Introduction
Soft-tissue sarcomas (STSs) are a heterogeneous
group of solid malignancies arising from the muscles, fat,
connective tissue, nerves and blood vessels. The global incidence
of STS is ~5 cases per 100,000 individuals (1,2). The most
common anatomical sites of occurrence are the extremities, followed
by the trunk and retroperitoneum (3).
En bloc resection of tumors along with a cuff of healthy tissue is
the typical treatment for STS, as incomplete excision with positive
microscopic surgical margins has been observed to be the strongest
risk factor for local recurrence and distant metastasis (4). Due to an increased understanding of the
pathology of STS, the evolution of surgical techniques and the
development of adjuvant therapies, including radiotherapy (RT) and
chemotherapy (CT), limb-sparing surgery has become the preferred
choice of treatment due to an improved preservation of limb
function and a post-operative prognosis equivalent to amputation
(3–5).
At present, only 10% of patients with STS of the extremities
undergo amputation surgery (5).
However, when the tumor is in close proximity to the
skin layer, with or without ulceration, bacterial colonization and
inflammation may exist pre-operatively within the surgical field,
which theoretically increases the risk of post-operative infection
(4–6).
Furthermore, wide excision of STS of the extremities results in a
large tissue deficit that is typically unsuitable for primary
closure; thus, soft-tissue reconstruction is necessary for
limb-sparing surgery in these cases (6). This adds to the difficulty of surgical
techniques, therefore, patients are expected to demonstrate an
increased risk of major wound complications. Previous studies have
reported a wide variety of wound complications following
reconstruction, including cellulitis, abscess, wound dehiscence,
seroma, hematoma and necrosis of the muscle flap (1–7). These
complications can lead to unfavorable outcomes of treatment and
postponed adjuvant therapy.
Therefore, proactive wound-care measures are
imperative to decrease the risk of wound complications associated
with reconstruction following wide resection of STSs with
ulceration or impending ulceration. Negative pressure wound therapy
(NPWT), also known as vacuum-assisted closure, is a revolutionary
technique for the management of complex wounds. Previous studies
have focused on the merits of NPWT compared with traditional
methods in the treatment of complex wound defects (8). Despite a lack of high-level evidence,
studies have indicated that NPWT may facilitate wound healing,
prepare the wound bed for skin grafts or flaps, decrease the risk
of infection and reduce the labor of clinicians (9–11).
Conventional indications for NPWT include traumatic wounds, wounds
with acute or chronic infections, ulcers, diabetic foot ulcers,
dehiscent wounds and burns (12).
However, to the best of our knowledge, there have been no reports
concerning the application of NPWT as an adjunct to the treatment
of extremity STS with skin involvement. In the present study, this
novel wound care measure was used on tissue-deficient wounds caused
by wide resection of extremity STS (with ulceration or impending
ulceration) as preparation for secondary wound closure. The
efficacy of NPWT in reducing major wound complications was
assessed.
Patients and methods
Patient data
Between February 2012 and January 2013, 5 patients
with extremity STS with skin involvement (ulceration or impending
ulceration) were enrolled in the present study. The data of the
patients is summarized in Table I.
The cohort consisted of 4 men and 1 woman, with a mean age of 48
years (range, 24–68 years) and diagnoses of undifferentiated
pleomorphic sarcoma (n=2), leiomyosarcoma (n=1), synovial sarcoma
(n=1) and epithelioid sarcoma (n=1). The tumor sites were the
forearm (n=2), thigh (n=1), knee (n=1) and leg (n=1). A total of 3
patients presented with ulcers on initial examination, and the
remaining patients exhibited excessive skin tension, marked skin
thinning, vanishing of subcutaneous fat and pigmentation, which
indicated impending ulceration. At diagnosis, 3 patients presented
with recurrent STS and previous surgery on the tumor sites. All
STSs were localized tumors, and no metastases or comorbidities were
detected. The mean duration of follow-up was 26 months (range,
12–36 months).
 | Table I.Patient data. |
Table I.
