Cancer remains a leading cause of mortality in
humans due to the limitations of current treatment. Surgery,
radiotherapy and chemotherapy are common methods of anticancer
treatment. Despite significant developments in therapeutic
strategies, cancer remains a major public health issue (1). The growth of early-stage tumors relies
on the diffusion of oxygen and nutrients from the surrounding
tissue, and under these conditions, tumor can grow to a size of 1–2
mm3. Further expansion of the tumor mass requires
establishment of new blood vessels to support the increased
metabolic demand (2). Tumor
development depends upon the formation of new blood vessels to
deliver oxygen and micronutrients, and to facilitate cancer
metastasis into the systemic circulation (3). The formation of blood vessels occurs by
two mechanisms: Angiogenesis and vasculogenesis. Angiogenesis is
the process via which new vessel formation occurs through
proliferation and migration of existing neighboring endothelial
cells (ECs) (4,5). It was previously generally believed to
be the only mechanism responsible for new blood vessel formation
during postnatal life and the exclusive result of the sprouting of
new vessels to support tumor development. This traditional concept
of blood vessel formation has been challenged by the identification
of the bone marrow (BM)-derived cell population endothelial
progenitor cells (EPCs) that could be recruited and differentiate
into mature ECs in injured endothelium (6). Moreover, EPCs can also secrete a series
of cytokines to promote new vessel formation (7). EPCs have been reported to migrate
actively to the tumor bed and incorporate directly in the
neovasculature with high specificity (8–10). Thus,
EPCs that mediate neovascularization may be crucial contributors to
the growth and spread of cancer, and have become an important
promising target for antineoplastic therapies in a variety of solid
tumors (2,11,12).
EPC-mediated neovascularization is a multiple step process, in
which numerous cytokines and modulators are involved to regulate
cell mobilization, recruitment and incorporation into the
endothelium (13). Although the
contribution of EPCs to new vessel formation has previously been
established, the underlying mechanisms remain unclear and require
further study, which may provide a potential target in therapeutic
management by blocking the process and signal pathways involved in
EPC-mediated tumor neovessel formation.
EPCs are a BM-derived cell population that
circulates in the peripheral circulation and homes to the sites of
injured vessels to participate in new blood vessel formation under
physiopathological conditions (6).
Vascular endothelial growth factor receptor-2 (VEGFR-2) and cluster
of differentiation (CD)34 were initially described as specific
expression markers of the cells; however, these markers are also
expressed on hemangioblast and hematopoietic progenitors, as well
as certain mature ECs (6). Putative
EPCs encompass different cell populations that all originate from
the hemangioblast overlap, phenotypically as well as functionally,
and present in different stages of endothelial differentiation in
the peripheral blood. Recently, an array of biomarkers has been
used to characterize the putative EPCs, including CD34, CD133,
CD31, VEGFR-2, von Willebrand factor (vWF), CD144, Tie2, CD117,
CD62E and CD45 (14–18). The glycosylated form of the CD133
protein has been accepted as a more appropriate marker for immature
progenitor cells, since it is expressed on immature stem cells, but
not on mature ECs (19,20). Thus,
CD133+/VEGFR-2+ cells more likely reflect
immature progenitor cells localized mainly in the BM, whereas
AC133−/CD34+/VEGFR-2+ cells may
represent more mature cells that are limited in their proliferative
capacity (21). There are two main
types of EPCs, early and late EPCs, which are isolated by different
culture methods (15,16). Early EPCs obtained from short-term
blood sample cultures possess numerous endothelial characteristics,
such as harboring markers of CD31, CD133, CD34 and VEGFR-2
(2), while late EPCs express
VE-cadherin and vWF, as well as endothelial markers such as CD31,
CD133, CD34 and VEGFR-2 (22).
Mounting evidence has suggested that early and late EPCs play a
critical role in postnatal angiogenesis and vasculogenesis under a
number of pathological conditions, including myocardial ischemia
and infarction, wound healing, atherosclerosis, limb ischemia and
tumor development (8,23–29).
Therefore, in the treatment of diseases such as solid tumors, EPCs
have been considered as potential targets that are closely
associated with neovascularization (30).
Tumors arise from several sequential genetic
mutations in DNA that result in the cell deregulation of normal
growth control mechanisms and uncontrolled proliferation.
