Introduction
Although there are currently a number of antitumor
treatments, surgery remains the most effective for use in solid
tumors. It has been suggested that tumor cells may be released from
the primary tumor into the vascular and lymphatic circulation
through tumor manipulation during surgery (1), ultimately resulting in an increased risk
of distant metastases and recurrence. Mortality in cancer patients
is mainly caused by metastases. The invasion and migration of tumor
cells are critical steps during tumor metastasis (2). In order to become invasive, tumor cells
must acquire capacities that enable enhanced migration through, and
proteolysis of the extracellular matrix. The ability of migration
is a prerequisite for a cancer cell to escape from the primary
tumor and be released into the circulation (3).
Calcium (Ca2+), as a second messenger, is
one of the critical regulators of cell migration. Previous findings
have shown that Ca2+ influx is essential for the
migration of various cell types, including tumor cells (4,5). Calcium
channel components, which are responsible for altered calcium
signaling in cancer cells, include the store-operated calcium (SOC)
channels, calcium release-activated calcium channel protein 1
(ORAI1) and a number of transient receptor potential (TRP) channel
family members. TRP subfamily V member 6 (TRPV6) (also known as
ECAC2, CaT1 and CaT-like) is a member of the TRP channel family,
and it has particularly high selectivity for Ca2+, as
well as constitutive activity (6).
Expression of TRPV6 mRNA and protein has been detected in ovarian
cancer and other cancer types, such as breast, prostate, thyroid
and colon cancer (7).
Dhennin-Duthille et al (8)
reported that selective silencing of TRPV6 inhibited the migration
and invasion of breast cancer MDA-MB-231 cells, as well as MCF-7
cell migration. It has been suggested that TRPV6 has the potential
for diagnostic, prognostic and therapeutic use in human breast and
prostate cancer (6,8–11).
Certain studies have suggested that the use of
regional anesthesia can reduce the possibility of cancer metastasis
and recurrence following surgical tumor excision, at least in
specific tumor types, such as breast and colorectal cancer
(12,13). Local anesthetics are known to block
the voltage-gated sodium channels (VGSC) in excitable cells, and
have been proven to have the effect of inhibiting tumor cell
invasion and migration in vitro (14,15).
However, the evidence of local anesthetics for the inhibition of
tumor progression through VGSC is limited (1). Local anesthetics not only block VGSC at
concentrations that are much lower than clinical concentrations
during regional anesthesia, but they also block potassium
(K+) channels and Ca2+ channels.
Lidocaine, the most commonly used local anesthetic,
is considered to effectively inhibit the invasive ability of tumor
cells at the concentrations used in surgical treatment (16). The present study therefore
investigated the inhibitory effect of lidocaine upon the cell
invasion and migration of TRPV6-expressing cancer cells, including
breast cancer MDA-MB-231 cells, prostatic cancer PC-3 cells and
ovarian cancer ES-2 cells. The study also investigated the
expression of TRPV6 mRNA and protein in the cells subsequent to the
treatment with lidocaine to determine whether lidocaine inhibits
TRPV6-expressing cancer cell invasion and migration via TRPV6
downregulation.
Materials and methods
Major reagents
Lidocaine hydrochloride with a purity of 2% was
purchased from Xinzheng Co., Ltd. (Tianjin, China). The
concentrations of the lidocaine local anesthetic are expressed in
molarity (mM) instead of percentages (%): 2%≈74 mM, 0.27%≈10 mM and
0.027%≈1 mM (17).
Cell culture
Breast cancer MDA-MB-231 cells, prostatic cancer
PC-3 cells and ovarian cancer ES-2 cells were obtained from and
stored at the Molecular Medicine and Cancer Research Center,
Chongqing Medical University (Chongqing, China). The cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS; Hyclone; GE Healthcare, Logan, UT, USA), 50 µg/ml
penicillin and 50 µg/ml streptomycin. The cells were incubated at
37°C in a humidified atmosphere containing 5% CO2.
MTT assay
Cell viability was assessed using the MTT assay.
Briefly, the MDA-MB-231, PC-3 and ES-2 cells were seeded into
96-well plates (1×104 cells/well), and treated with
fresh serum-free RPMI 1640 medium and different concentrations of
lidocaine (0, 10 and 100 µM, and 1, 2, 5 and 10 mM), respectively.
Once the cells had been incubated for 24 h, 200 µl MTT (Beyotime
Institute of Biotechnology, Haimen, China)-containing medium (0.5
mg/ml, diluted with fresh serum-free RPMI 1640 medium) was added
into each well, and the cells were incubated for 4 h at 37°C.
Finally, the MTT-containing medium was removed and 150 µl dimethyl
sulfoxide was added per well. The optical density (OD) was measured
using an iEMS Analyzer (Labsystems IEMS MF Type 1401; Thermo Fisher
Scientific Inc., Waltham, MA, USA) at a wavelength of 570 nm. The
percentage of viability was calculated using the following
equation: Viability (%) = (OD treatment group / OD control group) ×
100.
Wound healing assay
Cell migration was measured using a wound healing
assay. Briefly, the MDA-MB-231, PC-3 and ES-2 cells were seeded
into 6-well plates (1×106 cells/well). Once the
monolayer of cells had reached 100% confluence in the 6-well
plates, the cells were wounded with a sterile 10-µl plastic
micropipette tip to generate a clean scratch wound across the
center of the well. The floating cells were washed away and 2.5 ml
fresh serum-free RPMI 1640 medium was added together with different
concentrations of lidocaine (0 µM, 10 µM, 100 µM and 1 mM) in each
well. The 6-well plates were then placed in the CO2
incubator to allow the cells to migrate. The wound closure was
assessed at the 24-h time point by microscopic examination using
research inverted microscope IX83 (Olympus, Tokyo, Japan). Five
randomly selected points along each wound were marked, and the
shortest horizontal distance of the wound area was measured. The
migration distance of the treated cells was determined as the
difference between the shortest distance in the initial wound and
the shortest distance measured at the 24-h time point.