Patient data.
| Case no. | Gender | Age, years | Diagnosis | Tumor site | Skin involvement | Tumor size,
cm3 | AJCC stage |
|---|
| 1 | Male | 30 | UPS | Right thigh | IU | 461.5 | III |
| 2 | Male | 57 | LS | Right knee | Ulcer | 453.5 | III |
| 3 | Male | 62 | UPS | Left forearm | IU | 7.5 | IIA |
| 4 | Male | 24 | ES | Right leg | Ulcer | 20.1 | III |
| 5 | Female | 68 | SS | Right forearm | Ulcer | 113.8 | IIB |
Pre-operative assessment
The pre-operative evaluation assessed a variety of
factors, including overall health status, limb function, tumor
location, size and depth, tumor involvement with major
neurovascular structures, histological subtype of tumor and distant
metastasis (typically lung metastasis). Prophylactic intravenous
antibiotics [cephazolin, 1.0 g intravenously (IV), 30 min prior to
surgery] were used in all cases, and a routine bacterial culture
was performed pre-operatively in cases with ulceration as an
indication for intravenous antibiotic use. Fine-needle aspiration
biopsy was performed pre-operatively to determine the histological
diagnosis. In accordance with the American Joint Committee on
Cancer staging system (13), there
were 3 stage III cases, 1 case of stage IIA and 1 case of stage
IIB.
Surgery and NPWT procedure
Wide tumor resection with a radial 2 to 4-cm margin
was performed in all cases. Negative surgical margins were achieved
according to the findings of the post-operative pathological
examination. Immediate or secondary gastrocnemius muscle flap
reconstruction was performed in 2 patients to cover the exposed
bone and neurovascular structures. To reduce the risk of major
wound complications and to prepare the wound bed for secondary
soft-tissue reconstruction, a NPWT device (Smith & Nephew
Medical Ltd, Hull, UK) was applied as coverage on the wound
deficit. Polyurethane ether foam (15×10×1 cm) was cut in accordance
with the shape of the wound deficit and was used as a dressing. In
the majority of cases, 1 piece of foam was sufficient to span the
wound surface. The dressing was cut to cover the undetermined areas
and tracks for dead cavity closure. All foam dressings were fixed
to the skin edge by suturing. Continuous NPWT measuring at −200 to
−300 mmHg, as well as intravenous antibiotics (cephazolin, 1.0 g
IV, once every 8 h for 7–10 days), were administered for 7–10 days
post-operatively (mean time, 8 days). Close observation was
conducted for the early detection of complications (infection,
hemorrhage and blistering). During removal of the dressing, an
inspection of wound appearance, surface area, depth and exudate
amount was conducted. If the granulation formation was favorable
with no signs of infection, secondary soft-tissue reconstruction
(muscle flap or skin grafting) was performed on the wound
deficit.
Results
Outcomes of treatment
The outcomes of the treatment are summarized in
Table II. The mean wound area
following resection of the tumor was 73 cm2 (range,
45–110 cm2). Upon dressing removal, all cases
demonstrated a decreased wound size and depth, favorable
granulation formation and a clean skin edge, indicating that the
wound bed was feasible for secondary soft-tissue reconstruction.
Pain and minor bleeding during dressing removal was recorded in all
cases, which was attributable to the growth of granulation into the
foam dressing; however, no severe complications were detected. All
patients tolerated continuous NPWT well, and no interruption of
NPWT occurred due to discomfort. All muscle flaps and skin grafts
proved to be viable and healed favorably on discharge. No infection
or other major wound complications were recorded. Post-operative CT
(6–8 cycles) was administered in 2 patients, and neoadjuvant CT (2
cycles) was administered in 1 patient who presented with a
recurrent mass in close proximity to major nerves and vessels. Each
cycle of treatment consisted of 120 mg/m2 cisplatin, 30
mg/m2 doxorubicin and 2.0 g/m2 ifosfamide.
Post-operative functional and cosmetic outcomes were acceptable,
and all patients were satisfied with the outcome of their
treatment. During routine follow-up, 1 patient exhibited local
recurrence at 12 months after the initial surgery, presenting with
recurrent undifferentiated pleomorphic sarcoma at the previous
tumor site. Re-excision of the tumor was successfully performed,
allowing the limb to be spared. No evidence of remote metastasis
was detected, and all patients remained alive at the end of
follow-up, which was performed for a mean time of 26 months.
 | Table II.Treatment and prognosis of
patients. |
Table II.
Treatment and prognosis of
patients.
