Introduction
The mucin family of proteins are
high-molecular-weight glycoproteins, which provide protection for
the epithelial cell lining that is exposed to the external
environment (1). The mucin family is
classified into secreted and transmembrane mucins, and is involved
in the protection of the epithelial lining of the gastrointestinal
and respiratory tracts and duct linings present in the pancreas,
kidney, liver and mammary gland. Secreted mucins form a mucous
layer that provides a physical barrier, and transmembrane mucins
provide the physical barrier with ectodomains that are made up of
O-glycosylated tandem repeats (1).
Transmembrane mucins extend inside the cell through single
membranous regions and the cytoplasmic tail of the mucins transduce
signals to promote the growth and survival of the cell in response
to stress (1).
Mucin 1 (MUC1) is a transmembrane glycoprotein that
is overexpressed in various types of human carcinoma, including
breast, colon, lung and prostate cancer (2). MUC1 is synthesized as a single
polypeptide that undergoes auto-cleavage into two subunits, MUC1-N
and MUC1-C, which form a stable heterodimer at the cell surface
(3). The MUC1-N terminal subunit
contains variable numbers of glycosylated tandem repeats (4), while the MUC1-C terminal subunit
consists of a 58-amino acid extracellular domain, 28-amino acid
transmembrane domain and a 72-amino acid cytoplasmic domain
(2). MUC1 expression is induced by
certain cytokines, including tumor necrosis factor (TNF) α,
interferon (IFN) γ and interleukin (IL)-6. Alterations in MUC1
expression may contribute to chronic inflammation and cancer. The
activation of the nuclear factor kappa-light-chain-enhancer of
activated B cells (NF-κB) pathway is a clear mediator of
inflammation-induced cancer progression (5), and MUC1-C has been demonstrated to bind
IκB kinase (IKK) β (6) and NF-κB p65
(7). Helicobacter pylori
infection has been associated with MUC1 expression in inducing
inflammation and the development of gastric cancer (8). In addition, MUC1 interacts with
β-catenin and contributes to the activation of Wnt target genes
leading to tumorigenesis (9).
Prolonged MUC1 activation in chronic inflammation leads to growth
and survival of cells undergoing a stress response (8).
The synthesis of secretory mucins is also regulated
by cytokines, growth factors and bacterial products (10,11). Mucin
2 (MUC2) is a secretory mucin that forms the major component of the
intestinal mucus lining (1). MUC2 is
primarily expressed in colorectal goblet cells. Deregulation of
MUC2 expression at the epithelial cell surface provides a
microenvironment where bacteria initiate an inflammatory response
(12). Ulcerative colitis is a major
inflammatory bowel disease (IBD), which is characterized by
significant inflammation and depletion of mucin from goblet cells
(13). Therefore, IBD-associated
chronic inflammation increases the risk factor for colorectal
cancer (CRC), potentially by promoting genomic instability in a
microenvironment (14).
Overexpression of MUC2 and other secreted mucins by tumors also
protects tumor cells from recognition by anti-tumor immune
effectors and therefore contributes to cell transformation leading
to cancer (15). MUC2 in combination
with mucin 5AC (MUC5AC) is clustered on chromosome 11 (16). MUC5AC is predominantly expressed in
the mucus lining of the stomach and lung. Alterations in MUC2 and
MUC5AC expression are reported in lung, gastrointestinal,
pancreatic and liver cancer (17).
MUC5AC is downregulated in non-small cell lung carcinoma (NSCLC)
(18). Sialyl Lewis × antigen
expression is associated with MUC5AC in NSCLC, with those patients
exhibiting a poor survival time (19). MUC5AC expression in liver cancer has
been shown to be associated with high lymph node metastasis
(20). Alterations in mucin
expression may be significantly associated with histological grade,
clinical staging and prognosis of patients with CRC (17).
The majority of studies have reported that the
expression of mucins in CRC is primarily confined to the late stage
of the disease (20,21). The incidence of CRC is increasing in
young patients, specifically in the Middle East (22,23). The
average age of CRC detection in Saudi Arabia was 58 years between
the years 2000–2006; this is younger than the age of CRC detection
in the UK between the years 1996–2004, which was 68 years (22). Demographically, this indicates that
the occurrence of CRC develops earlier in patients from Saudi
Arabia. Although no studies have currently reported on CRC
detection in young patients, the publication of the present study,
and others, may mean that similar studies become more frequent.
Consequently, a biomarker is required to identify CRC in young
patients from Saudi Arabia. Due to the lack of data on mucin
expression in Saudi CRC patients, the present study aimed to
analyze the mucin expression profile in this ethnic group. The
study presented the expression profile of MUC1, MUC2 and MUC5AC in
various stages of CRC tissue using immunohistochemical staining. To
the best of our knowledge, this is the first study where
transmembrane MUC1 and secretory MUC2 and MUC5AC expression has
been compared at various stages of CRC in patients from Saudi
Arabia.
Materials and methods
Patient samples
The present study consisted of 22 patients that
underwent surgical resection of histologically confirmed CRC at
King Khalid University Hospital, King Saud University (Riyadh,
Saudi Arabia) between November 2012 and November 2013. The
demographics of the patients, including age, gender, tumor site and
histological stages were recorded in a database according to the
Union International Contre le Cancer-Tumor-Node-Metastais Staging
System and grading of CRC was in accordance with WHO classification
(24–26). Histologically adjacent normal tissue
from the margins of the tumors served as control tissue. All tissue
samples were diagnosed and classified by two pathologists and one
expert pathologist from the Department of Pathology, King Khalid
University Hospital, King Saud University. In order to minimize the
effect of radiotherapy and chemotherapy, all patients that had
undergone neoadjuvant or adjuvant therapy were excluded from the
present study. Control breast tumor samples and blood samples were
obtained from the Department of Surgery, King Khaled University
Hospital, King Saud University. Control blood samples were obtained
from Colorectal Research Center, Department of Surgery, College of
Medicine, King Khaled University Hospital, King Saud
University.