Cell invasion assay
Cell invasiveness was evaluated using Transwell
chambers, which incorporated a polycarbonate filter membrane
(Coring Costar, Corning Inc., Corning, NY, USA). The polycarbonate
filter membrane at the bottom of the upper chamber was coated with
50 µl Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) and
incubated for 30 min at 37°C prior to use. The MDA-MB-231, PC-3 and
ES-2 cells were re-suspended with fresh serum-free RPMI 1640 medium
and different concentrations of lidocaine (0 µM, 10 µM, 100 µM and
1 mM) and seeded in the upper chambers (200 µl; 1×105
cell/well), respectively. The bottom chamber was filled with RPMI
1640 medium enriched with 10% FBS. The cells were incubated in the
CO2 incubator for 24 h. The cells that did not penetrate
the polycarbonate filter membrane were removed using a clean cotton
swab. The cells on the lower surface of the polycarbonate filter
membrane were fixed with 70% ethanol for 15 min and then stained
with azure eosin methylene blue for 10 min. The cells from five
randomly selected fields were counted by light microscopy.
Measurement of intracellular-free
Ca2+ [Ca2+]I
Ca2+ measurements were performed using a
Fluo-3 AM fluorescence kit (Beyotime Institute of Biotechnology).
The MDA-MB-231, PC-3 and ES-2 cells were seeded into 6-well plates
(1×106 cells/well). The cell medium was replaced by 2.5
ml fresh serum-free RPMI 1640 medium with different concentrations
of lidocaine (0 and 100 µM), and then placed in the CO2
incubator for 12 h. The medium in each well was removed and the
cells were washed twice with PBS buffer (without CaCl2
and MgCl2), prior to the Fluo-3 AM fluorescence probes
(0.5 µM) being added to each well. The cells were incubated with
the probes for 60 min and protected from light. Following the
removal of the Fluo-3 AM dye-containing medium, the cells were
incubated in Tyrode's solution (145 mM NaCl, 1 mM NaOH, 2.5 mM KCl,
1.5 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES and
10 mM glucose; pH 7.4) for 5 min at room temperature. The cells
were then harvested and resuspended with Ca2+-free
Tyrode's solution. [Ca2+]I was analyzed using
a flow cytometry method according to the manufacturer's
instructions (Beyotime Institute of Biotechnology).
Quantitative-polymerase chain reaction
(PCR) analysis
The MDA-MB-231, PC-3 and ES-2 cells were seeded into
6-well plates (1×106 cells/well). Subsequent to being
left untreated or treated with 100 µM lidocaine for 12 h, the
expression of TRPV6 mRNA was analyzed by quantitative-PCR. Total
RNA was extracted from monolayer cells using TRIzol reagent
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions and quantified by spectrophotometry at
a wavelength of 260 nm. First-strand cDNAs were synthesized from 5
µg total RNA in a 20-µl reaction using the RevertAid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific Inc.), reverse
transcriptase, oligo(dT) primers and Taq DNA polymerase
(SYBR® Premix Ex Taq™ II; Takara Bio Inc., Shiga,
Japan). Reverse transcription-qPCR was performed in a CFX96
Real-Time PCR machine (Bio-Rad Laboratories Inc., Hercules, CA,
USA). The primers used were as follows: TRPV6 forward,
5′-TTTCCTGGGTGCATCAAAC-3′ and reverse, 5′-ACGTACATTCCTTGGCGTTC-3′;
and GAPDH (reference gene) forward, 5′-CAGGAGGCATTGCTGATGAT-3′ and
reverse, 5′-GAAGGCTGGGGCTCATTT-3′. The reaction was initiated with
denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec, 60°C for 60 sec (annealing), a terminal extension step at
95°C for 10 sec and a final holding stage at 4°C. Relative TRPV6
mRNA expression was defined as the ratio of TRPV6 gene expression
to GAPDH expression. The experiment was repeated three times.
Western blot analysis
The MDA-MB-231, PC-3 and ES-2 cells were seeded into
6-well plates (1×106 cells/well). After being left
untreated or treated or with 100 µM lidocaine for 12 h, the protein
expression of TRPV6 was assessed by western blot analysis. The
cells were lysed with lysis buffer and then centrifuged (14,000 × g
for 15 min) for the extraction of total proteins. The concentration
of total proteins was analyzed using a bicinchoninic acid assay.
Protein samples were separated by 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and then
electrotransferred onto a polyvinylidene difluoride (PVDF)
membrane. The PVDF membrane was blocked for 2 h at room temperature
in TBS-Tween 20 (TBST) buffer containing 5% skimmed milk, washed
with TBST three times (10 min each) and incubated overnight at 4°C
with polyclonal rabbit anti-mouse TRPV-6 antibodies (1:500
dilution; cat. no. ab117875; Abcam, Cambridge, UK) and mouse
monoclonal β-actin antibodies (1:10,000 dilution; cat. no. AA128;
Beyotime Institute of Biotechnology), respectively. Subsequent to
being washed with TBST three times (10 min each), the membranes
were incubated with horseradish peroxidase-labeled goat anti-rabbit
immunoglobulin G (H+L) secondary antibody (1;4,000 dilution; cat.
no. A0208; Beyotime Institute of Biotechnology) at 37°C for 1 h.