Eventually, these mutated cells expand to become a cell mass that
is able to obtain adequate nutrients and oxygen via the diffusion
from surrounding tissues to support growth at an early stage
(31). Once cell deposits reach a
size of 1–2 mm3, expansion of the tumor requires nascent
vasculature to deliver nutrients and oxygen for further cell
proliferation (32). In addition to
delivering oxygen and micronutrients to the tumor mass, the ECs
promote tumor growth via paracrine growth and survival signals
(33–35). Tumor angiogenesis can also facilitate
cancer metastasis by allowing cells to exit through the neovascular
network into the systemic circulation (36). The production of new blood vessels
from an existing vascular bed is known as angiogenesis. However,
recent attention has focused on tumor-associated vasculogenesis
that occurs through mature ECs via the proliferation and
differentiation of BM-derived EPCs. Accumulating data indicate that
EPCs provide not only structural support to nascent vessels
(37–39), but that they also regulate the
angiogenic process via paracrine secretion of a number of
proangiogenic growth factors (2).
Through these mechanisms, EPCs have a dual role in tumor
development. Thus EPCs may be an essential component in cancer
growth and progression, and the inhibition of EPC-mediated
neovascularization may efficiently block tumor development.
EPCs were first described as a subtype of stem cells
that gave rise to mature ECs in culture and were able to
incorporate themselves into sites of active neovascularization in
animal models (6). The cells were
then extensively studied regarding their role in
neovascularization. Accumulating data has confirmed that early and
late EPCs participate in the process of new vessel formation under
a wide range of physiopathological conditions. Early EPCs promote
angiogenesis by secreting a series of growth factors and cytokines,
such as VEGF, stromal cell-derived factor-1 (SDF-1), granulocyte
colony-stimulating factor (G-CSF) and insulin-like growth factor 1,
which enhance EC proliferation and survival, and direct endogenous
progenitor cell recruitment into nascent vessel sites (6,40). Late
EPCs contribute to vasculogenesis by providing structural support
via differentiation into mature ECs (16). Late EPCs can also promote angiogenesis
by the secretion of numerous cytokines (7). The contribution of EPCs in postnatal
endothelial repair and vasculogenesis has been documented in
physiological and pathological conditions, such as myocardial
ischemia (24), limb ischemia
(27), ischemic stroke (26), wound healing (28) and tumor vascularization (41). Accumulating evidence has suggested
that the number of circulating EPCs is elevated in a wide range of
cancer types, such as glioma, non-small cell lung cancer, myeloid
leukemia, hepatocellular carcinoma, colorectal cancer, lymphoma and
breast cancer (42,43). EPCs are considered to be a key
contributor to the new vessel formation of a tumor during its
development (2,44). Initial evidence of the involvement of
EPCs in tumor neovessels was discovered in
Id1+/−Id3−/− tumor
mouse models (41). The study
demonstrated that the defection in the recruitment of BM-derived
endothelial precursor cells blocks tumor angiogenesis and growth,
and that this could be reversed by the transplantation of
circulatory EPCs. Significantly, the donor-derived cells were
detected throughout the tumor neovessels in the animal cancer
models (41). Subsequent studies
showed that the blockage of EPC mobilization from the BM or
recruitment to the tumor bed can result in significant inhibition
of tumor neovasculogenesis and growth (45,46). These
data demonstrate that EPCs contribute to new vessel formation in
tumor development.
