year relative survival rate of <5%
(1), and almost all patients with PC
develop metastasis. The etiology of PC remains elusive; smoking is
the best known risk factor (2).
Advances in molecular biology have greatly improved
understanding the pathogenesis of PC. The development of PC
requires the transformation of normal pancreatic cells to precursor
pancreatic intraepithelial neoplasia, which is associated with gene
mutations, continuous alterations in nuclei, loss of polarity and
alterations to the architecture of cells (3). In addition, chromosome abnormalities are
involved in the pathophysiology and development of PC, which
usually presents as a loss or gain of alleles in various
chromosomes (4). It has been reported
that the development and progression of PC is caused by activation
of oncogenes and inactivation of tumor suppressor genes, as well as
deregulation of numerous signaling pathways, including epidermal
growth factor receptor, protein kinase B and nuclear factor kappa B
pathways (5). In addition, Hedgehog
signaling pathway, an essential pathway during embryonic pancreatic
development, is involved in several types of cancer and may be an
important mediator in human PC (6).
Previous studies indicate that PC has a complex genomic landscape
with frequent copy number alterations and point mutations (7). It has been demonstrated that common
mutated genes in PC include Kirsten rat sarcoma viral oncogene
homolog (K-ras; 74–100%), p16INK4a (≤98%), p53
(43–76%), deleted in pancreatic cancer, locus 4 (~50%), human
epidermal growth factor (HER)-2/neu (~65%) and
Fragile Histidine Triad (~70%) (8–12);
K-ras and HER-2/neu are proto-oncogenes, while
all the other genes are tumor suppressor genes (7). Through comprehensive genetic analysis of
24 samples of PC, Jones et al (13) demonstrated that PC contained an
average of 63 genetic alterations, the majority of which were point
mutations, and these alterations defined a core set of 12 cellular
signaling pathways, which were genetically altered in 67–100% of PC
tumors. Additionally, Biankin et al (14) defined 16 significant mutated genes,
reaffirmed known mutations [K-RAS, tumor protein p53,
cyclin-dependent kinase inhibitor 2A, SMAD4, myeloid/lymphoid or
mixed-lineage leukemia 3, transforming growth factor, beta receptor
II, AT-rich interaction domain (ARID) 1A and splicing factor
3b subunit 1] and uncovered novel mutated genes, including genes
involved in chromatin modification (enhancer of polycomb homolog 1
and ARID2), DNA damage repair (ATM serine/threonine kinase)
and other mechanisms in axon guidance (zinc finger imprinted 2,
mitogen-activated protein kinase kinase 4, sodium leak channel,
non-selective, solute carrier family 16 member 4 and MAGEA6). In a
humanized genetically modified mouse model of pancreatic ductal
adenocarcinoma, which accounts for >90% of PC, Rosenfeldt et
al (15) revealed that loss of
autophagy did not block tumor progression, but actually accelerated
tumor onset.
Genome-wide association study (GWAS) aims to detect
variants at genomic loci associated with complex traits in a
population and, in particular, detect associations between common
single-nucleotide polymorphisms (SNPs) and common diseases
(16). Gene expression is another
source of gene data for investigating complex genetic disease,
which describes the type and abundance of gene expression in
specific cells or tissues under certain conditions (17). Gene Expression Omnibus Series (GSE)
dataset GSE 23952 [human pancreatic carcinoma Panc-1 transforming
growth factor-β (TGF-β) treatment assay] was used by the present
study, which has also been used by previous studies. Kato et
al (18) analyzed two datasets
(GSE 17708 and 23952) to identify genes encoding secreted proteins
on GenePattern. Xu and Liu (19) used
several datasets to study the aberrant expression of cytoplasmic
polyadenylation element binding protein 4. Additionally, Gröger
(20) developed a comprehensive
meta-analysis combining 24 epithelial mesenchymal transition (EMT)
datasets, including GSE 23952, to investigate the effectors of EMT.
However, none of these studies focussed on pathway analysis in
PC.
The present study combined GWAS and gene expression
data to identify important pathways for the pathogenesis of PC.
Gene set enrichment analysis (GSEA) was used to identify
over-represented pathways in GWAS or gene expression profiles.
Meta-analysis was performed to select significant pathways in GWAS
and gene expression data.
Materials and methods
GWAS and gene expression profile
GWAS data (accession no., pha002874.1) were
downloaded from National Center for Biotechnology Information
(NCBI) database of Genotypes and Phenotypes (www.ncbi.nlm.nih.gov/projects/SNP/gViewer/gView.cgi?aid=2874).
The data was obtained by genotyping with the Illumina Hap 500
Infinium genotyping assay (Illumina, Inc., San Diego, CA, USA) on
1,896 PC patients and 1,939 control individuals drawn from 12
prospective cohorts plus one hospital-based case-control study
(21). A total of 522,293 SNPs were
used in this analysis.