| Case no. | Surgery | Deficit area,
cm2 | Suction mode | Pressure, mmHg | Surgical
margin | CT | Duration of NPWT,
days |
Recurrence/metastasis |
|---|
| 1 | WR+S+F | 110 | C | −250 | Negative | DIA | 10 | – |
| 2 | WR+S+F | 100 | C | −300 | Negative | – | 8 | – |
| 3 | WR+S | 60 | C | −200 | Negative | – | 7 | – |
| 4 | WR+S | 48 | C | −300 | Negative | DIA | 7 | Recurrence |
| 5 | WR+S | 45 | C | −300 | Negative | – | 7 | – |
Example cases
Case 1
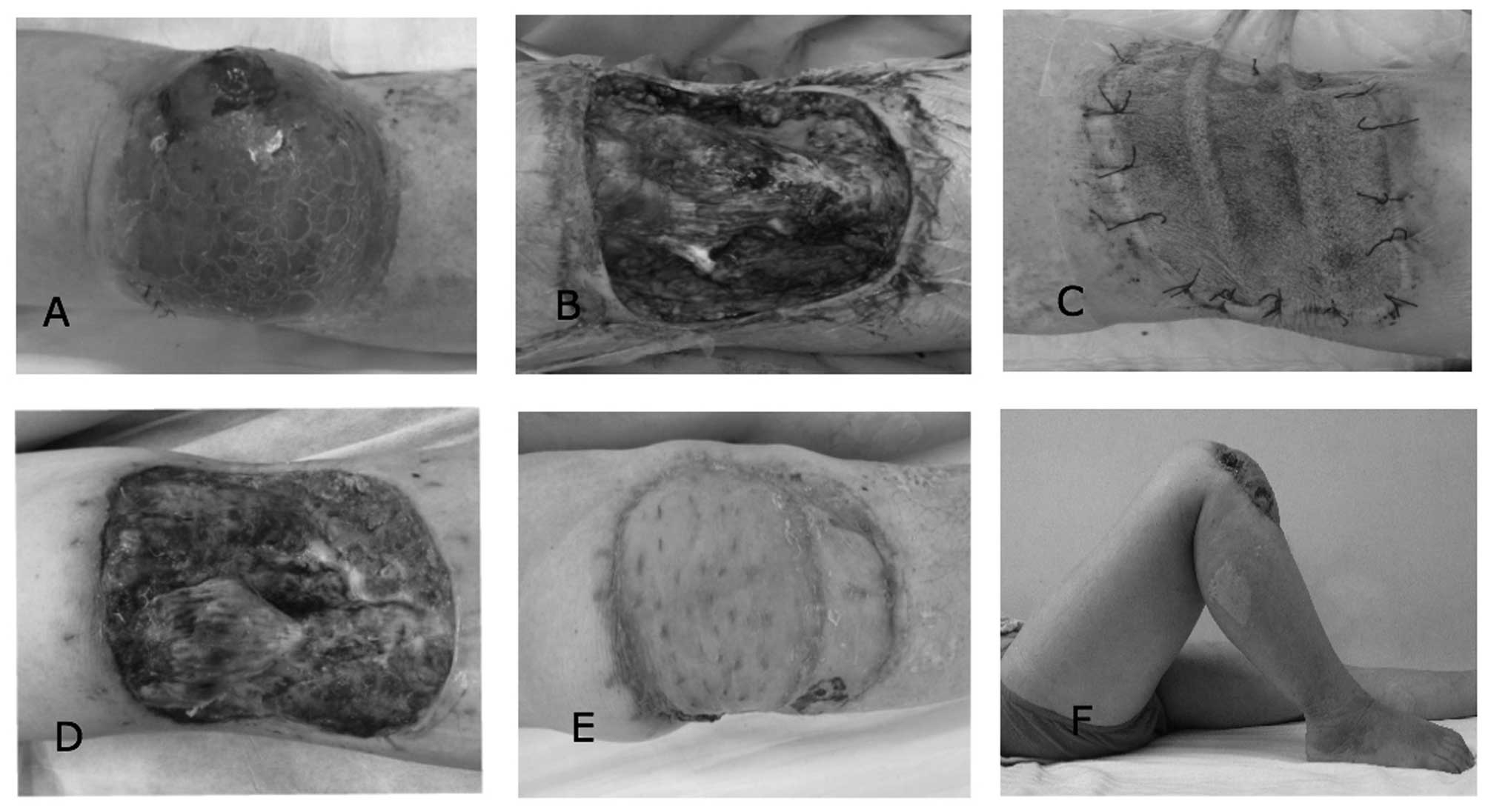
A 57-year-old male patient was referred to the
Department of Orthopedic Surgery, General Hospital of Jinan
Military Commanding Region (Jinan, Shandong) with a mass and
ulceration of the right knee (Fig.
1A). The function of the right knee was compromised (range of
motion, 5–35°), and histological findings revealed spindle cells
and myofibrils, indicating a diagnosis of leiomyosarcoma.