The present study was approved by the Ethics
Committee of King Saud University. Written informed consent was
obtained from the patients for this study.
Tissue microarray (TMA). The TMA was performed as
previously described (27).
Formalin-fixed paraffin-embedded (FFPE) CRC tissue blocks of the 22
patients were retrieved from the archives of King Khalid University
Hospital. The TMA was performed on the FFPE tumor blocks using a
manual tissue arrayer (Arraymold Tissue Microarrayer kit D; IHC
World LLC, Woodstock, MD, USA). Invasive carcinoma areas were
identified using hematoxylin (Leica Biosystems, Inc., Buffalo
Grove, IL, USA) and eosin Y (Sigma-Aldrich, St. Louis, MO, USA)
(H&E) stained slides by the aforementioned expert pathologist
from the Department of Pathology, King Khalid University Hospital,
King Saud University. To construct the TMA a 1 mm diameter needle
was used to take three cores from each FFPE block corresponding to
the rich tumor areas detected on the H&E slide. The cores were
inserted into the TMA paraffin block, which were subsequently
incubated at 37°C for 30 min to enhance the adhesion between the
cores and paraffin. TMA blocks were micro-dissected into 5-µm thick
sections using a semi-automatic microtome (Leica RM223; Leica
Microsystems GmbH, Wetzlar, Germany), and mounted on glass
slides.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (28). The slides with 5-µm
sections of tumor tissues and adjacent normal tissues were
deparaffinized in xylene (Sigma-Aldrich) and rehydrated using a
graded ethanol series (Sigma-Aldrich). Antigen was retrieved by
boiling the slides in a microwave oven for 15 min in 0.01 mol/l
citrate buffer (pH 6.0; Sigma-Aldrich). Endogenous peroxidase was
blocked with a 3% H2O2-methanol solution
(Sigma-Aldrich), and the slides were incubated in 10% normal goat
serum (Sigma-Aldrich) for 30 min to prevent nonspecific staining.
The tissue sections were incubated overnight at 4°C with the
following primary antibodies from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) at a dilution of 1:100: Rabbit polyclonal
anti-MUC1 (catalog no., sc-15333), rabbit polyclonal anti-MUC2
(catalog no., sc-15334) or mouse monoclonal anti-MUC5AC (catalog
no., sc-33667). The standard biotin-streptavidin-peroxidase method
was subsequently used with affinity purified goat anti-mouse IgM
secondary antibody (catalog no., RE7103; dilution, 5 µg/ml; Leica
Biosystems, Inc.), and the sections were lightly counterstained
with hematoxylin. Breast tumor was histologically used as positive
controls for MUC1, MUC2 and MUC5AC. As a negative control, the same
procedure was conducted without primary antibody. The expression of
MUC1, MUC2 and MUC5AC in tumor and adjacent normal samples was
analyzed using an eSlide capture device (Aperio CS2; Leica
Microsystems GmbH).
Image analysis
High-resolution, whole-slide digital scans of all
TMA glass slides were created using the eSlide capture device. The
digital slide images were viewed by ScanScope slide scanner
(ScanScope CS; Aperio ImageScope; Leica Microsystems GmbH), and
analyzed using Aperio's Image Analysis Algorithms (Leica
Microsystems GmbH). For each core, five fields of 0.2645
µm2 were randomly selected. Color Deconvolution
Algorithm (Aperio; Leica Microsystems GmbH) was run on the selected
area and this generated an intensity range color markup image,
segmenting and color-coding various parts of the image according to
the intensity of positive staining. The area for each of these four
intensity categories (expressed as a percent relative to the total
analysis area), together with the average positive intensity and
the average optical density, was also provided as numerical output.
The algorithm output also included a score (0–60) of mucin
expression based on the percent strong positive and percent medium
positive. These values were combined and named as ‘percent strong
positive’. The analysis output results were exported to Excel 2010
spreadsheets (Microsoft Corporation, Redmond, WA, USA) and
subjected to statistical analysis, focusing primarily on the
percentage of the total positive cells as the parameters to be
statistically analyzed and compared.
Serum MUC1 determination
Serum MUC1 levels were measured using Human
Carbohydrate Antigen 15-3 / Mucin-1 ELISA kit (catalog no.,
RAB0375; Sigma-Aldrich), according to the manufacturer's
protocol.
Statistical analysis
Data was presented with mean ± standard deviation.
Statistical analysis was performed using Microsoft Excel (Microsoft
Corporation). The means between the CRC tissue and normal adjacent
tissue were compared using Student's t-test. P≤0.05 was considered
to indicate a statistically significant difference.
Results
Clinicopathological features of the
patients
In total, ~90% of the tumor samples (20/22) were
advanced CRC at stage II and III. Additionally, the majority of the
tumors were high grade (grade 2 and Grade 3). A total of 8 patients
out of 22 already had developed lymph node metastases (Table I).
 | Table I.Clinicopathological characteristics
of patients with colorectal cancer. |
Table I.