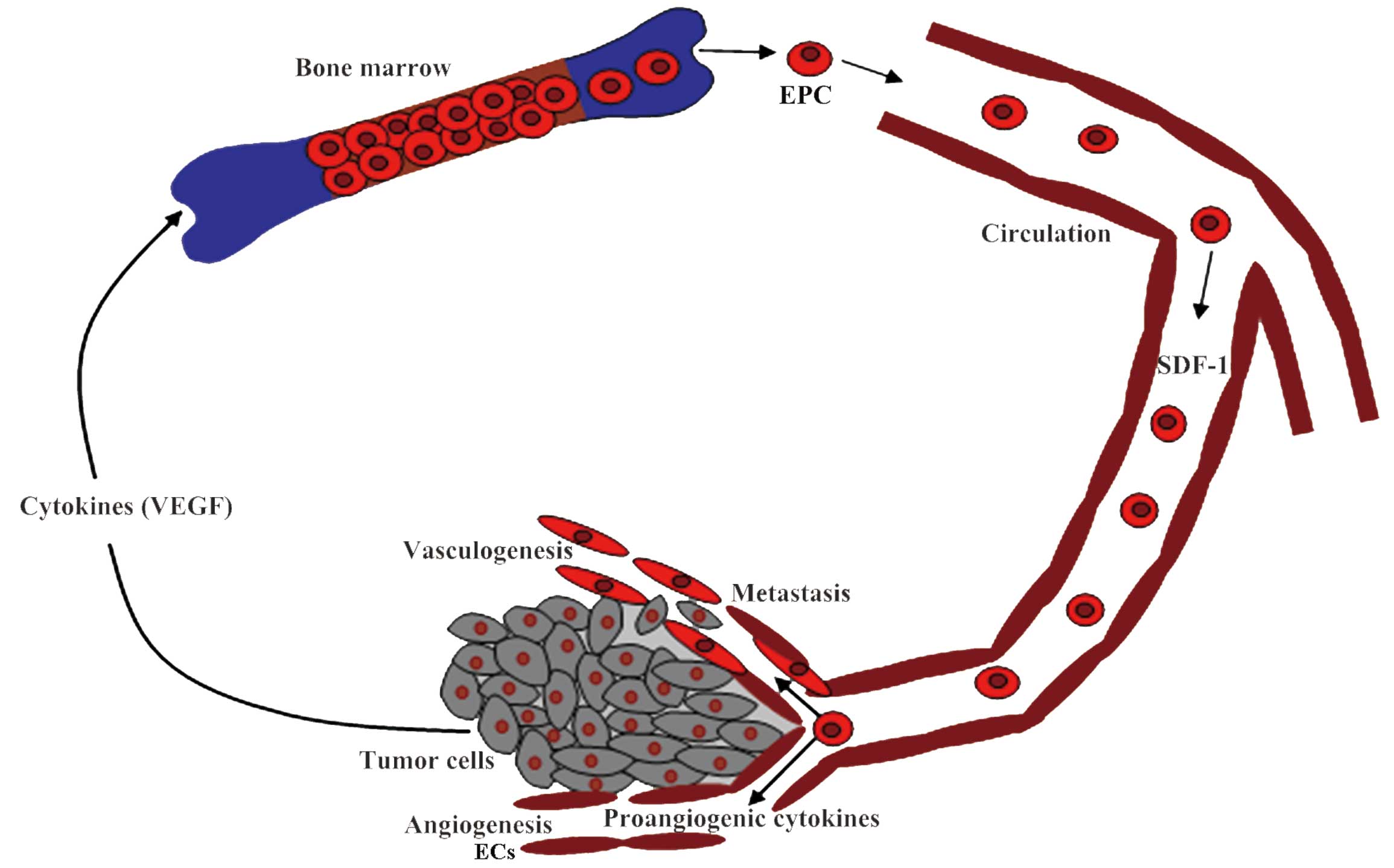
EPC-mediated neovascularization is a complex
process, including EPC mobilization from the BM into the peripheral
circulation, recruitment and adhesion to the sites of new vessel
formation, incorporation into the intima to form de novo
vessels, and paracrine support of the nascent microvasculature
(Fig. 1). These events are controlled
by multiple cytokines and modulators via different mechanisms
(47).
Mobilization of EPCs from the BM into the
circulation is the first step for EPC-mediated vasculogenesis. In
normal conditions, the number of circulating EPCs is extremely low
(15), and the majority of the cells
reside in the niche within the BM via the interaction of the
integrins expressed on these cells with stromal cells (48,49).
Tumor vasculogenesis requires signaling between
tumor cells and the EPCs residing in the BM, stimulating them to
mobilize into the peripheral circulation, home to the tumor sites
and invade the growing tumor (50).
The process involves multiple steps that are regulated by a wide
range of cytokines and chemokines. VEGF is a pleiotropic cytokine
that has been implicated in the mobilization of VEGFR-2+
EPCs from the BM, and VEGF gene transfer has been shown to augment
circulating EPCs in humans (51,52). VEGF
is an angiogenic cytokine that is expressed in the tumor bed. The
high levels of VEGF promote tumor vasculogenesis and progression by
mobilizing BM-resident EPCs into the peripheral circulation, and
enhance the recruitment of these cells to the tumor sites (51,53–55). VEGF
mechanism in EPC-mediated neovascularization involves a number of
enzyme and cytokines. VEGF activates BM nitric oxide (NO) synthase
and promotes NO production. This NO interacts with matrix
metalloproteinase-9, leading to the release of stem cell-active
soluble kit ligand, which enhances VEGFR-2+ EPC mobility
and stimulates cell mobilization from the BM into the peripheral
circulation (56). VEGF has the
ability to upregulate the levels of G-CSF, inducing the release of
progenitor cells from the BM (57).