Pre-operative bacterial culture determined positive results for
Staphylococcus epidermidis within the deep tissue of the
surgical region. Wide excision of the tumor resulted in a wound
defect measuring 100 cm2 and exposed the proximal tibia
bone surface (Fig. 1B). To reduce the
risk of major wound complications, immediate wound closure was
avoided and NPWT was used to cover the wound defect, with
simultaneous administration of intravenous antibiotics (Fig. 1C). The microscopic negative surgical
margin was determined by pathological examination. Wound inspection
at dressing removal 8 days after surgery indicated that granulation
formation was favorable, with no evidence of infection (Fig. 1D). Secondary muscle flap
(gastrocnemius muscle) followed by free split-thickness skin
grafting was performed. No evidence of recurrence or metastasis was
detected. The patient demonstrated improved knee function (range of
motion, 0–100°), and the appearance of the closed wound defect was
acceptable (Fig. 1E and F). The
patient was satisfied with the outcome of treatment at 36 months
post-surgery.
Case 2
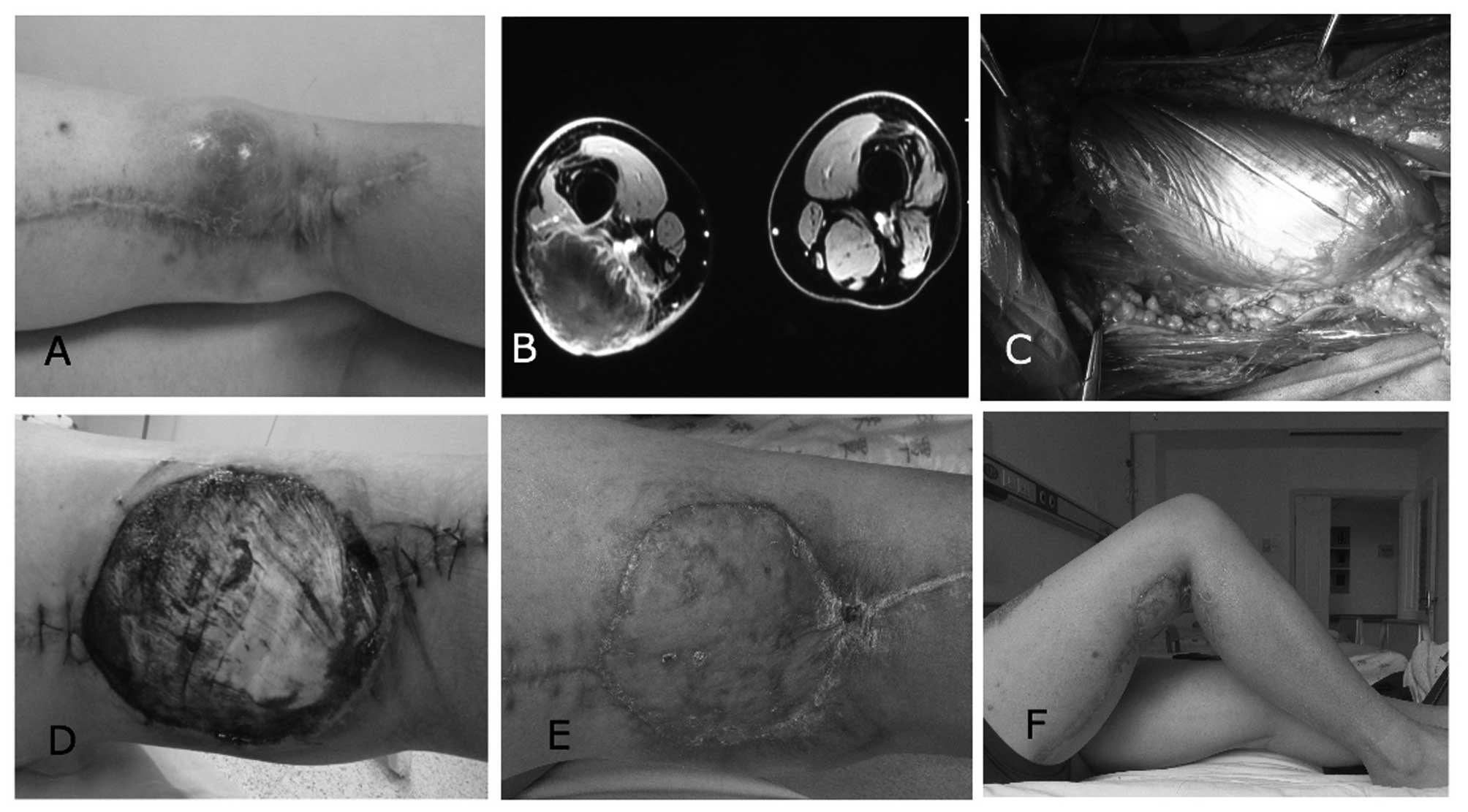
A 30-year-old male patient was diagnosed with
recurrent undifferentiated pleomorphic sarcoma and demonstrated
impending ulceration following previous surgery on the right distal
posterior thigh (Fig. 2A). The
function of the patient's right knee was compromised (range of
motion, 0–40°). Magnetic resonance imaging indicated that the tumor
was in close proximity to neurovascular structures, and no tumor
encasement of major nerves and vessels was detected (Fig. 2B). To achieve favorable surgical
margins, neoadjuvant CT was commenced (120 mg/m2
cisplatin, 30 mg/m2 doxorubicin and 2.0 g/m2
ifosfamide, for 2 cycles pre-operatively at an interval of 7 days).