Protein signals were detected using enhanced chemiluminescence 32
reagent (EMD Millipore, Billerica, MA, USA) and quantified by
densitometry using Quantity One software (Bio-Rad Laboratories
Inc.).
Statistical analysis
Statistical analysis was conducted using SPSS 20.0
for Windows software (IBM SPSS, Armonk, NY, USA). Data are
presented as the mean ± standard deviation. Differences between
groups were analyzed using a repeated measure analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Lidocaine decreases cell viability at
high concentrations
To observe the inhibitory effect of lidocaine on the
viability of TRPV6-expressing cancer cells (MDA-MB-231, PC-3 and
ES-2), the OD of cells treated with different concentrations of
lidocaine (0 µM, 10 µM, 100 µM, 1 mM, 2 mM, 5 mM and 10 mM) was
measured and the percentage viability was calculated. As shown in
Fig. 1A, lidocaine was able to
significantly decreased cell viability in a concentration-dependent
manner. However, lower concentrations (≤1 mM) of lidocaine
exhibited no marked cytotoxicity. The study subsequently aimed to
determine whether lidocaine at lower concentrations (10 µM, 100 µM
and 1 mM) inhibited the invasion and migration of TRPV6-expressing
cancer cells.
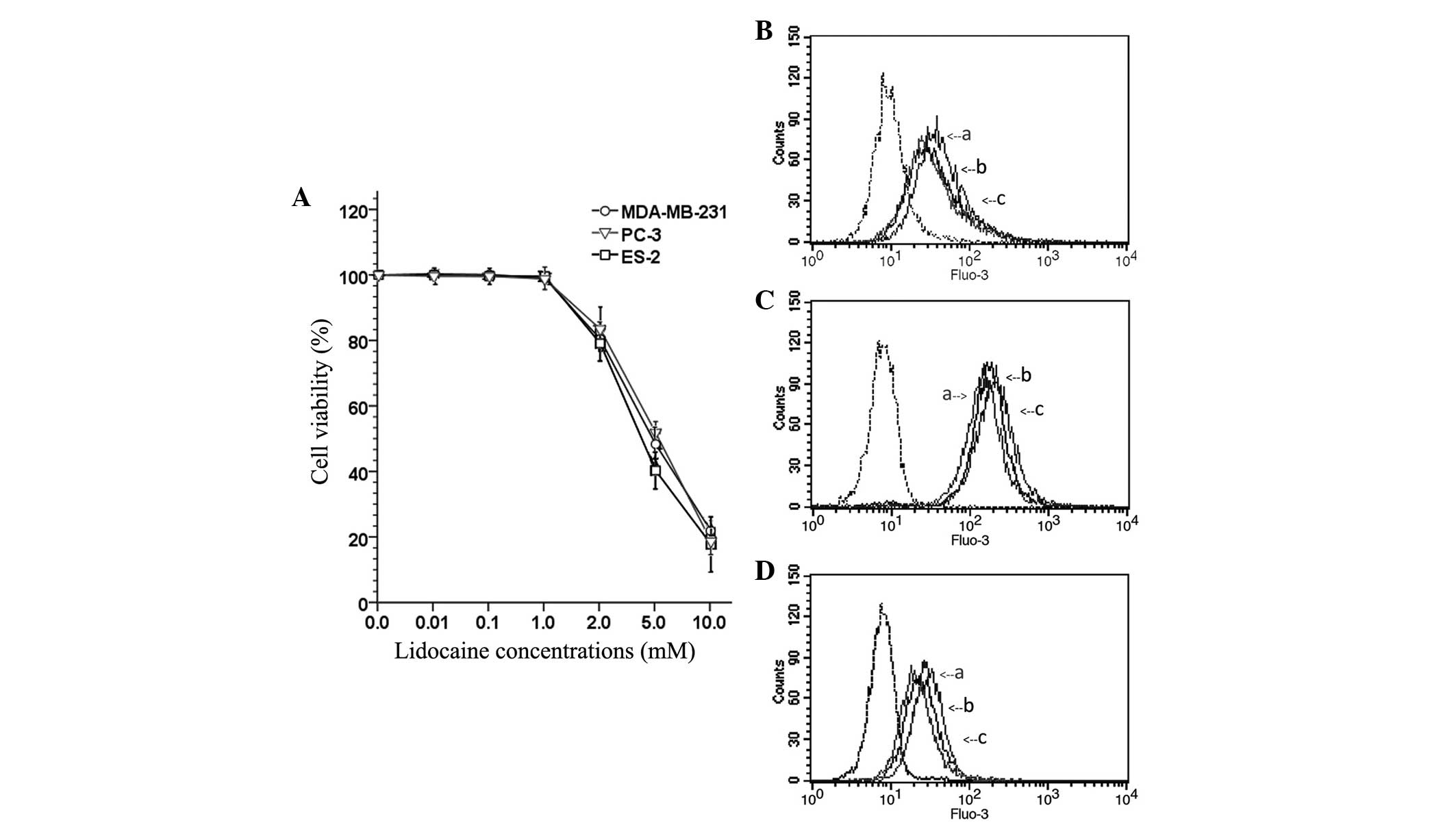 | Figure 1.(A) Cell viability of transient
receptor potential cation channel subfamily V member 6-expressing
cancer cells (MDA-MB-231, PC-3 and ES-2 cells) after treatment with
a series of concentrations of lidocaine (0, 10 and 100 µM, and 1,
2, 5 and 10 mM) for 24 h. The fluorescence curves of the (B)