For gene expression analysis, the present study
utilized the microarray data set submitted by Maupin et al
(22). The data were downloaded from
NCBI Gene Expression Omnibus (www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS4106;
accession no., GSE23952; human pancreatic carcinoma Panc-1 TGF-β
treatment assay). In the study by Maupin et al, the human
pancreatic adenocarcinoma Panc-1 cell line was treated with TGF-β
to induce EMT, and the study was repeated three times. Samples were
assayed using Affymetrix Human Genome U133 Plus 2.0 Array
(Affymetrix, Santa Clara, CA, USA), and probes with the largest
differential expression value were selected. A total of 54,623
probe-sets were obtained following normalization. Differentially
expressed genes (DEGs) derived from probes were used for further
analysis.
GWAS data analysis: Mapping SNPs to
genes
SNPs in the GWAS data were mapped to corresponding
genes. The SNPs were annotated based on hg19 (hgdownload.cse.ucsc.edu/goldenPath/hg19/bigZips/).
Genes were identified according to their sitting priority [exon
region > intron region > 5′ untranslated region (UTR) > 3′
UTR]. If there were no genes identified in any genetic locus, genes
closest to one side of the SNP were included. If more than one SNP
was mapped to a gene, genes with the smallest P-value were selected
in from the GWAS data.
GSEA pathway analysis
Pathway analysis was performed using GSEA on GWAS
and gene expression profile data. GSEA statistically tests whether
members of a predefined gene set are randomly distributed
throughout a ranked list of genes or whether the members of the
gene set cluster toward the top of the list provided by the Broad
Institute (www.broadinstitute.org/gsea/index.jsp) (23–25).