Clinicopathological characteristics
of patients with colorectal cancer.
| Characteristic | Value |
|---|
| Total, n | 22 |
| Gender, n (%) |
|
|
Male | 11 (50) |
|
Female | 11 (50) |
| Age, years |
|
|
Median | 57 |
|
Range | 36–81 |
| Site of cancer, n
(%) |
|
|
Colon | 9
(41) |
|
Rectum | 2 (9) |
|
Sigmoid | 5
(23) |
|
Rectosigmoid | 6
(27) |
| Adenocarcinoma, n
(%) | 22
(100) |
| Histological grade,
n (%) |
|
| 2 | 19 (86) |
| 3 | 3
(14) |
| Clinical staging, n
(%) |
|
| I | 1 (5) |
| II | 12 (55) |
|
III | 8
(36) |
| IV | 1 (5) |
| Tumor staging, n
(%) |
|
| T2 | 3
(14) |
| T3 | 17 (77) |
| T4 | 2 (9) |
| Lymph node status,
n (%) |
|
| N0 | 14 (64) |
| N1 | 6
(27) |
| N2 | 2 (9) |
| Metastasis, n
(%) |
|
|
Yes | 9
(41) |
| No | 13 (59) |
Mucin staining
Mucin protein expression in the CRC and normal
adjacent tissues was observed using immunohistochemical staining,
and the results are summarized in Table
II. The expression of MUC1 and MUC2 varied between normal
tissues and different stages of colorectal tumor. The majority of
the staining was prominently characterized by diffuse cytoplasmic
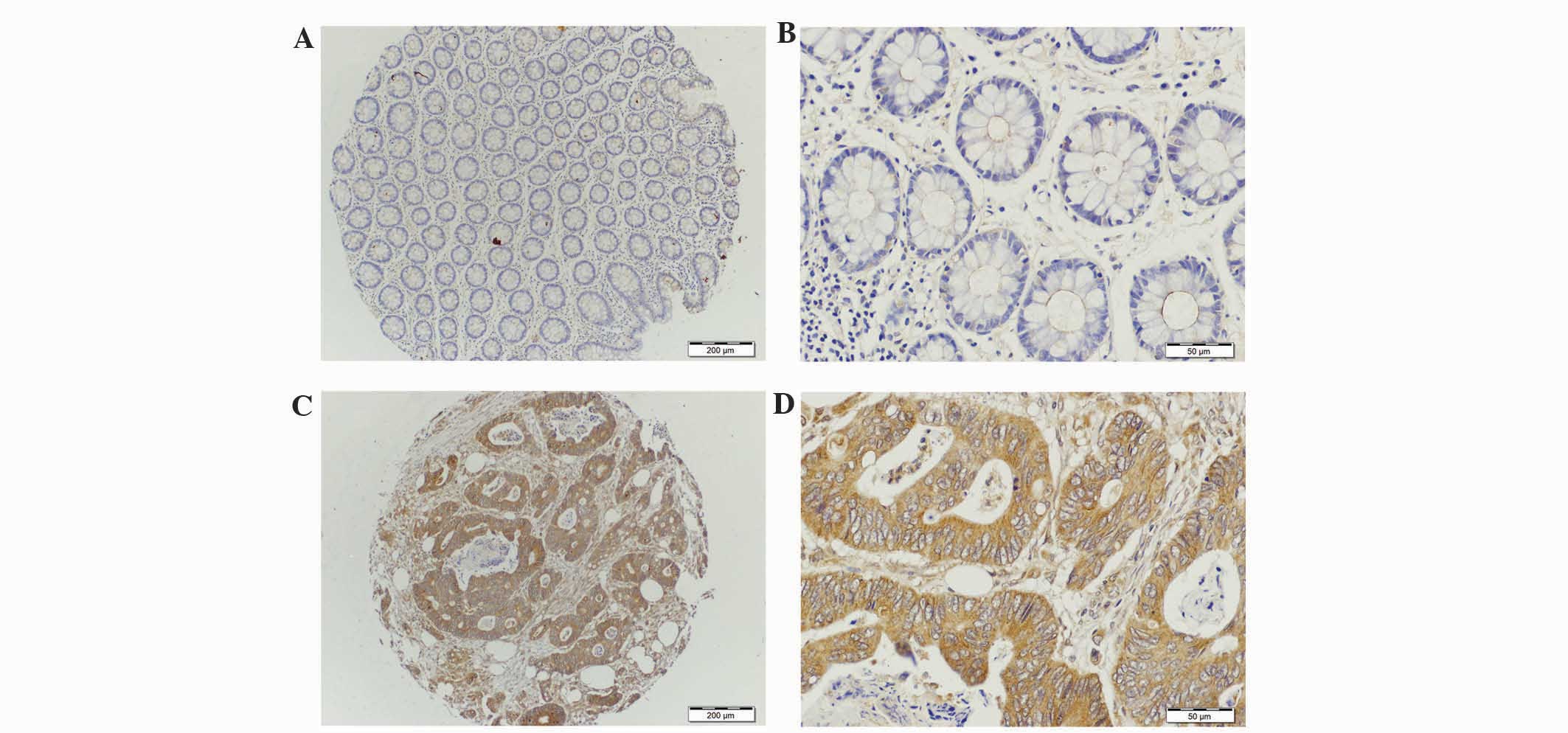
staining. MUC1 protein was observed to be weakly expressed in the
adjacent normal tissue group (Fig. 1A and
B). However in the colorectal tumor group, the majority of