MDA-MB-231, (C) PC-3 and (D) ES-2 cells. ‘a’, cells that did not
receive any lidocaine treatment, and were exposed to
Ca2+-free Tyrode's solution after uploaded the Fluo-3 AM
fluorescence kit. The fluorescence curve of those cells is used as
baseline for [Ca2+]I; ‘b’, the cells were
treated with lidocaine (100 µM) for 12 h, and exposed to Tyrode's
solution (with CaCl2) after using the Fluo-3 AM
fluorescence kit; ‘c’, the cells did not receive any lidocaine
treatment, but were exposed to Tyrode's solution (with
CaCl2) after using the Fluo-3 AM fluorescence kit;
dotted line, the cells did not receive any lidocaine treatment and
after using the Fluo-3 AM fluorescence kit, the fluorescence curve
of those cells was used as a negative control. Following exposure
to Tyrode's solution (with CaCl2), the fluorescence
curve of [Ca2+]I of the cells that received
the lidocaine treatment was higher than baseline, but lower than
the cells that did not receive the lidocaine treatment. This
indicated that lidocaine (100 µM) treatment led to a significant
decrease in the [Ca2+]I of the MDA-MB-231,
PC-3 and ES-2 cells. |
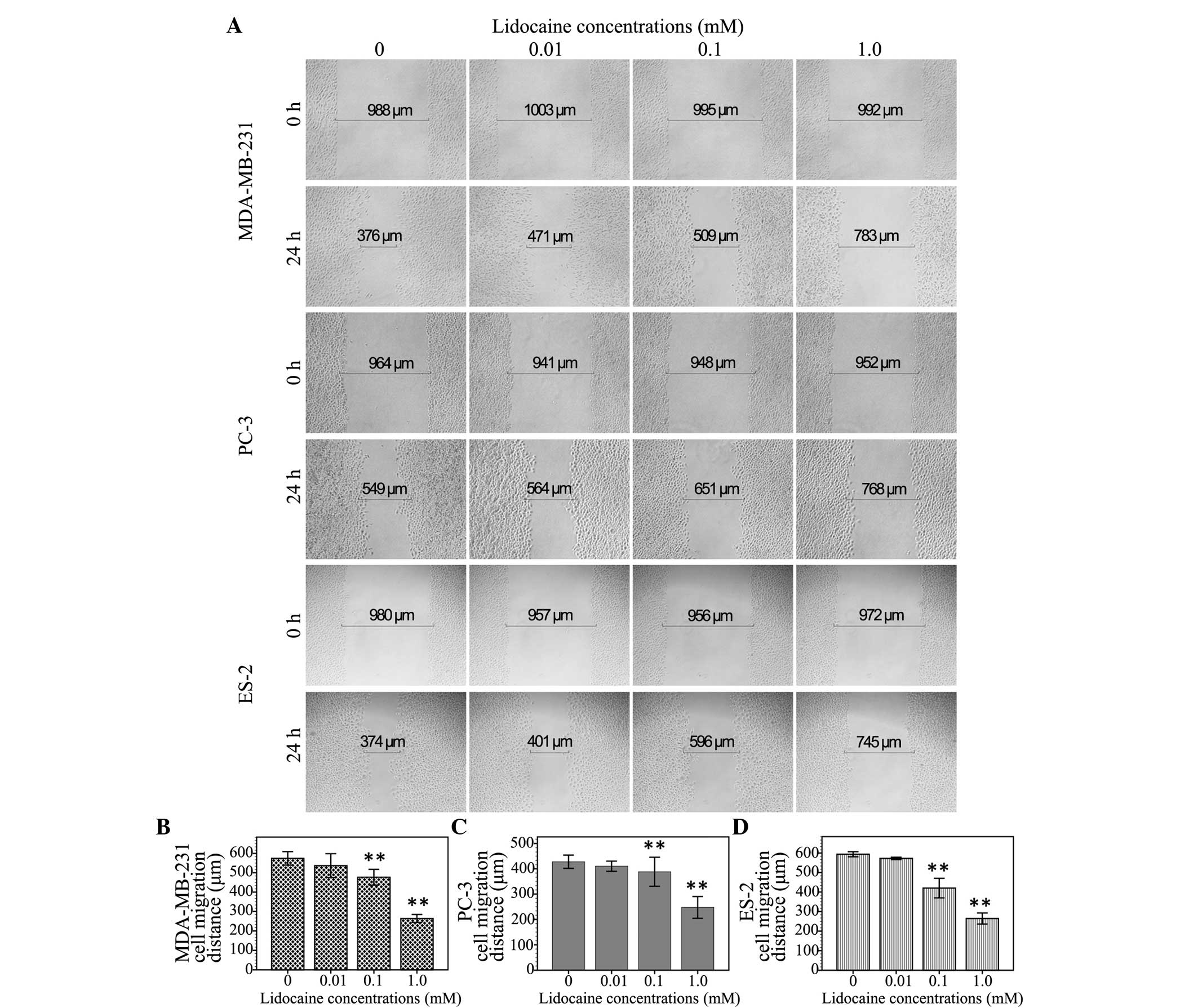
Lidocaine inhibits cell migration
The inhibitory effect of lidocaine on
TRPV6-expressing cancer cells was assessed by measuring the
migration distance of the wound area using the wound healing assay.
As shown in Fig. 2, the migration
distance of the MDA-MB-231 cells treated with different
concentrations of lidocaine (0 µM, 10 µM, 100 µM and 1 mM) was
574.3±17.5, 525.3±13.3, 477.1±20.5 and 264.0±10.2 µm, respectively.