Pathway analysis of gene sets was performed through Gene Ontology
(GO) pathways (c5.all.v4.0.symbols.gmt) from the Molecular
Signatures Database (www.broadinstitute.org/msigdb) (23) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathways (www.genome.jp/kegg/) (26).
Meta-analysis
Pathways with a significant difference in GWAS and
gene expression data were selected to perform meta-analysis. Meta
P-values were obtained by Fisher's combined probability test
(27). The combined P-value was
calculated by adding −2ln (P-value) of the two tests for a pathway.
Subsequently, a χ2-test distribution was performed,
which was used to determine the meta P-value (28). P<0.05 was considered to indicate a
statistically significant difference. All statistical tests were
performed using Perl language version 5.24.0 (www.perl.org/).
Results
Meta-analysis of over-represented
pathways in PC
The 522,293 SNPs loci were contained in GWAS data
and were mapped to 18,910 genes. In total, 58 over-represented
pathways were selected by GSEA pathway analysis. For gene
expression data, 54,623 probes were obtained and the probes were
mapped to 31,620 genes. Subsequently, 230 pathways were identified
by GSEA (P<0.05).
A meta-analysis of over-represented pathways was
performed to identify statistically significant pathways in the
combined GWAS and gene expression PC data. A total of 7
over-represented pathways from the GWAS and gene expression data
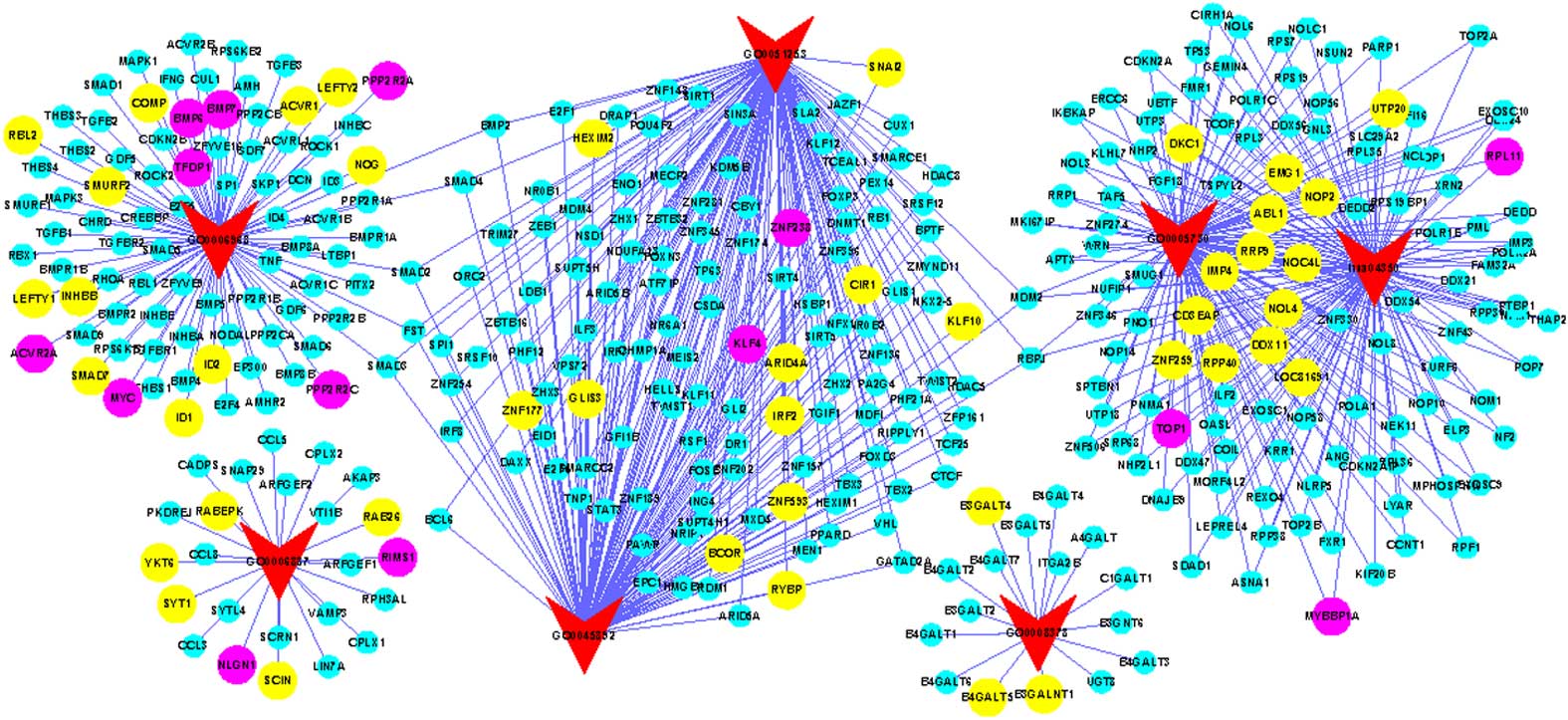
were identified (Fig. 1; Table I), of which 6 pathways were GO signal
pathways, and 1 pathway (KEGG ID, hsa 04350) was from the KEGG
database (Fig. 2). Additionally, a
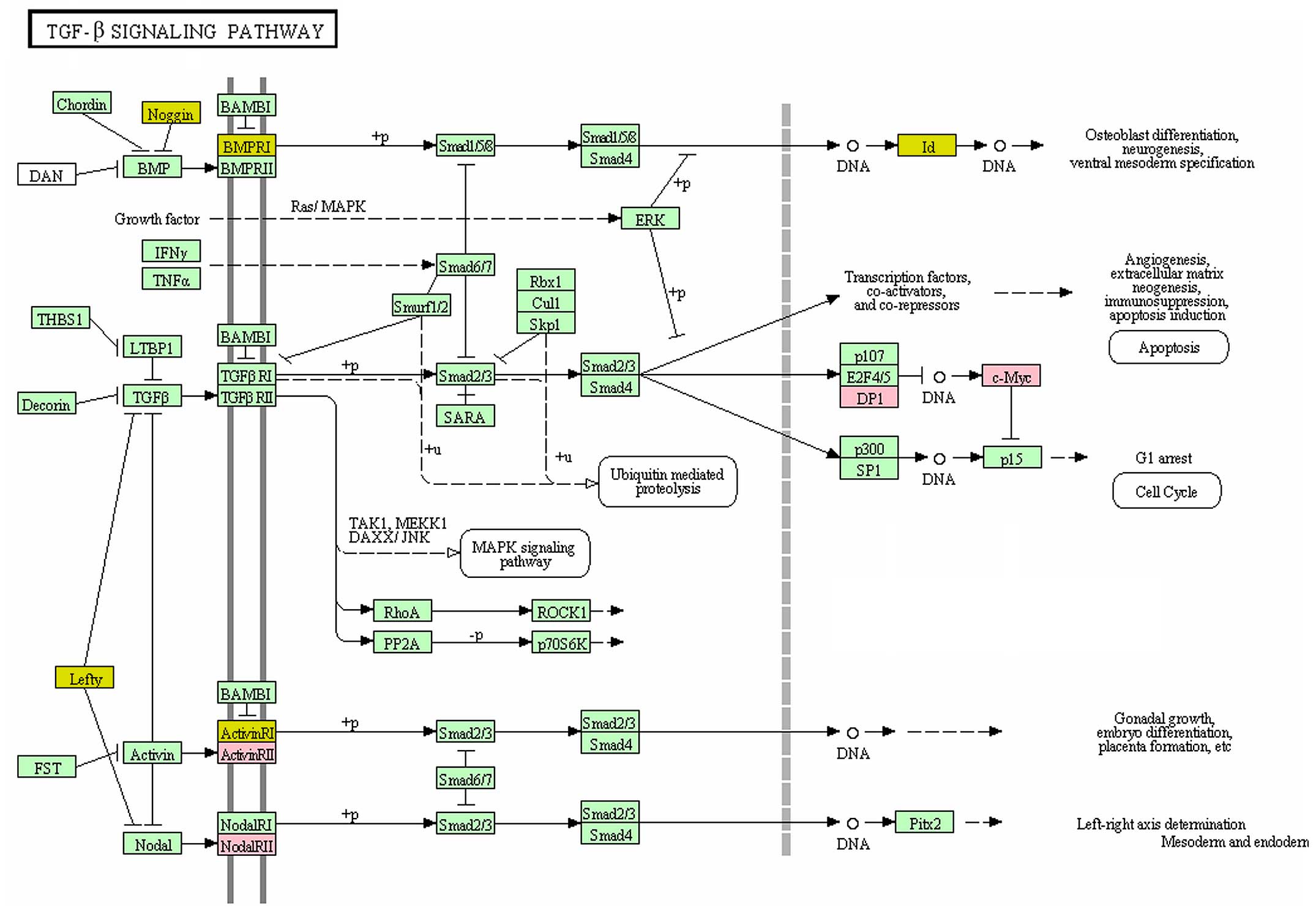
TGF-β signaling pathway (hsa 04350) had the smallest meta P-value.