G-CSF mechanisms in EPC mobilization are correlated with
BM-neutrophil-released elastase and cathepsin G, which induce the
proteolytic cleavage of vascular cell adhesion molecule-1,
expressed by BM stromal cells, followed by progenitor cell
mobilization (58). The CXC motif
chemokine family is another well-known inducer of EPC mobilization.
SDF-1 is the most well-characterized component of EPC mobilization
and a effective chemokine in the adhesion and migration of the
cells. The expansion of the tumor causes surrounding tissue
hypoxia, which through elevated levels of hypoxia-inducible
factor-1 (HIF-1), upregulates the responsive of chemokines such as
SDF-1 and VEGF, and stimulates the release and recruitment of EPCs
from the BM into the circulation (52,59,60).
Tumors can also produce chemokine (C-C motif) ligand (CCL)2 and
CCL5, which are involved in EPC mobilization (61). In addition, the cells surrounding the
tumor produce other factors to mobilize EPCs and recruit them to
the tumor bed. Adiponectin, for example, is a peptide hormone
secreted by adipocytes that has been shown to promote EPC numbers
and mammary tumor growth (62–64).
The recruitment of EPCs from the circulation to the
site of the tumor bed is an essential step for EPC-mediated new
vessel formation in neoplasm growth and development. Tumor and
ischemic tissues have the potential to direct EPCs from the
circulation into vasculogenic sites in order to increase the number
of sprouting vessels for the blood supply via secretion of
cytokines, of which SDF-1 is the most potent (65,66). SDF-1
functions as a major chemokine to direct various types of chemokine
(C-X-C motif) receptor 4 (CXCR4)+ cells to the tumor bed
(65–69). In normal conditions, the levels of
SDF-1 are low in the circulation, BM and other tissues (49). As a secretory protein, the level of
SDF-1 is upregulated in the tumor microenvironment, where it can
create a gradient between the peripheral blood and the tumor
microenvironment, directing the migration of EPCs from the
circulation into the tumor bed (30).
The process requires HIF, a heterodimeric transcription factor
sensitive to oxygen concentrations in tissues (70). Malignant tumor growth results in
neoplastic tissue hypoxia, which then induces HIF production. The
SDF-1 promoter contains HIF-1 binding sites, and the binding leads
to SDF-1 production (66). There is
an increasing amount of evidence suggesting that the overexpression
of SDF-1 is closely associated with neovascularization by mediating
the homing and retention of pro-vasculogenic stem cells to areas of
new vessel formation (49,71,72).
Histological analysis in rat glioma and melanoma models revealed
that the majority of implanted, magnetically-labeled cord
blood-derived AC133+ EPCs were expressed in peripheral
regions of the tumor, and the high levels of HIF-1 and SDF-1 were
also disturbed in regions, indicating a more hypoxic
microenvironment. The studies also showed the incorporation of
human AC133+ cells into the tumor neovasculature upon
immunofluorescent staining (73). In
diabetes, a wound site is characterized by low levels of SDF-1 and
delayed healing. This could be reversed by the administration of
SDF-1 to improve the efficiency of EPC migration, consequently
enhancing neovascularization and wound healing. This indicates the
correlation of SDF-1 with the homing of EPCs to the neovessel and
subsequently, EPC-mediated new vessel formation (74). The CXC/CXCR4 pathway pays a crucial
role in these processes. SDF-1 is a member of the CXC chemokine
family, which plays an important role in chemotaxis (71,72). SDF-1
exerts a chemoattractant role through binding with its receptor,
CXCR4, expressed on the surface of EPCs. Blocking the SDF-1/CXCR4
pathway inhibits the ability of EPCs to home and their adhesion to
the sites of ischemic tissues, indicating the involvement of the
SDF-1/CXCR4 pathway in the process of EPC-mediated
neovascularization (75,76). The interaction of SDF-1 with CXCR4
activates multiple downstream targets, ultimately leading to
cytoskeletal rearrangements, actin polymerization, polarization,
pseudopodia formation and integrin-dependent adhesion to ECs
(77).