In the PC3 cells, following treatment with these different
concentrations of lidocaine, the migration distance was 427.9±13.1,
410.3±10.1, 388.3±28.8 and 247.3±21.5 µm, respectively. Moreover,
the migration distance of the ES-2 cells treated with these
different concentrations of lidocaine was 593.8±6.5, 572.6±2.9,
420.1±25.1 and 264.3±14.2 µm, respectively. Compared with the
control group, the 10-µM treatment group showed no significant
inhibition of cell migration at the 24-h time point (MDA-MB-231,
P=0.059; PC3, P=0.305; ES-2, P=0.119), but 100 µM (MDA-MB-231,
P<0.01; PC3, P=0.039; ES-2, P<0.01) and 1 mM lidocaine
demonstrated a significant inhibitory effect (P<0.01).
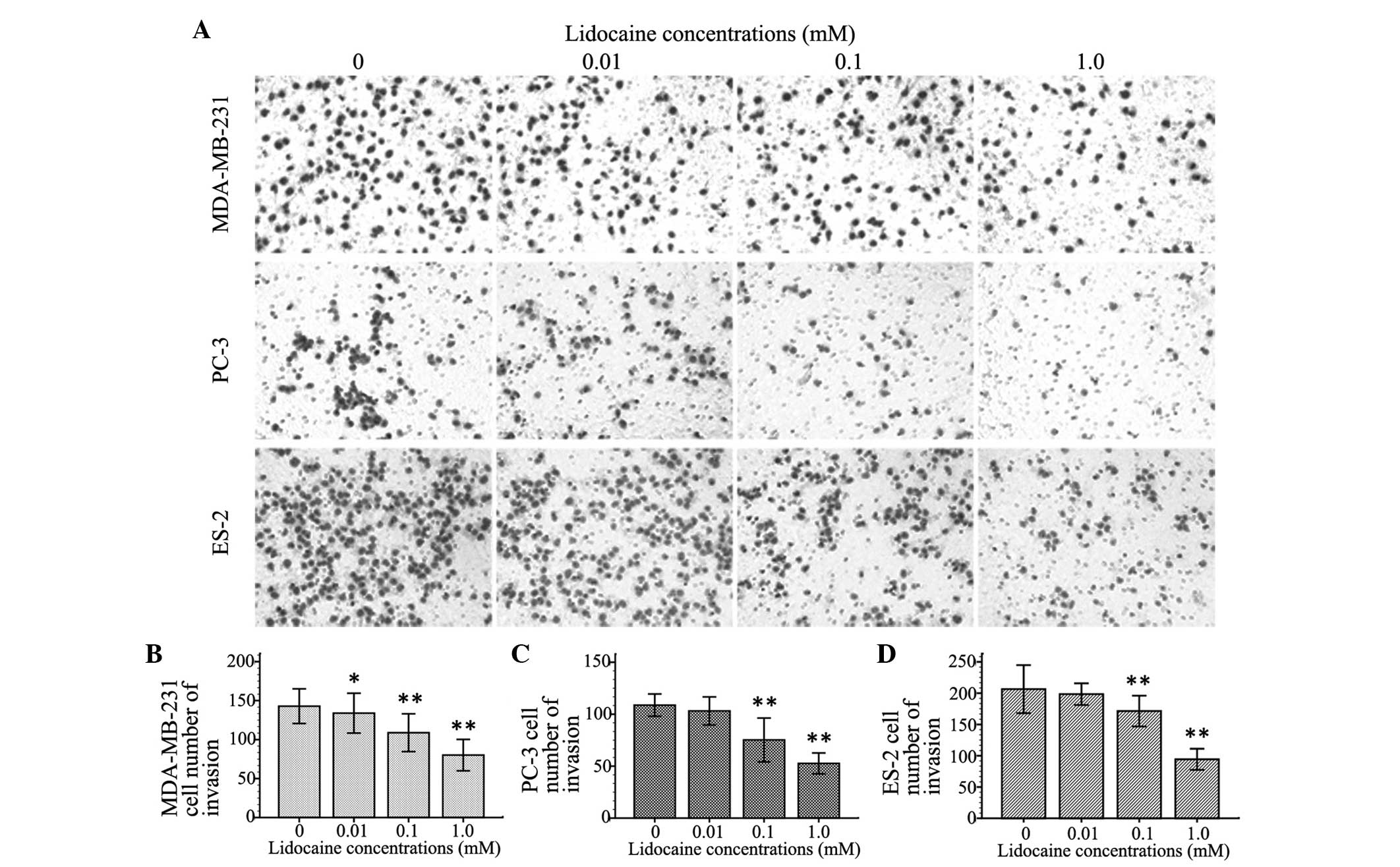
Lidocaine inhibits cell invasion
To observe the inhibitory effect of lidocaine on the
invasion of TRPV6-expressing cancer cells, the number of invasive
cells was counted using the cell invasion assay. Cell invasion
following treatment with different concentrations of lidocaine (10
µM, 100 µM and 1 mM) decreased by different degrees in the three
cell lines compared with control group. The inhibitory effect on
cell invasion was enhanced with increasing concentrations of
lidocaine. As shown in Fig. 3, At 100
µM and 1 mM, lidocaine remarkably inhibited cell migration of the
MDA-MB-231, PC-3 and ES-2 cells (P<0.01). Additionally, 10 µM
lidocaine caused a significant inhibitory effect on migration in
the MDA-MB-231 cells (P=0.039), but not in the PC-3 (P=0.111) and
ES-2 cells (P=0.092).
Lidocaine reduces the rate of
[Ca2+]I
The inhibitory effect of lidocaine on the function
of the calcium channel TRPV6 basal calcium influx in
TRPV6-expressing cancer cells was investigated using a Fluo-3 AM
fluorescence kit. After Fluo-3 AM staining, flow cytometry
investigation revealed that the spectral shift of the fluorescence
curve of treated cells following exposure to Tyrode's solution
(with 1.5 mM CaCl2) was lower than that of the control
group. This indicated that lidocaine (100 µM) treatment led to a
significant decrease in the [Ca2+]I of the
MDA-MB-231 (Fig. 1B), PC-3 (Fig. 1C) and ES-2 (Fig. 1D) cells.