staining for MUC1 was observed within the cytoplasm and cell
membrane (Fig. 1C and D). For MUC1,
positive staining was observed in 82% (18/22) of tumor tissues and
no staining was observed in 18% (4/22) tumor tissues (Table II). There was a significant
difference between MUC1 positive staining in tumor and negative
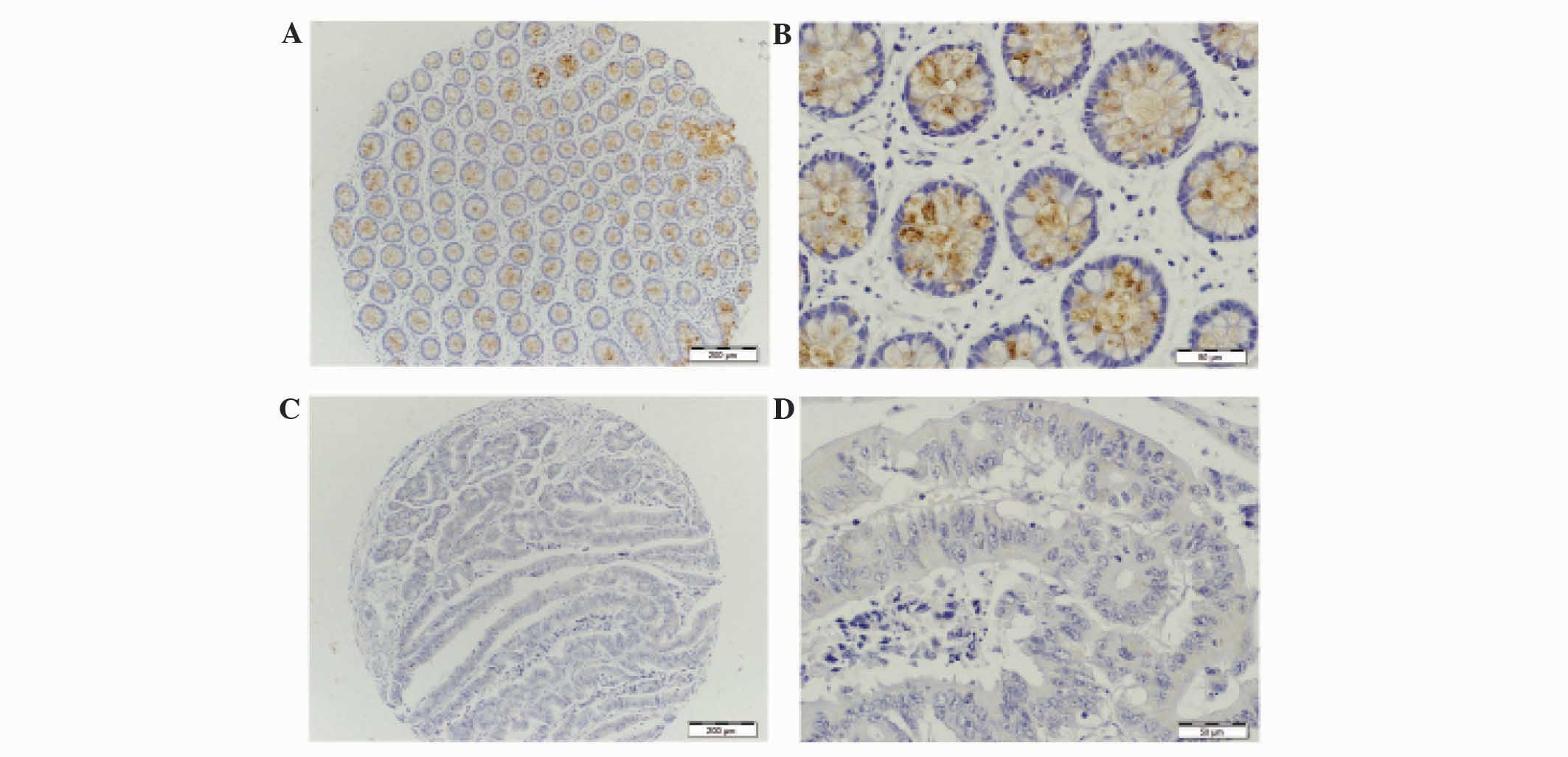
adjacent normal tissue (P<0.0001). There was clear MUC2 staining
in the lumen and cytoplasm of goblet cells in adjacent normal
tissue (Fig. 2A and B); however it
was negative or weak in colorectal tumor tissues (Fig. 2C and D). The majority of the tumor
tissues (90%) exhibited negative MUC2 staining. In total, 86% of
the adjacent normal tissues exhibited clear positive staining for
MUC2. There was a significant difference between MUC2 negative
staining in tumor tissues and positive staining in normal tissue
(P=0.0005). The MUC5AC staining was observed to be negative in
tumor and adjacent normal tissues. Collectively, these findings
demonstrate that MUC1 was highly expressed in colorectal tumor
compared to normal tissue, and MUC2 protein expression was
downregulated in tumor tissues compared with adjacent normal
tissues. Colorectal tumors did not express MUC5AC.
 | Table II.Frequency of mucin expression in
colorectal tumors and adjacent normal tissues from 22 patients with
colorectal cancer. |
Table II.