In this signaling pathway, transcription factor Dp-1
(TFDP1), activin A receptor type (ACVR) 2A and v-myc
avian myelocytomatosis viral oncogene homolog (MYC) were
differentially expressed in GWAS data, while noggin (NOG),
inhibitor of DNA binding 1, HLH protein (ID1), left-right
determination factor 1 (LEFTY1) and ACVR1 were
differentially expressed in gene expression data. The other
significant pathways in the two data sources were as follows:
Negative regulation of DNA-dependent transcription (GO: 0045892);
the nucleolus (GO: 0005730); negative regulation of RNA metabolic
process (GO: 0051253); the cellular defense response (GO: 0006968);
exocytosis (GO: 0006887); and galactosyltransferase activity (GO:
0008378).
 | Table I.A total of 7 pathways were determined
by meta-analysis using combined genome-wide association study and
gene expression data. |
Table I.
A total of 7 pathways were determined
by meta-analysis using combined genome-wide association study and
gene expression data.
| Pathway | Term | Meta P-value |
|---|
| Hsa 04350 | TGF-β signaling
pathway | 0.00037 |
| GO: 0005730 | Nucleolus | 0.00045 |
| GO: 0006968 | Cellular defense
response | 0.00145 |
| GO: 0008378 |
Galactosyltransferase activity | 0.00182 |
| GO: 0006887 | Exocytosis | 0.00219 |
| GO: 0051253 | Negative regulation
of RNA metabolic process | 0.00331 |
| GO: 0045892 | Negative regulation
of DNA-dependent transcription | 0.00333 |
According to the constructed gene-pathway network,
exocytosis (GO: 0006887) and galactosyltransferase activity (GO:
0008378) had no connection with the other pathways, while the other
5 pathways were closely associated with each other (Fig. 1). As shown in the network, the TGF-β
signaling pathway (hsa 04350) and nucleolus pathway (GO: 0005730)
were closely connected, with numerous genes overlapping each other,
as well as negative regulation of RNA metabolic process pathway
(GO: 0051253) and negative regulation of DNA-dependent
transcription pathway (GO: 0045892). Additionally, 4 pathways,
including negative regulation of RNA metabolic process (GO:
0051253), negative regulation of DNA-dependent transcription (GO:
0045892), the nucleolus (GO: 0005730) and TGF-β signaling pathway
(hsa 04350), were connected via recombination signal binding
protein for immunoglobulin kappa J region (RBPJ) and MDM2
proto-oncogene (MDM2), while cellular defense response (GO:
0006968), negative regulation of RNA metabolic process (GO:
0051253) and negative regulation of DNA-dependent transcription
(GO: 0045892) were associated via SMAD2, SMAD3,
SMAD4, bone morphogenetic protein (BMP) 2 and
follistatin.
Identification of key genes for PC
pathways
To identify specific genes within the 7 pathways
identified by the present study, individual genes with P<0.05
were selected from GWAS and gene expression data. Among the 7
pathways, 11 key genes (2.9% out of a total of 380 genes) from GWAS
data were differentially expressed (Table II), including neuroligin 1,
regulating synaptic membrane exocytosis 1, protein phosphatase 2
regulatory subunit 2C, BMP6, zinc finger protein 238, Kruppel-like
factor 4, MYB Binding Protein (P160) 1a, ABL proto-oncogene 1,
non-receptor tyrosine kinase (ABL1), ribosomal protein L11,
topoisomerase (DNA) I. For the gene expression data, ~43 genes
(10.5% out of a total of 409 genes) were identified as being
significantly expressed (Table
III). Among these significant genes, only ABL1 from the
nucleolus pathway (GO: 0005730) was significantly expressed in both
GWAS [P=0.002085; P-value < min (0.5*N), where N = gene number]
and gene expression data [value=1.7271; value<max (0.5*N)].
 | Table II.DEGs from genome-wide association
study data of each pathway identified by meta-analysis. |
Table II.