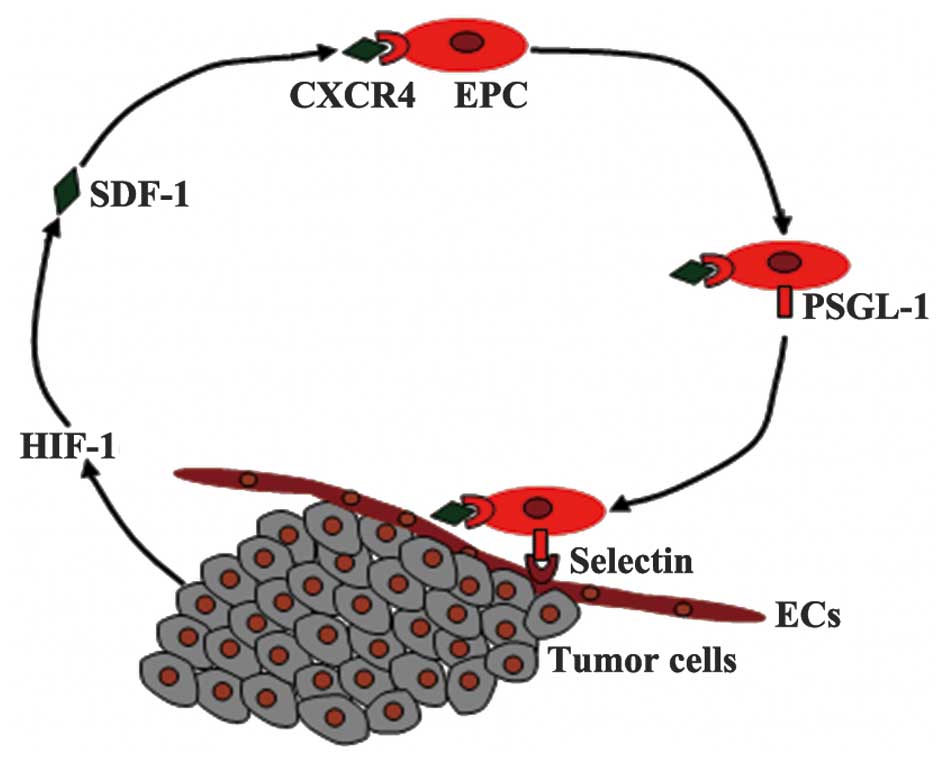
EPC adhesion requires the molecular targets
expressed by EPCs and by the angiogenic tissues that they home to
(78). P selectin glycoprotein
ligand-1 (PSGL-1) is a binding protein expressed on EPCs involved
in cell adhesion; it is a major ligand of P-selectin and E-selectin
expressed on ECs. The binding results in EPC transendothelial
migration into the blood vessel wall where vascular remodeling is
required (79). In the
microenvironment of angiogenic sites, released SDF-1 may be vital
in the modulation of this process via the SDF-1/CXCR4 pathway
(80). The interaction of SDF-1 and
EPC CXCR4 upregulates the expression of PSGL-1 on the EPC surface,
leading to the adhesion and rolling of the cells to the blood
vessel wall (Fig. 2). The adhesion of
the EPCs to the endothelial monolayer is also strengthened by
integrins, a type of cell adhesion molecule expressed on the cell
surface (81). The interaction of
integrins with intercellular adhesion molecule-1 and fibrinogen
mediates the adhesion of the EPCs to active angiogenic sites and
facilitates the cells transendothelial migration (82,83). The
release of high-mobility group box 1 by necrotic cells can also
promote EPC adhesion to the sites of new vessel formation via the
activation of β1- and β2-integrins (84,85). This
process is initiated by the activation of proangiogenic factors,
such as VEGF, followed by the conversion of inactive enzyme
plasminogen into the active serine protease plasmin and degradation
of the extracellular matrix (86). In
addition, upregulation of α4-integrin can also improve circulating
progenitor cell invasion into the neovasculature and augment
ischemic neovascularization (87,88).
Overall, the process of EPC-mediated neovascularization is complex,
including multiple steps in which numerous cytokines and modulators
are involved.