Frequency of mucin expression in
colorectal tumors and adjacent normal tissues from 22 patients with
colorectal cancer.
| Staining score | Tumor, n (%) | Adjacent normal, n
(%) | P-value |
|---|
| MUC1 |
|
| <0.0001 |
|
Positive | 18 (82) | 5
(23) |
|
Negative | 4
(18) | 17 (77) |
| MUC2 |
|
|
0.0005 |
|
Positive | 2 (9) | 19 (86) |
|
Negative | 20 (91) | 3
(14) |
MUC1 positive staining was similar across all ages
of CRC patients (Table III). It was
observed that MUC1 expression was also positive in young CRC
patients and old CRC patients from Saudi Arabia. There was no
significant difference between MUC1 staining the ages of CRC
patients (P=0.0820).
 | Table III.Tumor stage and MUC1 expression in
patients with colorectal carcinoma according to age. |
Table III.
Tumor stage and MUC1 expression in
patients with colorectal carcinoma according to age.
| Pathological
stage | Young patients, ≤50
years, n (%) | Old patients,
>50 years, n (%) |
|---|
| Total | 7 (31) | 15 (68) |
| Clinical
staging |
|
|
|
| Early
(I and II) | 3 (43) | 10 (67) |
| Late
(III and IV) | 4 (57) | 5
(33) |
| MUC1 positive
expression | 7
(100) | 14 (93) |
Immunohistochemical analysis of MUC1,
MUC2 and MUC5AC
MUC1 was demonstrated to have an increased
expression in colorectal tumor compared with adjacent normal
tissue. In adjacent normal tissue, MUC1 staining was extremely weak
(Fig. 1A and B). MUC1 expression in
colorectal tumor was clearly observed to be localized in the
cytoplasm (Fig. 1C and D). The
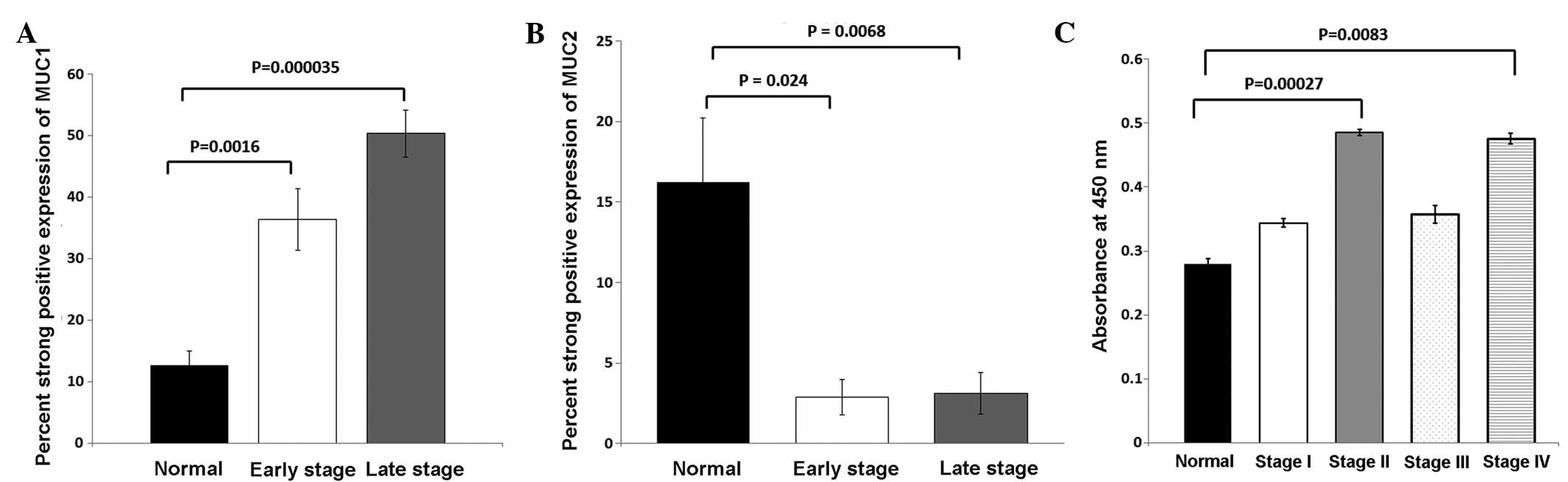
expression frequency and strong positive staining of MUC1 was
significantly higher in early (stage I and II) and late stage
(stage III and IV) tumor tissue compared with adjacent normal
tissue (Fig. 3A). When MUC1
expression was compared between adjacent normal and early stage
tumor tissue, MUC1 was found to be overexpressed (P=0.0016).
However, MUC1 expression was significantly increased in late stage
colorectal tumors compared with normal adjacent tissue
(P<0.0001). The frequency of MUC2 strong positive staining was
increased in adjacent normal tissue compared with colorectal tumor
tissue. MUC2 staining was primarily in the lumen and cytoplasm of
adjacent normal tissue (Fig. 2A and
B). MUC2 positive staining was found to be significantly
downregulated in early stage (P=0.0240) and late stage (P=0.0068)
colorectal cancer compared with adjacent normal tissue (Fig. 3B). The MUC2 expression profile for
colorectal tumor and adjacent normal tissue identified in the
present study are consistent with other reports (17,29). In
the present study, MUC5AC expression was undetected in tumor and
adjacent normal tissue (data not shown). These results demonstrate
that MUC1 was highly expressed in tumor samples and localized in
the cytoplasm; however, MUC2 expression was high in normal samples
and was decreased in tumor samples. Therefore, MUC1 and MUC2
expression are inversely associated in Saudi patients with CRC and
these colorectal tumors are negative for MUC5AC expression.