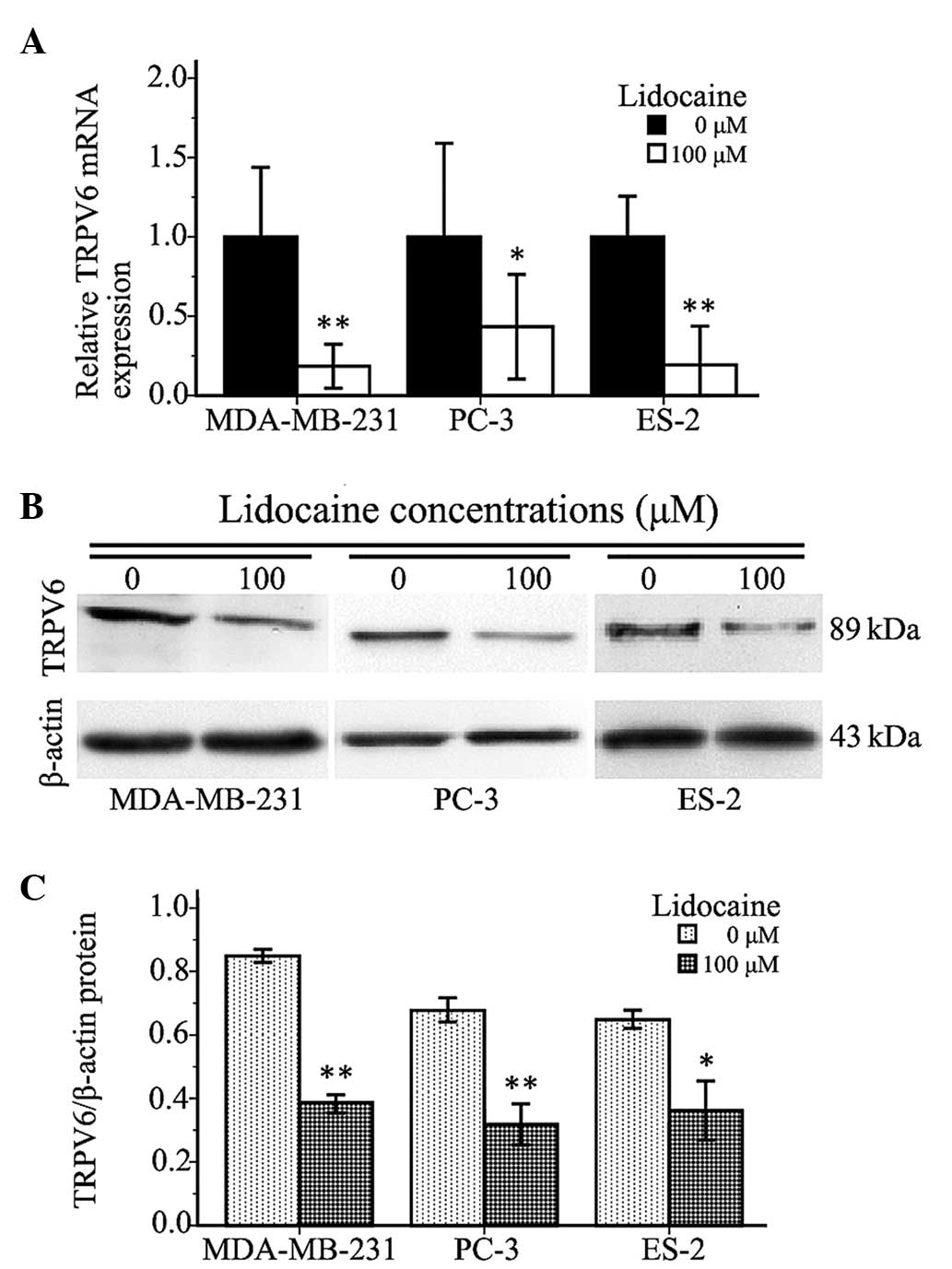
Lidocaine downregulates TRPV6
expression
The present study found that a 100-µM lidocaine
treatment could significantly inhibit cell invasion and cell
migration, and decrease intracellular-free Ca2+ in
TRPV6-expressing cells. Therefore, the expression of TRPV6 mRNA and
protein in the cells treated with lidocaine was investigated to
determine whether lidocaine inhibited TRPV6-expressing cancer cell
invasion and migration through TRPV6 downregulation. As shown in
Fig. 4A, compared with control group,
the expression of TRPV6 mRNA in the MDA-MB-231, PC-3 and ES-2 cells
treated with 100 µM lidocaine for 12 h was decreased by 81.54±5.47%
(P<0.01), 56.63±5.65% (P=0.002) and 78.82±5.75% (P<0.01),
respectively. The protein expression of TRPV6 in the treated cells
was lower than that in the control group (MDA-MB-231, P<0.01;
PC3, P=0.001; ES-2, P=0.012) (Fig. 4B and
C). The quantitative PCR and western blot analyses revealed
that 100 µM lidocaine downregulated TRPV6 at the mRNA and protein
levels.