The human vascular system forms an intricate and
dynamic network in the body for the delivery of oxygen and
micronutrients to tissues, and for the removal of carbon dioxide
and metabolic by-products. The system also exerts effects on
adjacent nonvascular cells by secreting paracrine growth and
survival signals (33). Pathological
excessive neovascularization can promote the growth of diseased
tissues, which can be observed in the majority of cancers (89,90). Tumor
progression depends largely on new vessel formation to deliver
oxygen and nutrients for tumor growth, expansion and metastasis;
thus inhibiting tumor neovascularization may be an effective
treatment strategy for various solid cancer types. The first
hypothesis stating that the inhibition of neovascularization may be
an effective strategy in cancer treatment was proposed in 1971
(91). Subsequent studies in animal
models indicated that anti-angiogenesis at an early stage could
prevent tumor growth and development in a wide spectrum of cancers
(92). Preclinical, clinical and
epidemiological data have shown that the inhibition of angiogenesis
achieves the prevention of tumor development (93,94). A
number of agents have been shown to exhibit effective
antiangiogenic activities and exert preventative roles in the
growth of a series of cancer types, including colorectal, renal,
liver, lung, brain, pancreatic and neuroendocrine tumors, and
multiple myeloma (95). It is now
accepted that postnatal neovessel formation depends not only on
angiogenesis, but also on vasculogenesis, which requires BM-derived
EPC incorporation into the endothelium and differentiation into
mature ECs to provide structural support to nascent vessels
(96). Similarly, tumors obtain their
vasculature through not only angiogenesis, but also through
EPC-mediated vasculogenesis (41,97). In
addition to structural support to nascent vessels (37,38), EPCs
can also regulate the angiogenic process via the paracrine
secretion of a number of proangiogenic growth factors and have a
dual role in tumor development (30).
Inhibiting EPC-mediated vasculogenesis, similar to
anti-angiogenesis, may block tumor progression. This hypothesis is
supported by studies that blocked EPC mobilization from BM or
recruitment to the tumor bed, resulting in significant inhibition
of tumor neovasculogenesis and growth (45,46).
Disruption of SDF-1/CXCR4 via the CXCR4 blocking antibody, for
example, reduced the recruitment of BM-derived progenitor cells to
the tumor bed and resulted in the inhibition of tumor growth in
mouse models (98). A complete
understanding of the mechanisms underlying EPC-mediated
neovascularization is therefore required, as this may result in
potential effective methods of cancer treatment.
EPC-mediated neovascularization may be an essential
component of cancer growth and development. In addition to
providing structural support to nascent vessels in tumor expansion,
EPCs can also promote tumor progression via independent
vasculogenesis pathways. EPCs release a series of cytokines that
exert effects on angiogenesis and vasculogenesis. The process of
EPC-mediated neovascularization involves multiple steps, including
cell mobilization, recruitment and adhesion to neovessel sites, and
incorporation into the endothelium. Numerous cytokines are
involved. Blockage of the process may reduce new vessel formation
in tumors, followed by the blockage of tumor growth, invasion and
metastasis. Thus, a comprehensive understanding of the mechanisms
by which EPCs participate in neovascularization is required, which
may provide potential targets in cancer treatment by blocking
EPC-mediated tumor neovascularization.
This study was supported by the Natural Science
Foundation for Young Scientists of Jilin Province, China (grant no.
20140520035JH) to L.X.L., “13th Five-Year” and technology research
of the Education Department of Jilin Province, China (grant no.
2016-483) to Z.X. and the Health and Family Planning Commission for
Young Scientists of Jilin Province, China (grant no. 2015Q008) to
L.X.L.
|
1
|
Global Burden of Disease Cancer
Collaboration. Fitzmaurice C, Dicker D, Pain A, et al: The global
burden of cancer 2013. JAMA Oncol. 1:505–527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janic B and Arbab AS: The role and
therapeutic potential of endothelial progenitor cells in tumor
neovascularization. Scientific World Journal. 10:1088–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kerbel RS: Tumor angiogenesis: Past,
present and the near future. Carcinogenesis. 21:505–515. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
5
|
Folkman J: Seminars in medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moubarik C, Guillet B, Youssef B,
Codaccioni JL, Piercecchi MD, Sabatier F, Lionel P, Dou L,
Foucault-Bertaud A, Velly L, et al: Transplanted late outgrowth
endothelial progenitor cells as cell therapy product for stroke.