Serum MUC1 levels in colorectal
tumor
Serum MUC1 levels were measured in control
individuals and patients with various stages of CRC (stage I, II,
III and IV). MUC1 serum levels were increased in stage II
(P=0.0003) and stage IV (P=0.0083) serum compared with control
serum (Fig. 3C). Serum MUC1 levels
were associated with higher MUC1 staining in early and late stage
CRC. These findings indicate that MUC1 is highly expressed in early
and late stage tumors and was demonstrated to be cleaved during
early and late stage CRC. Overall, the present results collectively
indicated that MUC1 is clearly expressed in CRC tissues and MUC2 is
downregulated during tumor transformation in CRC. MUC1 gene
expression was demonstrated to be increased in tumor tissue and
serum MUC1 levels were high in early and late stage patients with
CRC.
Discussion
Mucins are high molecular weight glycoproteins with
20 amino acid tandem repeats, which undergo glycosylation (1). Mucins are known to be aberrantly
expressed in CRC with aggressive phenotypes (17,20,21).
However, the mucin expression profile of patients with CRC is not
known in the Middle East, specifically in Saudi Arabia and, to the
best of our knowledge, there has only been one report that studied
the impact of mucin production on the prognosis of patients with
CRC (30). Therefore, the present
study investigated the expression of MUC1, MUC2 and MUC5AC as a
biomarker in various stages of CRC in Saudi patients. In the
present study population, ~40% of tumors were identified in the
colon, and ~60% were in the rectum, sigmoid and rectosigmoid. All
these tumors were high-grade adenocarcinomas consisting of early
stage (stage I and II, ~60%) and late stage (stage III and IV,
~40%). In total, ~37% of the patients were positive for lymph node
metastases. Almost 81% of the CRC tumor samples were positive for
MUC1 in the present study. The majority of these positive MUC1
tumors expressed MUC1 in the cytoplasm of tumor cells. Adjacent
normal tissues from the same patients were negative for MUC1
staining. In addition, MUC1 staining was positive in patients with
early-stage CRC (stage I and II; P=0.0016) compared with normal
adjacent tissues. This is in contrast to previous studies performed
in Europe, which reported that MUC1 is only upregulated in
late-stage metastatic CRC (20,31);
however, in the present study, it is significantly higher in late
stage tumor (P<0.0001). In the present study, the expression of
MUC1 in late stages is significantly higher than early stage
colorectal cancer (P=0.0460). It appears that the expression
profile of MUC1 in Saudi CRC patients is different, since there is
MUC1 expression in early-stage CRC tumors. Therefore, MUC1
expression may be dependent on ethnicity and demographic location
of individuals.
MUC1 forms the mucosal barrier of the intestinal
tract and protects the epithelial lining from ingested toxins,
pathogenic bacteria and inflammatory cytokines (1). However, pro-inflammatory cytokines,
including TNFα, IFNγ and IL-6, are known to induce MUC1 expression
during chronic inflammation. Overexpression of MUC1 during chronic
inflammation induces pro-tumorigenic effects and leads to the
development of CRC (1,15). The IKKβ-NFκB pathway is a primary
mediator of inflammation-induced cancer progression (1), and notably, MUC1 binds IKKβ (6) and NFκB p65 (7) and contributes to the constitutive
activation of the NFκB pathway. In this regard, during chronic
inflammation MUC1-induced activation of the NFκB pathway may lead
to CRC. In addition, MUC1 interacts with β-catenin during cell
adhesion (9). NFκB is constitutively
activated in CRC, which is important in promoting tumor growth
(32), and β-catenin is has been
demonstrated to be involved in CRC tumorigenesis (33). Consequently, it is possible that MUC1
may be involved in activating β-catenin and NFκB signaling
pathways; thereby contributing to CRC progression.
The expression of the secretory mucin MUC2 is
restricted to normal tissue, mostly in the lumen and cytoplasm. In
the present study, MUC2 was expressed in >85% of adjacent normal
tissue, and was not expressed or had an extremely low expression in
early and late stage CRC tumor tissues when compared with normal
tissue (P=0.0240 and P=0.0068, respectively). This is consistent
with other studies that demonstrated that there was a reduced
expression of MUC2 in patients with CRC (17,29,31,34).
This decrease or absence of MUC2 expression may be due to MUC2
promoter methylation in CRC cells (35). A reduced MUC2 expression in colon
cancer contributes to the pro-survival pathway in cells; p53
protein regulates MUC2 transcription as MUC2 staining is reported
to be inversely associated with p53 expression in mucinous
carcinoma (36). The intestinal
epithelial lining is covered by a mucous layer, which is primarily
composed of secreted mucins. This mucous layer acts as a barrier
that prevents the epithelial lining from damage and blocks the
activation of the immune response. MUC2 is a primary component of
the mucous layer of the normal intestine. In the absence of MUC2,
an inflammatory process is initiated at the cell surface, and a
loss of MUC2 in goblet cells during chronic inflammation is
associated with ulcerative colitis (1). Therefore, depletion of MUC2 production
results in the increased risk of colorectal cancer.
In the present study, MUC5AC was not identified in
either normal adjacent or CRC tumor tissues. This is not notable
considering that previous studies have demonstrated that MUC5AC is
not expressed in poorly-differentiated colorectal tumors (16,17,19,21).
By contrast, MUC5AC has been reported to be expressed in
well-differentiated tumors (17,21). CRC
patients with MUC5AC negative tumors have a poor prognosis with a
low survival rate compared with patients with MUC5AC positive
tumors (17,21). This indicates that an absence of
MUC5AC expression is a prognostic factor for highly aggressive
colorectal cancer.