DEGs from genome-wide association
study data of each pathway identified by meta-analysis.
| Pathway | DEGs | P-value |
|---|
| GO: 0008378 | – | 0.005 |
| GO: 0051253 | ZNF238,
KLF4 | 0.031 |
| Hsa 04350 | PPP2R2C,
BMP6, TFDP1, ACVR2A, PPP2R2A,
BMP7, MYC | 0.033 |
| GO: 0045892 | ZNF238,
KLF4 | 0.034 |
| GO: 0006968 | – | 0.037 |
| GO: 0006887 | NLGN1,
RIMS1 | 0.039 |
| GO: 0005730 | MYBBP1A,
ABL1, RPL11, TOP1 | 0.040 |
 | Table III.DEGs from gene expression profiles of
each pathway identified by meta-analysis. |
Table III.
DEGs from gene expression profiles of
each pathway identified by meta-analysis.
| Pathway | DEGs | P-value |
|---|
| Hsa 04350 | NOG, ID1,
LEFTY2, INHBB, LEFTY1, SMAD7, COMP, RBL2, ID2, SMURF2,
ACVR1 | 0.001 |
| GO: 0005730 | CD3EAP, NOL4,
IMP4, DDX11, ZNF259, EMG1, RRP9, ABL1, LOC81691, DKC1, RPP40,
UTP20, NOC4L, NOP2 | 0.001 |
| GO: 0006968 | KLRC3, KLRC4,
FOSL1, LGALS3BP, MICB | 0.004 |
| GO: 0006887 | RAB26, SYT1,
RABEPK, YKT6, SCIN | 0.006 |
| GO: 0045892 | SNAI2, RYBP,
GLIS3, IRF2, HEXIM2, KLF10, CIR1, BCOR, ZNF177, ZNF593,
ARID4A | 0.011 |
| GO: 0051253 | SNAI2, RYBP,
GLIS3, IRF2, HEXIM2, KLF10, CIR1, BCOR, ZNF177, ZNF593,
ARID4A | 0.012 |
| GO: 0008378 | B3GALT4,
B4GALT5, B3GALNT1 | 0.038 |
Discussion
According to meta-analysis based on GSEA pathway
analysis, 7 pathways were identified by the present study to be
significant in GWAS and gene expression profiles. These pathways
were associated with the TGF-β signaling pathway (hsa 04350),
negative regulation of DNA-dependent transcription (GO: 0045892),
the nucleolus (GO: 0005730), negative regulation of RNA metabolic
process (GO: 0051253), the cellular defense response (GO: 0006968),
exocytosis (GO: 0006887) and galactosyltransferase activity (GO:
0008378). The TGF-β signaling pathway had the smallest meta
P-value. By constructing the gene-pathway network, 5 pathways were
identified as closely connected, apart from exocytosis and
galactosyltransferase activity pathways. Among the 7 pathways, 11
key genes (2.9% out of a total of 380 genes) from GWAS data and 43
genes (10.5% out of a total of 409 genes) from gene expression data
were differentially expressed. Only ABL1 from the nucleolus
pathway was significantly expressed in the both data sources.
In total, 3 genes (TFDP1, ACVR2A and
MYC) from GWAS data and 4 genes (NOG, ID1,
LEFTY1 and ACVR1) from gene expression profile were
differentially expressed in the TGF-β signaling pathway. TGF-β
family members include TGF-betas, activins and BMPs, which are
structurally associated with secreted cytokines (29). In addition, the TGF-β family regulates
numerous cellular processes, including cell proliferation,
recognition, differentiation and apoptosis (30). MYC, an over-expressed
proto-oncogene in the TGF-β signaling pathway, encodes a
DNA-binding factor that activates or represses transcription
(31). Via this mechanism, MYC
regulates the expression of numerous target genes, which control
key cellular functions, such as cell growth and cell cycle
progression (32). Therefore,
deregulated MYC expression, resulting from various types of
genetic alterations, results in constitutive MYC activity in
a variety of cancers and promotes oncogenesis (33). If PC cells are stably transfected with
a dominant-negative mutant of MYC (c-Myc), their
proliferation is markedly inhibited (34). Grippo et al (35) studied myc-associated acinar-to-ductal
metaplasia in Ela-c-myc transgenic rats, and demonstrated that
c-myc was associated with human pancreatic neoplasms, which was
sufficient to induce acinar hyperplasia. Additionally, Köenig et
al (36) revealed a novel
mechanism regulating cell growth in PC: Serum promotes the
occurrence of PC through the induction of proliferative NFAT/c-Myc
axis by impaired c-Myc expression and reduces tumor growth upon
nuclear factor of activated T-cells depletion in vitro and
in vivo. TFDP1 is the first member of the E2F
transcription factor family that regulates the expression of
various cellular promoters, particularly those involved in the cell
cycle (37). Abba et al
(38) identified that TFDP1
exhibits the highest frequency of amplification affecting primary
breast cancer samples. In addition, meta-analysis reveals a strong
association between a high expression of TFDP1 or NOG
and the decreased overall survival in patients with breast cancer
(39). Furthermore, overexpression of
TFDP1 may contribute to the progression of certain
hepatocellular carcinomas by promoting the growth of tumor cells
(40). Additionally, ID1, a
gene associated with cell growth, senescence, differentiation and
angiogenesis, participates in numerous tumor processes (41,42). Other
genes, including ACVR2A, LEFTY1 and ACVR1, are
mostly associated with pituitary tumors (43), left-right axis malformations (44) and fibrodysplasia ossificans
progressive (45), respectively.