Stem Cell Rev. 7:208–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi
JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA,
Benezra R and Mittal V: Bone marrow-derived endothelial progenitor
cells are a major determinant of nascent tumor neovascularization.
Genes Dev. 21:1546–1558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folkins C, Shaked Y, Man S, Tang T, Lee
CR, Zhu Z, Hoffman RM and Kerbel RS: Glioma tumor stem-like cells
promote tumor angiogenesis and vasculogenesis via vascular
endothelial growth factor and stromal-derived factor 1. Cancer Res.
69:7243–7251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HR, Chen FL, Xu CP, Ping YF, Wang
QL, Liang ZQ, Wang JM and Bian XW: Incorporation of endothelial
progenitor cells into the neovasculature of malignant glioma
xenograft. J Neurooncol. 93:165–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Fang J, Wang S, Liu H, Du X, Chen
J, Li X, Yang Y, Zhang B and Zhang W: A new mosaic pattern in
glioma vascularization: Exogenous endothelial progenitor cells
integrating into the vessels containing tumor-derived endothelial
cells. Oncotarget. 5:1955–1968. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russell JS and Brown JM: Circulating mouse
Flk1+/c-Kit+/CD45− cells function
as endothelial progenitors cells (EPCs) and stimulate the growth of
human tumor xenografts. Mol Cancer. 13:1772014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee PS and Poh KK: Endothelial progenitor
cells in cardiovascular diseases. World J Stem Cells. 6:355–366.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rouhl RP, van Oostenbrugge RJ, Damoiseaux
J, Tervaert JW and Lodder J: Endothelial progenitor cell research
in stroke: A potential shift in pathophysiological and
therapeutical concepts. Stroke. 39:2158–2165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fadini GP, Losordo D and Dimmeler S:
Critical reevaluation of endothelial progenitor cell phenotypes for
therapeutic and diagnostic use. Circ Res. 110:624–637. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ,
Hwang KK, Oh BH, Lee MM and Park YB: Characterization of two types
of endothelial progenitor cells and their different contributions
to neovasculogenesis. Arterioscler Thromb Vasc Biol. 24:288–293.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: Mobilization, differentiation and homing.
Arterioscler Thromb Vasc Biol. 23:1185–1189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walenta KL, Bettink S, Böhm M and
Friedrich EB: Differential chemokine receptor expression regulates
functional specialization of endothelial progenitor cell
subpopulations. Basic Res Cardiol. 106:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gehling UM, Ergün S, Schumacher U, Wagener
C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, et
al: In vitro differentiation of endothelial cells from
AC133-positive progenitor cells. Blood. 95:3106–3112.
2000.PubMed/NCBI
|
|
20
|
Peichev M, Naiyer AJ, Pereira D, Zhu Z,
Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA and Rafii
S: Expression of VEGFR-2 and AC133 by circulating human CD34(+)
cells identifies a population of functional endothelial precursors.
Blood. 95:952–958. 2000.PubMed/NCBI
|
|
21
|
Khakoo AY and Finkel T: Endothelial
progenitor cells. Annu Rev Med. 56:79–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shantsila E, Watson T and Lip GY:
Endothelial progenitor cells in cardiovascular disorders. J Am Coll
Cardiol. 49:741–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi
JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM and
Asahara T: Therapeutic potential of ex vivo expanded endothelial
progenitor cells for myocardial ischemia. Circulation. 103:634–637.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawamoto A, Tkebuchava T, Yamaguchi J,
Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma
H, et al: Intramyocardial transplantation of autologous endothelial
progenitor cells for therapeutic neovascularization of myocardial
ischemia. Circulation. 107:461–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang ZG, Zhang L, Jiang Q and Chopp M:
Bone marrow-derived endothelial progenitor cells participate in
cerebral neovascularization after focal cerebral ischemia in the
adult mouse. Circ Res. 90:284–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu
J, Chen Y, Su H, Young WL and Yang GY: Endothelial progenitor cell
transplantation improves long-term stroke outcome in mice. Ann
Neurol. 67:488–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi J, Kusano KF, Masuo O, Kawamoto
A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner
JM and Asahara T: Stromal cell-derived factor-1 effects on ex vivo
expanded endothelial progenitor cell recruitment for ischemic
neovascularization. Circulation. 107:1322–1328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu ZJ and Velazquez OC: Hyperoxia,
endothelial progenitor cell mobilization, and diabetic wound
healing. Antioxid Redox Signal. 10:1869–1882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dome B, Timar J, Ladanyi A, Paku S,
Renyi-Vamos F, Klepetko W, Lang G, Dome P, Bogos K and Tovari J:
Circulating endothelial cells, bone marrow-derived endothelial
progenitor cells and proangiogenic hematopoietic cells in cancer:
From biology to therapy. Crit Rev Oncol Hematol. 69:108–124. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de la Puente P, Muz B, Azab F and Azab AK:
Cell trafficking of endothelial progenitor cells in tumor
progression. Clin Cancer Res. 19:3360–3368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tazzyman S, Lewis CE and Murdoch C:
Neutrophils: Key mediators of tumour angiogenesis. Int J Exp
Pathol. 90:222–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(6 Suppl 16): S15–S18. 2002.