The majority of the present study sample consisted
of patients with higher grade colorectal tumors. Mucin expression
has been correlated with high levels of microsatellite instability,
in particular an increased expression of MUC2 and MUC5AC in
sporadic cancer (31). Mucin gene
expression may contribute to cell transformation, and consequently
tumorigenesis, due to the loss of tumor suppressor genes; mucin
expression has been associated with mutations in mismatch repair
genes or mutL homolog 1 (MLH1) hypermethylation (29). MUC1 is a well-known interacting
protein that may interact with certain histone methyl transferases,
and therefore lead to hypermethylation of MLH1. In the present
study, serum MUC1 levels were higher in early and late stage CRC
tissues compared with normal adjacent tissues. MUC1 expression has
been reported in the serum of patients with head and neck squamous
cell carcinoma (HNSCC); MUC1 serum levels were higher in HNSCC
patients compared with control individuals, and was positively
associated with MUC1 staining in the HNSCC tumors (37).
The incidence of CRC and mortality rates are
decreasing among in patients aged >50 years worldwide; however,
the incidence of CRC is increasing in young patients and is termed
as young onset CRC. A similar trend has been observed in Saudi
Arabia, where there has been an increase in the incidence of young
onset CRC (21). Young onset CRC is
characterized by microsatellite stability primarily identified in
the distal colon and rectum with poor differentiation (38). Furthermore, patients with young onset
CRC present with advance staged CRC with mucinous and signet ring
features.
The present study demonstrates a role for MUC1 in
the progression of CRC using a TMA in CRC patients from Saudi
Arabia. Therefore, MUC1 expression may be used as an independent
prognostic marker in CRC. The present findings implicate MUC1
expression as possessing diagnostic, prognostic and therapeutic
significance in CRC. MUC1 overexpression in early and late stage
CRC may be a useful biomarker, particularly in young onset CRC
patients. Specifically in Saudi CRC patients, MUC1 appears to be
expressed significantly in early stage cancer, which will aid in
the early diagnosis of CRC. MUC1 expression in young CRC patients
from Saudi Arabia may be due to a differences observed in the
ethnic population, including genetic predisposition, diet and
smoking. In conclusion, the present study demonstrates that MUC1
may be a useful biomarker for the detection of early as well as
late stage CRC. Additional studies are required with a larger
sample size to evaluate the biomarker capability of MUC1 in CRC,
particularly in patients with a young onset CRC phenotype.
Acknowledgements
The authors would like to thank Dr Amer Mahmood of
Stem Cell Unit (King Saud University) for valuable scientific input
that greatly improved this manuscript. The present study was
supported by the Vice Deanship Research Chair, King Saud University
Deanship of Scientific Research.
References
|
1
|
Kufe DW: Mucins in cancer: Function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kufe DW: MUC1-C oncoprotein as a target in
breast cancer: Activation of signaling pathway and therapeutic
approaches. Oncogene. 32:1073–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macao B, Johansson DG, Hansson GC and Härd
T: Autoproteolysis coupled to protein folding in the SEA domain of
the membrane-bound MUC1 mucin. Nat Struct Mol Biol. 13:71–76. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siddiqui J, Abe M, Hayes D, Shani E, Yunis
E and Kufe D: Isolation and sequencing of a cDNA coding for the
human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci
USA. 85:2320–2323. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 10:749–759. 2005. View
Article : Google Scholar
|
|
6
|
Ahmad R, Raina D, Trivedi V, Ren J, Rajabi
H, Kharbanda S and Kufe D: MUC1 oncoprotein activates the I kappaB
kinase complex and constitutive NF-kappaB signaling. Nat Cell Biol.
9:1419–1427. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmad R, Raina D, Joshi MD, Kawano T, Ren
J, Kharbanda S and Kufe D: MUC1-C oncoprotein functions as a direct
activator of the nuclear factor-kappaB p65 transcription factor.
Cancer Res. 69:7013–7021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vinall LE, King M, Novelli M, Green CA,
Daniels G, Hilkens J, Sarner M and Swallow DM: Altered expression
and allelic association of the hypervariable membrane mucin MUC1 in
Helicobacter pylori gastritis. Gastroenterology. 123:41–49. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang L, Chen D, Liu D, Yin L, Kharbanda S
and Kufe D: MUC1 oncoprotein blocks glycogen synthase kinase 3
beta-mediated phosphorylation and degradation of beta-catenin.
Cancer Res. 65:10413–10422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanoh A, Takeuchi H, Kato K, Waki M, Usami
K and Irimura T: Interleukin-4 induces specific pp-GalNAc-T
expression and alterations in mucin O-glycosylation in colonic
epithelial cells. Biochim Biophys Acta. 1780:577–584. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Byrd JC and Bresalier RS: Mucins and mucin
binding proteins in colorectal cancer. Cancer Metastasis Rev.
23:77–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johansson ME, Phillipson M, Petersson J,
Velcich A, Holm L and Hansson GC: The inner of the two Muc2
mucin-dependent mucus layers in colon is devoid of bacteria. Proc
Natl Acad Sci USA. 105:15064–15069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feagins LA, Souza RF and Spechler SJ:
Carcinogenesis in IBD: Potential targets for the prevention of
colorectal cancer. Nat Rev Gastroenterol Hepatol. 6:297–305. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: Protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vincent A, Perrais M, Desseyn JL, Aubert
JP, Pigny P and Van Seuningen I: Epigenetic regulation (DNA
methylation, histone modifications) of the 11p15 mucin genes (MUC2,
MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene.