Aggressive excision of the tumor resulted in a wound defect
measuring 110 cm2, with exposed neurovascular
structures. A gastrocnemius muscle flap was immediately used to
separate the neurovascular structures from the subsequent negative
pressure (Fig. 2C). NPWT was used to
cover the wound defect as preparation for secondary wound closure.
Microscopic negative surgical margins were determined by
pathological examination. Wound inspection at dressing removal 10
days after surgery indicated that the wound bed was feasible for
skin grafts, with favorable granulation formation (Fig. 2D). Following split-thickness skin
grafting, the patient was administered 6 cycles of post-operative
CT (120 mg/m2 cisplatin, 30 mg/m2 doxorubicin
and 2.0 g/m2 ifosfamide). The cosmetic and functional
outcomes (range of motion, −5 to 90°) were acceptable (Fig. 2E and F). The patient returned to
normal daily activities and was satisfied with the outcome of
treatment at 12 months post-surgery.
Discussion
The surgical approach to the treatment of STS of the
extremities has undergone significant change over the past decade.
Aggressive resection of the tumor along with the pseudocapsule may
assist in achieving microscopic negative surgical margins, which is
deemed to be the strongest predictor of patient prognosis (14). With advances in multidisciplinary
personalized treatment of STS, limb-sparing surgeries have become
the preferred choice of treatment over amputations (5). However, extensive tissue defects
following surgery have challenged the efficacy and feasibility of
limb salvage, as under certain circumstances primary closure is
infeasible due to insufficient soft tissue and exposed bone,
tendons, nerves or vessels (15).
Conventional measures to treat such wound defects following the
wide excision of tumors are primarily composed of a variety of
soft-tissue reconstruction methods. Previous studies have reported
the use of various flaps (local, regional, distant and free) and
skin grafts (split-thickness and full-thickness) as an important
adjunct to treatment for STS (16,17).
However, the aforementioned complex techniques may predispose
patients to an increased risk of major wound complications,
including infection, seromas, hematomas and necrotic flaps. A
number of studies have reported that the complication rates
associated with reconstruction may be as high as 16–56% (18,19). In
the present study, all STS patients presented with ulceration or
impending ulceration. This increased the risk of post-operative
infection due to potential bacterial colonization or inflammation
within the surgical field. These major wound complications may
result in delayed post-operative adjuvant therapy and an increased
risk of amputation, and may have a detrimental impact on
oncological outcomes.
In the present study, in order to manage wound
defects while preventing the occurrence of major wound
complications, NPWT was applied to facilitate wound closure. NPWT
was used as a bridge for wound closure in STS affecting the skin,
as the cases in the present study were expected to demonstrate
bacterial contamination or inflammation within the surgical field.
Such patients are exposed to a considerable risk of post-operative
infection; therefore, proactive wound care measures reducing the
risk of infection are imperative. The present study used NPWT as a
reliable method of assisting in reducing the risk of infection, as
it is able to separate the wound from contamination and has been
observed to be efficient in controlling bacterial proliferation
(20). Li et al (20) investigated the efficacy of regulated
NPWT in the treatment of infected blast injury in swine. The
results of this study indicated that NPWT reduced the bacterial
load efficiently compared with gauze dressing treatments (20). Blum et al (21) compared the efficacy of NPWT and
conventional dressings for the treatment of open tibial fractures
that underwent delayed soft-tissue coverage, and NPWT was observed
to reduce the risk of deep infection by almost 80% (21). As well as preventing infection and
decreasing the bacterial load, NPWT has demonstrated a wide variety
of merits in the treatment of complex wound defects. Previous
studies have reported that NPWT is able to decrease wound size,
maintain a moist atmosphere for wound healing, reduce edema and
assist with the closure of areas not fully closed in the wound,
also termed dead cavities (8,22). Notably, microscopically NPWT is able
to promote granulation, vascularization, epithelialization and the
synthesis of fibrin within the wound (23,24).