These genes, including TFDP1, NOG, ID1 ACVR2A,
LEFTY1 and ACVR1, were identified as being associated
with PC in the present study, which is rarely reported in PC
pathogenesis. Thus, further study is required verify these genes in
a PC context.
According to the constructed gene-signal pathway
network, 5 pathways were demonstrated to be closely connected in
the present study, apart from exocytosis and galactosyltransferase
activity pathway. RBPJ and MDM2 were bridges that
connected 4 of these pathways. Masui et al (46) demonstrated that pancreas specific
transcription factor, 1a (PTFLA), a basic helix-loop-helix
transcription factor required for pancreatic development, interacts
with RBPJ within a stable trimeric DNA-binding complex,
PTF1, during early PC development in mice. Introduction of a
PTFLA mutant, which is unable to bind RBPJ, truncated
pancreatic development at an immature stage and acini or islets
were not formed. MDM2 is an E3 ubiquitin ligase that targets
the tumor suppressor p53 protein for proteasomal degradation. p53
induces cell cycle arrest or apoptosis in response to cellular
stress (47). The MDM2 oncoprotein
promotes cell survival and cell cycle progression by inhibiting the
p53 tumor suppressor protein (48).
ABL1 (OMIM entry, *189980) was first
identified as an oncogene from the ABL family of nonreceptor
tyrosine kinases, and it transduces diverse extracellular signals
to protein networks that control proliferation, survival, migration
and invasion of cells (49).
ABL1 encodes a cytoplasmic and nuclear protein tyrosine
kinase, which is involved in cell differentiation, division and
adhesion, and the stress response (50). Alterations of ABL1 by
chromosomal rearrangement or viral transduction leads to malignant
transformation (51). The
overexpression of microRNA (miR)-203 leads to a poor survival of
cancer, due to its oncogenic function; however, miR-203 exhibits
tumor-suppressor qualities in PC by inhibiting the expression of
ABL1 and BCR-ABL1, resulting in an inhibition of cell
proliferation (52).
Overall, through the combined pathway analysis of
GWAS and gene expression, 7 pathways were demonstrated to be
significant by meta-analysis performed by the present study. Among
all the significantly expressed genes, only one gene, ABL1,
was differentially expressed in the both data sources. The present
study identified MYC as the most probable gene associated
with the TGF-β signaling pathway in PC. In conclusion, the results
of the present analysis provide possible factors for the occurrence
of PC, and the identification of pathways and genes provides
valuable data for investigating the pathogenesis of PC. However,
bioinformatics analysis generally lacks experimental support, so
additional study is required to verify the results of the present
study.
Acknowledgements
The authors wish to express their warm thanks to
Fenghe Information Technology Co., Ltd (Shanghai, China), whose
ideas and help provided valuable dimensions to the present
study.
References
|
1
|
Thomas J, Kim M, Balakrishnan L, Nanjappa
V, Raju R, Marimuthu A, Radhakrishnan A, Muthusamy B, Khan AA,
Sakamuri S, et al: Pancreatic cancer database: An integrative
resource for pancreatic cancer. Cancer Biol Ther. 15:963–967. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lowenfels AB and Maisonneuve P:
Epidemiology and risk factors for pancreatic cancer. Best Pract Res
Clin Gastroenterol. 20:197–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hruban RH, Adsay NV, Albores-Saavedra J,
Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G,
Longnecker DS, et al: Pancreatic intraepithelial neoplasia: A new
nomenclature and classification system for pancreatic duct lesions.