Discussion
Retrospective studies have suggested that regional
anesthesia plays a beneficial role in the prevention of cancer
metastasis and recurrence, and that this may be attributed to the
inhibition of the surgical stress response and a decrease in opioid
analgesia requirement after cancer surgery. Local anesthetics are
not only used for nerve blocks, pain control, and anti-arrhythmia
and anti-inflammatory purposes, but can also cause neuronal cell
death and the death of other cell types (18–20). It
has even been speculated that the cytotoxicity of local anesthetics
could play an important antitumor role. Chang et al
(16) found that lidocaine and
bupivacaine may be ideal infiltration anesthetics for breast cancer
surgery as they could induce apoptosis of human breast cancer cells
at clinically relevant concentrations, and since they demonstrated
potential benefits of local anesthetics. Subsequently, after
investigating lidocaine and bupivacaine treatment in human thyroid
cancer cells, the study also found a significant decrease in cell
viability and colony formation in a dose-dependent manner as the
result of treatment with lidocaine and bupivacaine at higher
concentrations, and suggested that lidocaine and bupivacaine may
induce cell apoptosis through the mitogen-activated protein kinase
pathway (21). The present study
assessed the cytotoxicity of lidocaine at 1 to 10 mM and also found
that lidocaine was able to significantly decreased cell viability
of MDA-MB-231, PC-3 and ES-2 cells in a concentration-dependent
manner. Moreover, lidocaine inhibited the migration and invasion of
the cancer cells at concentrations that are much lower than
clinical concentrations. The migration distance and the number of
invading cells following treatment with 100 µM lidocaine was
markedly decreased.
Local anesthetics can block Ca2+
channels, as well as voltage-dependent sodium and K+
channels during regional anesthesia (17). Ca2+, as an extracellular
and intracellular signaling ion, is essential for the growth,
proliferation and survival of normal and malignant cells. The
increase in [Ca2+]I that is involved in cell
migration has been known about for a long time (22). In the present study, the
[Ca2+]I was measured using a Fluo-3 AM
fluorescence kit and the fluorescence spectra was analyzed using
flow cytometry. It was found that the calcium homeostasis of the
MDA-MB-231, PC-3 and ES-2 cells had been altered following exposure
to Tyrode's solution (with CaC12). The fluorescence
spectra of [Ca2+]I was lower than that of the
control cells, indicating that lidocaine (100 µM) treatment led to
a significant decrease in the [Ca2+]I of the
MDA-MB-231, PC-3 and ES-2 cells, and was accompanied by a
concomitant inhibition of the migration and invasion of the treated
cells.
Recent findings have shown that TRP and SOC
channels, ORAI1 and stromal interaction molecule 1 (STIM1), are
involved in cancer cell metastasis (3). A study by Yang et al (2) demonstrated that ORAI1 and STIM1 were
essential for breast cancer cell migration in vitro and
metastasis in mice, and suggested that SOC channels have the
potential as a therapeutic target in breast cancer. TRP channels,
as well as SOC channels, have become a focus of attention due to
their potential role as diagnostic, prognostic and therapeutic
targets in human tumors. Compared with other TRP channels, TRPV6
channels participate in the regulation of calcium homeostasis in
the body and have high calcium selectivity. TRPV6 channels have a
direct affect upon intestinal calcium absorption, renal calcium
excretion and bone metabolism (3,11). TRPV6
also appears to play a role as encoded by a possible oncogene in
tumor development and progression, as it was observed to be
upregulated and correlated with tumor grade in prostate, breast,
ovarian, thyroid, colon and pancreatic tumors (8,9,23). The exact role of TRPV6 in tumor
development and progression for the majority of cancer types is not
clear, but it has been demonstrated that calcium signaling controls
cancer cell proliferation and apoptosis via the TRPV6 channel.
Schwarz et al (24) suggested
that TRPV6 increases the rate of prostate cancer HEK-293 cell
proliferation in a Ca2+-dependent manner and correlates
with slightly increased [Ca2+]I. Lehen'kyi
et al (25) found that TRPV6
silencing in prostate cancer LNCaP cells decreased the cell
proliferation rate and the percentage of cells in the S-phase of
the cell cycle. Peters et al (6) also found a reduced percentage of
estrogen receptor-negative breast cancer cells in the S-phase after
TRPV6 small interfering RNA treatment. Furthermore, it was found
that the cells accumulated in the G1-phase, and this was
attributed to the attenuation of the calcium influx associated with
TRPV6 inhibition. In the present study, lidocaine was shown to
downregulate TRPV6 expression. Compared with that of the control
group, the expression of TRPV6 mRNA and protein in the
TRPV6-expressing cells with lidocaine treatment was decreased
significantly. The inhibition of cell invasion and migration
resulted in the attenuation of calcium influx associated with
lidocaine, impacting TRPV6 expression in the MDA-MB-231, PC-3 and
ES-2 cells. There are other potential calcium permeable channels,
e.g., TRPC4 and TRPC6, which could also be contributing to the
calcium uptake in the cancer cells (26). Therefore, their positive contribution
to this study could not be ruled out; however, the present results
indicate that TRPV6 has a major impact on cellular calcium
entry.
In summary, lidocaine inhibits the cell invasion and
migration of MDA-MB-231, PC-3 and ES-2 cells at lower than clinical
concentrations. The inhibitory effect of lidocaine on
TRPV6-expressing cancer cells was associated with a reduced rate of
calcium influx, and could occur partly as a result of the
downregulation of TRPV6 expression. The use of appropriate local
anesthetics may confer potential benefits in clinical practice for
the treatment of patients with TRPV6-expressing cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81171859) and by a Chongqing
Municipal Healthcare Department Medical Research Grant (no.
2010-1-20:2). The authors would like to express their sincere
thanks to Dr Yi Yuan (The Affiliated Hospital of Traditional
Chinese Medicine, Southwest Medical University, Luzhou, China), Dr
Wenfang Li (Shiyan Taihe Hospital, Shiyan, China), Dr Jianguo Hu
(The Second Affiliated Hospital, Chongqing Medical University,
Chonqing, China), Mr. Meicai Li (Chongqing Three Gorges Central
Hospital, Chonqing, China) and Miss Zunzhen Zhou (Chongqing Medwise
Anorectal Hospital, Chonqing, China) for providing useful
assistance.