26:6566–6576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bu XD, Li N, Tian XQ, Li L, Wang JS, Yu XJ
and Huang PL: Altered expression of MUC2 and MUC5AC in progression
of colorectal carcinoma. World J Gastroenterol. 16:4089–4094. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López-Ferrer A, Curull V, Barranco C,
Garrido M, Lloreta J, Real FX and de Bolós C: Mucins as
differentiation markers in bronchial epithelium. Squamous cell
carcinoma and adenocarcinoma display similar expression patterns.
Am J Respir Cell Mol Biol. 24:22–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu CJ, Shih JY, Lee YC, Shun CT, Yuan A
and Yang PC: Sialyl Lewis antigens: Association with MUC5AC protein
and correlation with post-operative recurrence of non-small cell
lung cancer. Lung Cancer. 47:59–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duncan TJ, Watson NF, Al-Attari AH,
Scholefield JH and Durrant LG: The role of MUC1 and MUC3 in the
biology and prognosis of colorectal cancer. World J Sur Oncol.
5:312007. View Article : Google Scholar
|
|
21
|
Kocer B, Soran A, Erdogan S, Karabeyoglu
M, Yildirim O, Eroglu A, Bozkurt B and Cengiz O: Expression of
MUC5AC in colorectal carcinoma and relationship with prognosis.
Pathol Int. 52:470–477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mosli MH and Al-Ahwal MS: Colorectal
cancer in the kingdom of Saudi Arabia: Need for screening. Asian
Pac J Cancer Prev. 13:3809–3813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gado A, Ebeid B, Abdelmohsen A and Axon A:
Colorectal cancer in Egypt is commoner in young people: Is this
cause for alarm. Alexandria J Med. 50:197–201. 2014. View Article : Google Scholar
|
|
24
|
Greene FL: TNM staging for malignancies of
the digestive tract: 2003 changes and beyond. Semin Surg Oncol.
21:23–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours (6th). John Wiley & Sons.
Hoboken, NJ: 2002.
|
|
26
|
Hamilton SR and Aaltonen L: Tumours of
small intestine. In: World Health Organization Classification of
Tumours. Pathology and Genetics of Tumours of the Digestive System.
IARC Press. (Lyon). 69–91. 2000.
|
|
27
|
Rimm DL, Camp RL, Charette LA, Costa J,
Olsen DA and Reiss M: Tissue microarray: A new technology for
amplification of tissue resources. Cancer J. 7:24–31.
2001.PubMed/NCBI
|
|
28
|
Biemer-Hüttmann AE, Walsh MD, McGuckin MA,
et al: Mucin core protein expression in colorectal cancers with
high levels of microsatellite instability indicates a novel pathway
of morphogenesis. Clin Cancer Res. 6:1909–1916. 2000.PubMed/NCBI
|
|
29
|
Farhat MH, Barada KA, Tawil AN, Itani DM,
Hatoum HA and Shamseddine AI: Effect of mucin production on
survival in colorectal cancer: A case-control study. World J
Gastroenterol. 14:6981–6985. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baldus SE, Mönig SP, Hanisch FG, Zirbes
TK, Flucke U, Oelert S, Zilkens G, Madejczik B, Thiele J, Schneider
PM, et al: Comparative evaluation of the prognostic value of MUC1,
MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal
adenocarcinoma. Histopathology. 40:440–449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Voloshanenko O, Erdmann G, Dubash TD,
Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T,
Anchang B, et al: Wnt secretion is required to maintain high levels
of Wnt activity in colon cancer cells. Nat Commun. 4:26102013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ajioka Y, Allison LJ and Jass JR:
Significance of MUC1 and MUC2 mucin expression in colorectal
cancer. J Clin Pathol. 49:560–564. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gratchev A, Siedow A, Bumke-Vogt C, Hummel
M, Foss HD, Hanski ML, Kobalz U, Mann B, Lammert H, Mansmann U, et
al: Regulation of the intestinal mucin MUC2 gene expression in
vivo: Evidence for the role of promoter methylation. Cancer
Lett. 168:71–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ookawa K, Kudo T, Aizawa S, Saito H and
Tsuchida S: Transcriptional activation of the MUC2 gene by p53. J
Biol Chem. 277:48270–48275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biemer-Hüttmann AE, Walsh MD, McGuckin MA,
Simms LA, Young J, Leggett BA and Jass JR: Mucin core protein
expression in colorectal cancers with high levels of microsatellite
instability indicates a novel pathway of morphogenesis. Clin Cancer
Res. 6:1909–1916. 2000.PubMed/NCBI
|
|
36
|
Lugli A, Zlobec I, Baker K, Minoo P,
Tornillo L, Terracciano L and Jass JR: Prognostic significance of
mucins in colorectal cancer with different DNA mismatch-repair
status. J Clin Pathol. 60:534–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rabassa ME, Croce M, Pereyra A and
Segal-Eiras A: MUC1 expression and anti-MUC1 serum immune response
in head and neck squamous cell carcinoma (HNSCC): A multivariate
analysis. BMC Cancer. 6:2532006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahnen DJ, Wade SW, Jones WF, Sifri R,
Silveiras Mendoza J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A
and You YN: The increasing incidence of young onset colorectal
cancer: A call to action. Mayo Clinic Proc. 89:216–224. 2014.
View Article : Google Scholar
|