Am J Surg Pathol. 25:579–586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griffin CA, Hruban RH, Long PP, Morsberger
LA, Douna-Issa F and Yeo CJ: Chromosome abnormalities in pancreatic
adenocarcinoma. Genes Chromosomes Cancer. 9:93–100. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarkar FH, Banerjee S and Li Y: Pancreatic
cancer: Pathogenesis, prevention and treatment. Toxicol Appl
Pharmacol. 224:326–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strimpakos A, Saif MW and Syrigos KN:
Pancreatic cancer: From molecular pathogenesis to targeted therapy.
Cancer Metastasis Rev. 27:495–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hingorani SR, Petricoin EF, Maitra A,
Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD,
Hitt BA, et al: Preinvasive and invasive ductal pancreatic cancer
and its early detection in the mouse. Cancer Cell. 4:437–450. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein AM, Fraser MC, Struewing JP,
Hussussian CJ, Ranade K, Zametkin DP, Fontaine LS, Organic SM,
Dracopoli NC, Clark WH Jr, et al: Increased risk of pancreatic
cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J
Med. 333:970–975. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pellegata N, Sessa F, Renault B, Bonato M,
Leone BE, Solcia E and Ranzani GN: K-ras and p53 gene mutations in
pancreatic cancer: Ductal and nonductal tumors progress through
different genetic lesions. Cancer Res. 54:1556–1560.
1994.PubMed/NCBI
|
|
11
|
Grau AM, Zhang L, Wang W, Ruan S, Evans
DB, Abbruzzese JL, Zhang W and Chiao PJ: Induction of p21waf1
expression and growth inhibition by transforming growth factor beta
involve the tumor suppressor gene DPC4 in human pancreatic
adenocarcinoma cells. Cancer Res. 57:3929–3934. 1997.PubMed/NCBI
|
|
12
|
Sorio C, Baron A, Orlandini S, Zamboni G,
Pederzoli P, Huebner K and Scarpa A: The FHIT gene is expressed in
pancreatic ductular cells and is altered in pancreatic cancers.
Cancer Res. 59:1308–1314. 1999.PubMed/NCBI
|
|
13
|
Jones S, Zhang X, Parsons DW, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et
al: Core signaling pathways in human pancreatic cancers revealed by
global genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Biankin AV, Waddell N, Kassahn KS, Gingras
MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J,
et al: Pancreatic cancer genomes reveal aberrations in axon
guidance pathway genes. Nature. 491:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosenfeldt MT, O'Prey J, Morton JP, Nixon
C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, et al:
p53 status determines the role of autophagy in pancreatic tumour
development. Nature. 504:296–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cantor RM, Lange K and Sinsheimer JS:
Prioritizing GWAS results: A review of statistical methods and
recommendations for their application. Am J Hum Genet. 86:6–22.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Croteau-Chonka DC, Qiu W, Carey VJ, Weiss
ST and Raby BA: Gene set enrichment analyses of gene expression
associations with asthma control hint at candidate drug pathways.
Am J Respir Crit Care Med. 189:A52742014.
|
|
18
|
Kato S, Hayakawa Y, Sakurai H, Saiki I and
Yokoyama S: Mesenchymal-transitioned cancer cells instigate the
invasion of epithelial cancer cells through secretion of WNT3 and
WNT5B. Cancer Sci. 105:281–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu H and Liu B: CPEB4 is a candidate
biomarker for defining metastatic cancers and directing
personalized therapies. Med Hypotheses. 81:875–877. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gröger C: The role of Neuropilin 2 in
hepatocellular carcinoma: From meta-analysis to target
characterization. Journal. 2013.
|
|
21
|
Amundadottir L, Kraft P,
Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA,
Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, et al:
Genome-wide association study identifies variants in the ABO locus
associated with susceptibility to pancreatic cancer. Nat Genet.
41:986–990. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maupin KA, Sinha A, Eugster E, Miller J,
Ross J, Paulino V, Keshamouni VG, Tran N, Berens M, Webb C and Haab
BB: Glycogene expression alterations associated with pancreatic
cancer epithelial-mesenchymal transition in complementary model
systems. PloS One. 5:e130022010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joslyn G, Ravindranathan A, Brush G,
Schuckit M and White RL: Human variation in alcohol response is
influenced by variation in neuronal signaling genes. Alcohol Clin
Exp Res. 34:800–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40(Database
Issue): D109–D114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rice WR: A consensus combined P-value test
and the family-wide significance of component tests. Biometrics.
303–308. 1990. View
Article : Google Scholar
|
|
28
|
Edwards YJ, Beecham GW, Scott WK, Khuri S,
Bademci G, Tekin D, Martin ER, Jiang Z, Mash DC, ffrench-Mullen J,
et al: Identifying consensus disease pathways in Parkinson's
disease using an integrative systems biology approach. PLoS One.
6:e169172011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Unsicker K, Spittau B and Krieglstein K:
The multiple facets of the TGF-β family cytokine
growth/differentiation factor-15/macrophage inhibitory cytokine-1.