However, there is little relevant literature regarding the use of
NPWT as an adjunct to wound closure in patients with tumors. Oh
et al (25) assessed the
efficacy of NPWT in conjunction with secondary full-thickness skin
grafting for the treatment of melanoma on the foot, and observed
acceptable functional and cosmetic outcomes. Katz et al
(26) used NPWT as an adjunct to
surgery for lymphangioma in children. The results of this study
indicated that NPWT was effective and safe for post-operative wound
closure, and that NPWT also decreased the risk of recurrence and
infection (26). NPWT means that
daily dressing changes are unnecessary, as accumulation of fluid at
wound sites is deterred by the drainage; thus, the labor required
from clinicians is reduced (22).
Therefore, the aforementioned characteristics make NPWT an ideal
adjunct to the management of wound defects following excision of
STS of the extremities with potential risk of major wound
complications.
Treatment of extremity STSs consists of a meticulous
pre-operative evaluation, complete removal of the tumor and
surrounding healthy tissue, soft-tissue reconstruction and adjuvant
therapies, including RT or CT (2,3). As STSs
are composed of a heterogeneous group of disease subtypes with
various manifestations, a pre-operative evaluation is of importance
and determines the treatment strategy. Evaluation must assess tumor
involvement with peripheral major neurovascular structures and
whether the tumor arises from or is encasing the major nerves or
blood vessels; if the expected function of the distal limb is
predicted to be poor, amputation may be preferable to limb-sparing
surgery (27). Previously, a number
of attempts have been made to reconstruct the nerves and vessels,
so as to expand the indications for limb-sparing surgery among
patients with STSs of the extremities. In cases with tumor
involvement of the vessels, reversed saphenous vein grafts, femoral
venous grafts or synthetic grafts have been used as options for
limb salvage surgery (28). However,
post-operative functional results are less predictable, and the
patients exhibit high risks of complications and amputation
(28). Nerve transfer may be a
promising alternative for amputation in cases with nerve
encasement; however, this is currently a complex technique, which
is only used for brachial plexus reconstruction (29). The feasibility of nerve transfer in
treating defects following a wide resection of an STS remains to be
demonstrated. Notably, all choices regarding limb-sparing surgery
should not be made until the goal of negative surgical margins is
achievable. In the present study, a patient with recurrent
undifferentiated pleomorphic sarcoma in close proximity to major
nerves and vessels of the lower extremities was administered
neoadjuvant CT to achieve favorable surgical margins. This
procedure proved to be effective during surgery, and the goal of a
wide excision of the tumor was achieved.
When managing large wound defects with or without
exposed bone, tendons, nerves and vessels following resection of
the tumor, soft-tissue reconstruction should be conducted. In
clinical practice, cases with indications for reconstruction are
not rare in the treatment of STS of the extremities, and a previous
study reported that 40/100 consecutive patients with STS underwent
immediate soft-tissue reconstruction (18). The spectrum of associated surgical
techniques involves flaps (local, regional, distant and free flaps)
and split-thickness or full-thickness skin grafts (6). The specific techniques selected are
dependent on a comprehensive evaluation of the defect size and
depth, the location of tumor, the tumor proximity to neurovascular
structures and the overall health status (30). In the current study, NPWT allowed for
secondary soft-tissue reconstruction for cases with a high risk of
post-operative wound complications. Furthermore, using a muscle
flap as coverage on the exposed neurovascular structures allowed
for application of NPWT on the wound defect, as exposed nerves and
vessels are vulnerable to negative pressure, and are considered to
be a contraindication for NPWT (22).
A number of factors should be taken into account
when implementing NPWT on wound defects following wide tumor
resection. The goal of using NPWT in the current study was to
prepare the wound bed for secondary soft-tissue reconstruction,
while reducing the risk of infection and other major wound
complications. However, the optimal suction protocol to be applied
to this situation remains to be elucidated, and the reported
methods are primarily empirical. Previous studies regarding the use
of NPWT primarily concern traumatic wounds with or without
infection, and the most frequently used pressure is −80 to −125
mmHg (31). In cases with fragile
wound edges, low perfusion, poor tolerability or skin grafts, a
minor pressure (<125 mmHg) is often attempted (8). Furthermore, negative pressure protocols
are typically used at one of two settings, namely, continuous or
intermittent. In one previous study, intermittent suction was
observed to cause a more profound tissue response compared with
conventional methods; however, this suction mode may cause
discomfort and intolerability in patients (32). In the present study, continuous
negative pressure at a relatively high level of −200 to 300 mmHg
was used on the wound beds. This suction protocol was used as it
had previously been observed to be effective in clinical practice
and as stronger suction was not considered since intense negative
pressure may increase the risk of hemorrhage (10). Intermittent suction was additionally
avoided due to potential patient intolerability. The duration of
NPWT is dependent on the goal of treatment and the wound status. In
the present study, devices were removed on post-operative days
7–10, when granulation formation was favorable. According to
observations made at the point of foam dressing removal, the
drainage protocol used in the present study was effective in
reducing edema, removing exudation, promoting granulation formation
and assisting with the prevention of wound infection. An increased
duration of NPWT was avoided, as the risk of infection increases
along with prolonged drainage duration, and early initiation of
post-operative adjuvant therapy additionally required a shortened
drainage duration (18).