References
|
1
|
Mao L, Lin S and Lin J: The effects of
anesthetics on tumor progression. Int J Physiol Pathophysiol
Pharmacol. 5:1–10. 2013.PubMed/NCBI
|
|
2
|
Yang S, Zhang JJ and Huang XY: Orai1 and
STIM1 are critical for breast tumor cell migration and metastasis.
Cancer Cell. 15:124–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prevarskaya N, Skryma R and Shuba Y:
Calcium in tumour metastasis: New roles for known actors. Nat Rev
Cancer. 11:609–618. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee J, Ishihara A, Oxford G, Johnson B and
Jacobson K: Regulation of cell movement is mediated by
stretch-activated calcium channels. Nature. 400:382–386. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang S and Huang XY: Ca2+
influx through L-type Ca2+ channels controls the
trailing tail contraction in growth factor-induced fibroblast cell
migration. J Biol Chem. 280:27130–27137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peters AA, Simpson PT, Bassett JJ, Lee JM,
Da Silva L, Reid LE, Song S, Parat MO, Lakhani SR, Kenny PA, et al:
Calcium channel TRPV6 as a potential therapeutic target in estrogen
receptor-negative breast cancer. Mol Cancer Ther. 11:2158–2168.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bowen CV, DeBay D, Ewart HS, Gallant P,
Gormley S, Ilenchuk TT, Iqbal U, Lutes T, Martina M and Mealing G:
In Vivo detection of human TRPV6-Rich tumors with anti-cancer
peptides derived from soricidin. PLoS One. 8:e588662013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhenin-Duthille I, Gautier M, Faouzi M,
Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H and
Ouadid-Ahidouch H: High expression of transient receptor potential
channels in human breast cancer epithelial cells and tissues:
Correlation with pathological parameters. Cell Physiol Biochem.
28:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng JB, Zhuang L, Berger UV, Adam RM,
Williams BJ, Brown EM, Hediger MA and Freeman MR: CaT1 expression
correlates with tumor grade in prostate cancer. Biochem Biophys Res
Commun. 282:729–734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolanz KA, Kovacs GG, Landowski CP and
Hediger MA: Tamoxifen inhibits TRPV6 activity via estrogen
receptor-independent pathways in TRPV6-expressing MCF-7 breast
cancer cells. Mol Cancer Res. 7:2000–2010. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gkika D and Prevarskaya N: TRP channels in
prostate cancer: The good, the bad and the ugly? Asian J Androl.
13:673–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Exadaktylos AK, Buggy DJ, Moriarty DC,
Mascha E and Sessler DI: Can anesthetic technique for primary
breast cancer surgery affect recurrence or metastasis?
Anesthesiology. 105:660–664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gottschalk A, Ford JG, Regelin CC, You J,
Mascha EJ, Sessler DI, Durieux ME and Nemergut EC: Association
between epidural analgesia and cancer recurrence after colorectal
cancer surgery. Anesthesiology. 113:27–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yildirim S, Altun S, Gumushan H, Patel A
and Djamgoz MB: Voltage-gated sodium channel activity promotes
prostate cancer metastasis in vivo. Cancer Lett. 323:58–61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brackenbury WJ: Voltage-gated sodium
channels and metastatic disease. Channels (Austin). 6:352–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang YC, Liu CL, Chen MJ, Hsu YW, Chen
SN, Lin CH, Chen CM, Yang FM and Hu MC: Local anesthetics induce
apoptosis in human breast tumor cells. Anesth Analg. 118:116–124.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perez-Castro R, Patel S, Garavito-Aguilar
ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ and Xu F:
Cytotoxicity of local anesthetics in human neuronal cells. Anesth
Analg. 108:997–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Li YH, Yu HZ, Wang RX and Fan TJ:
Local anesthetic lidocaine induces apoptosis in human corneal
stromal cells in vitro. Int J Ophthalmol. 6:766–771.
2013.PubMed/NCBI
|
|
19
|
Breu A, Ecki S, Zink W, Kujat R and Angele
P: Cytotoxicity of local anesthetics on human mesenchymal stem
cells in vitro. Arthroscopy. 29:1676–1684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HT, Xu H, Siegel CD and Krichevsky IE:
Local anesthetics induce human renal cell apoptosis. Am J Nephrol.
23:129–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC
and Cheng SP: Local anesthetics induce apoptosis in human thyroid
cancer cells through the mitogen-activated protein kinase pathway.
PLoS One. 9:e895632014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bolanz KA, Hediger MA and Landowski CP:
The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther.
7:271–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raphaël M, Lehen'kyi V1, Vandenberghe M,
Beck B, Khalimonchyk S, Vanden Abeele F, Farsetti L, Germain E,
Bokhobza A and Mihalache A: TRPV6 calcium channel translocates to
the plasma membrane via Orai1-mediated mechanism and controls
cancer cell survival. Proc Natl Acad Sci USA. 111:E3870–E3879.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwarz EC, Wissenbacn U, Niemeyer BA,
Strauss B, Philipp SE, Flockerzi V and Hoth M: TRPV6 potentiates
calcium-dependent cell proliferation. Cell Calcium. 39:163–173.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lehen'kyi V, Raphaël M and Prevarskaya N:
The role of the TRPV6 channel in cancer. J Physiol. 590:1369–1376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bödding M: TRP proteins and cancer. Cell
Signal. 19:617–624. 2007. View Article : Google Scholar : PubMed/NCBI
|