Cytokine Growth Factor Rev. 24:373–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murre C, McCaw PS and Baltimore D: A new
DNA binding and dimerization motif in immunoglobulin enhancer
binding, daughterless, MyoD, and myc proteins. Cell. 56:777–783.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dominguez-Sola D, Ying CY, Grandori C,
Ruggiero L, Chen B, Li M, Galloway DA, Gu W, Gautier J and
Dalla-Favera R: Non-transcriptional control of DNA replication by
c-Myc. Nature. 448:445–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL
and Reddy SA: The PI 3-kinase/Akt signaling pathway is activated
due to aberrant Pten expression and targets transcription factors
NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene.
23:8571–8580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grippo PJ and Sandgren EP:
Acinar-to-ductal metaplasia accompanies c-myc-induced exocrine
pancreatic cancer progression in transgenic rodents. Int J Cancer.
131:1243–1248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Köenig A, Linhart T, Schlengemann K,
Reutlinger K, Wegele J, Adler G, Singh G, Hofmann L, Kunsch S, Büch
T, et al: NFAT-induced histone acetylation relay switch promotes
c-Myc-dependent growth in pancreatic cancer cells.
Gastroenterology. 138:1189–1199, e1–e2. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Calzone L, Gelay A, Zinovyev A, Radvanyi F
and Barillot E: A comprehensive modular map of molecular
interactions in RB/E2F pathway. Mol Syst Biol. 4:1732008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abba MC, Fabris VT, Hu Y, Kittrell FS, Cai
WW, Donehower LA, Sahin A, Medina D and Aldaz CM: Identification of
novel amplification gene targets in mouse and human breast cancer
at a syntenic cluster mapping to mouse ch8A1 and human ch13q34.
Cancer Res. 67:4104–4112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tarragona M, Pavlovic M, Arnal-Estapé A,
Urosevic J, Morales M, Guiu M, Planet E, González-Suárez E and
Gomis RR: Identification of NOG as a specific breast cancer bone
metastasis-supporting gene. J Biol Chem. 287:21346–21355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yasui K, Okamoto H, Arii S and Inazawa J:
Association of over-expressed TFDP1 with progression of
hepatocellular carcinomas. J Hum Genet. 48:609–613. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kebebew E, Peng M, Treseler PA, Clark OH,
Duh QY, Ginzinger D and Miner R: Id1 gene expression is
up-regulated in hyperplastic and neoplastic thyroid tissue and
regulates growth and differentiation in thyroid cancer cells. J
Clin Endocrinol Metab. 89:6105–6111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gumireddy K, Li A, Gimotty PA,
Klein-Szanto AJ, Showe LC, Katsaros D, Coukos G, Zhang L and Huang
Q: KLF17 is a negative regulator of epithelial-mesenchymal
transition and metastasis in breast cancer. Nat Cell Biol.
11:1297–1304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
D'Abronzo FH, Swearingen B, Klibanski A
and Alexander JM: Mutational Analysis of activin/transforming
growth factor-beta type I and type II receptor kinases in human
pituitary tumors. J Clin Endocrinol Metab. 84:1716–1721. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Casey B and Hackett BP: Left-right axis
malformations in man and mouse. Curr Opin Genet Dev. 10:257–261.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shore EM, Xu M, Feldman GJ, Fenstermacher
DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, et
al: A recurrent mutation in the BMP type I receptor ACVR1 causes
inherited and sporadic fibrodysplasia ossificans progressiva. Nat
Genet. 38:525–527. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Masui T, Long Q, Beres TM, Magnuson MA and
MacDonald RJ: Early pancreatic development requires the vertebrate
Suppressor of Hairless (RBPJ) in the PTF1 bHLH complex. Genes Dev.
21:2629–2643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sasaki M, Kawahara K, Nishio M, Mimori K,
Kogo R, Hamada K, Itoh B, Wang J, Komatsu Y, Yang YR, et al:
Regulation of the MDM2-P53 pathway and tumor growth by PICT1 via
nucleolar RPL11. Nat Med. 17:944–951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mayo LD and Donner DB: A
phosphatidylinositol 3-kinase/Akt pathway promotes translocation of
Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA.
98:11598–11603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Greuber EK, Smith-Pearson P, Wang J and
Pendergast AM: Role of ABL family kinases in cancer: From leukaemia
to solid tumours. Nat Rev Cancer. 13:559–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shaul Y and Ben-Yehoyada M: Role of c-Abl
in the DNA damage stress response. Cell Res. 15:33–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Barilá D and Superti-Furga G: An
intramolecular SH3-domain interaction regulates c-Abl activity. Nat
Genet. 18:280–282. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Greither T, Grochola LF, Udelnow A,
Lautenschläger C, Würl P and Taubert H: Elevated expression of
microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated
with poorer survival. Int J Cancer. 126:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|