Potential complications associated with NPWT should
be taken into account during clinical practice. The associated
complications include hemorrhage, blistering, odors, skin erosion
around the suction tube, sepsis and obstruction of the suction tube
(33). A number of the aforementioned
complications, although rarely observed, may be severe; thus, close
observation and assessment of the wound throughout the entire
process of NPWT is necessary. In the present study, frequent
observation of exudation, wound edges and the patency of the
drainage tube was conducted. No major complications were detected,
whereas pain and minor bleeding during dressing removal were
recorded in all cases; this was in accordance with the majority of
the outcomes in previous studies (11,22). Pain
and minor bleeding were attributed to the growth of granulation
tissue into the foam dressing; however, all patients responded to
these complications with good tolerability.
Notably, the sample size in the current study was
small, as extremity STSs with skin involvement account for a small
fraction of the total STS patients admitted by the Department of
Orthopedic Surgery, General Hospital of Jinan Military Commanding
Region. In the present study, when NPWT was used as an adjunct to
the treatment of extremity STS with ulceration or impending
ulceration, the outcomes of treatment were favorable. However,
studies on larger sample sizes and with a control group are
required to test the efficacy of NPWT in decreasing major wound
complications compared with conventional treatment.
In conclusion, when wide tumor excision and negative
surgical margins are achievable, NPWT in conjunction with
soft-tissue reconstruction may be a reliable and safe adjunct to
the management of large soft-tissue deficits caused by aggressive
excision of extremity STS with ulceration or impending
ulceration.
References
|
1
|
Rosenberg AE: WHO classification of soft
tissue and bone, fourth edition: Summary and commentary. Curr Opin
Oncol. 25:571–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kneisl JS, Coleman MM and Raut CP:
Outcomes in the management of adult soft tissue sarcomas. J Surg
Oncol. 110:527–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gronchi A, Colombo C and Raut CP: Surgical
management of localized soft tissue tumors. Cancer. 120:2638–2648.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trovik CS, Bauer HC, Alvegård TA, Anderson
H, Blomqvist C, Berlin O, Gustafson P, Saeter G and Wallöe A:
Surgical margins, local recurrence and metastasis in soft tissue
sarcomas: 559 surgically-treated patients from the Scandinavian
Sarcoma Group Register. Eur J Cancer. 36:710–716. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williard WC, Collin C, Casper ES, Hajdu SI
and Brennan MF: The changing role of amputation for soft tissue
sarcoma of the extremity in adults. Surg Gynecol Obstet.
175:389–396. 1992.PubMed/NCBI
|
|
6
|
Talbot SG, Athanasian EA, Cordeiro PG and
Mehrara BJ: Soft tissue reconstruction following tumor resection in
the hand. Hand Clin. 20(vi): 181–202. 2004. View Article : Google Scholar
|
|
7
|
Baldini EH, Lapidus MR, Wang Q, Manola J,
Orgill DP, Pomahac B, Marcus KJ, Bertagnolli MM, Devlin PM, George
S, et al: Predictors for major wound complications following
preoperative radiotherapy and surgery for soft-tissue sarcoma of
the extremities and trunk: Importance of tumor proximity to skin
surface. Ann Surg Oncol. 20:1494–1499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong HS, Lee BH, Lee HK, Kim HS, Moon MS
and Suh IS: Negative pressure wound therapy of chronically infected
wounds using 1% acetic Acid irrigation. Arch Plast Surg. 42:59–67.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orgill DP, Manders EK, Sumpio BE, Lee RC,
Attinger CE, Gurtner GC and Ehrlich HP: The mechanisms of action of
vacuum assisted closure: More to learn. Surgery. 146:40–51. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karaaslan F, Erdem Ş and Mermerkaya MU:
Wound management with vacuum-assisted closure in postoperative
infections after surgery for spinal stenosis. Int Med Case Rep J.
8:7–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu CC, Chew KY, Chen CC and Kuo YR:
Antimicrobial-impregnated dressing combined with negative-pressure
wound therapy increases split-thickness skin graft engraftment: A
simple effective technique. Adv Skin Wound Care. 28:21–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Putnis S, Khan WS and Wong JM: Negative
pressure wound therapy - a review of its uses in orthopaedic
trauma. Open Orthop J. 8:142–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lahat G, Tuvin D, Wei C, Anaya DA, Bekele
BN, Lazar AJ, Pisters PW, Lev D and Pollock RE: New perspectives
for staging and prognosis in soft tissue sarcoma. Ann Surg Oncol.
15:2739–2748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gronchi A, Verderio P, De Paoli A, Ferraro
A, Tendero O, Majò J, Martin J, Comandone A, Grignani G,
Pizzamiglio S, et al: Quality of surgery and neoadjuvant combined
therapy in the ISG-GEIS trial on soft tissue sarcomas of limbs and
trunk wall. Ann Oncol. 24:817–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barwick WJ, Goldberg JA, Scully SP and
Harrelson JM: Vascularized tissue transfer for closure of
irradiated wounds after soft tissue sarcoma resection. Ann Surg.
216:591–595. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boyce DE and Shokrollahi K: Reconstructive
surgery. BMJ. 332:710–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Turner AJ and Parkhouse N: Revisiting the
reconstructive ladder. Plast Reconstr Surg. 118:267–268. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lohman RF, Nabawi AS, Reece GP, Pollock RE
and Evans GR: Soft tissue sarcoma of the upper extremity: A 5-year
experience at two institutions emphasizing the role of soft tissue
flap reconstruction. Cancer. 94:2256–2264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geller DS, Hornicek FJ, Mankin HJ and
Raskin KA: Soft tissue sarcoma resection volume associated with
wound-healing complications. Clin Orthop Relat Res. 459:182–185.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Topaz M, Tan H, Li Y, Li W, Xun W,
Yuan Y, Chen S and Li X: Treatment of infected soft tissue blast
injury in swine by regulated negative pressure wound therapy. Ann
Surg. 257:335–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blum ML, Esser M, Richardson M, Paul E and
Rosenfeldt FL: Negative pressure wound therapy reduces deep
infection rate in open tibial fractures. J Orthop Trauma.
26:499–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Laat EH, van den Boogaard MH, Spauwen
PH, van Kuppevelt DH, van Goor H and Schoonhoven L: Faster wound
healing with topical negative pressure therapy in difficult-to-heal
wounds: A prospective randomized controlled trial. Ann Plast Surg.
67:626–631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim BS, Choi WJ, Baek MK, Kim YS and Lee
JW: Limb salvage in severe diabetic foot infection. Foot Ankle Int.
32:31–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scherer SS, Pietramaggiori G, Mathews JC,
Prsa MJ, Huang S and Orgill DP: The mechanism of action of the
vacuum-assisted closure device. Plast Reconstr Surg. 122:786–797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh BH, Lee SH, Nam KA, Lee HB and Chung
KY: Comparison of negative pressure wound therapy and secondary
intention healing after excision of acral lentiginous melanoma on
the foot. Br J Dermatol. 168:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katz MS, Finck CM, Schwartz MZ, Moront ML,
Prasad R, Timmapuri SJ and Arthur LG: Vacuum-assisted closure in
the treatment of extensive lymphangiomas in children. J Pediatr
Surg. 47:367–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferguson PC, Kulidjian AA, Jones KB,
Deheshi BM and Wunder JS: Peripheral nerve considerations in the
management of extremity soft tissue sarcomas. Recent Results Cancer
Res. 179:243–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mahendra A, Gortzak Y, Ferguson PC,
Deheshi BM, Lindsay TF and Wunder JS: Management of vascular
involvement in extremity soft tissue sarcoma. Recent Results Cancer
Res. 179:285–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SK and Wolfe SW: Nerve transfers for
the upper extremity: New horizons in nerve reconstruction. J Am
Acad Orthop Surg. 20:506–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong JC and Abraham JA: Upper extremity
considerations for oncologic surgery. Orthop Clin North Am.
45:541–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borgquist O, Ingemansson R and Malmsjö M:
Individualizing the use of negative pressure wound therapy for
optimal wound healing: A focused review of the literature. Ostomy
Wound Manage. 57:44–54. 2011.PubMed/NCBI
|
|
32
|
Younan G, Ogawa R, Ramirez M, Helm D,
Dastouri P and Orgill DP: Analysis of nerve and neuropeptide
patterns in vacuum-assisted closure-treated diabetic murine wounds.
Plast Reconstr Surg. 126:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang C, Leavitt T, Bayer LR and Orgill
DP: Effect of negative pressure wound therapy on wound healing.
Curr Probl Surg. 51:301–331. 2014. View Article : Google Scholar : PubMed/NCBI
|