View Article : Google Scholar
|
|
33
|
Rak J, Filmus J and Kerbel RS: Reciprocal
paracrine interactions between tumour cells and endothelial cells:
The ‘angiogenesis progression’ hypothesis. Eur J Cancer.
32A:2438–2450. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Folkman J: Angiogenesis-dependent
diseases. Semin Oncol. 28:536–542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Folkman J: Angiogenesis and apoptosis.
Semin Cancer Biol. 13:159–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Calzi S, Neu MB, Shaw LC, Kielczewski
JL, Moldovan NI and Grant MB: EPCs and pathological angiogenesis:
When good cells go bad. Microvasc Res. 79:207–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu XH, Pan W, Kang LH, Feng H and Song YQ:
Association of annexin A2 with cancer development (Review). Oncol
Rep. 33:2121–2128. 2015.PubMed/NCBI
|
|
40
|
Urbich C, Aicher A, Heeschen C, Dernbach
E, Hofmann WK, Zeiher AM and Dimmeler S: Soluble factors released
by endothelial progenitor cells promote migration of endothelial
cells and cardiac resident progenitor cells. J Mol Cell Cardiol.
39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lyden D, Hattori K, Dias S, Costa C,
Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et
al: Impaired recruitment of bone-marrow-derived endothelial and
hematopoietic precursor cells blocks tumor angiogenesis and growth.
Nat Med. 7:1194–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hristov M and Weber C: Endothelial
progenitor cells: Characterization, pathophysiology, and possible
clinical relevance. J Cell Mol Med. 8:498–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: Isolation and characterization. Trends Cardiovasc
Med. 13:201–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stoll BR, Migliorini C, Kadambi A, Munn LL
and Jain RK: A mathematical model of the contribution of
endothelial progenitor cells to angiogenesis in tumors:
Implications for antiangiogenic therapy. Blood. 102:2555–2561.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shojaei F, Wu X, Malik AK, Zhong C,
Baldwin ME, Schanz S, Fuh G, Gerber HP and Ferrara N: Tumor
refractoriness to anti-VEGF treatment is mediated by
CD11b+Gr1+ myeloid cells. Nat Biotechnol.
25:911–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li DW, Liu ZQ, Wei J, Liu Y and Hu LS:
Contribution of endothelial progenitor cells to neovascularization
(Review). Int J Mol Med. 30:1000–1006. 2012.PubMed/NCBI
|
|
48
|
Lapidot T and Petit I: Current
understanding of stem cell mobilization: The roles of chemokines,
proteolytic enzymes, adhesion molecules, cytokines and stromal
cells. Exp Hematol. 30:973–981. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lapidot T, Dar A and Kollet O: How do stem
cells find their way home? Blood. 106:1901–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ferrara N and Alitalo K: Clinical
applications of angiogenic growth factors and their inhibitors. Nat
Med. 5:1359–1364. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M, Isner JM, et al: VEGF
contributes to postnatal neovascularization by mobilizing bone
marrow-derived endothelial progenitor cells. EMBO J. 18:3964–3972.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kalka C, Masuda H, Takahashi T, Gordon R,
Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM
and Asahara T: Vascular endothelial growth factor(165) gene
transfer augments circulating endothelial progenitor cells in human
subjects. Circ Res. 86:1198–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hattori K, Dias S, Heissig B, Hackett NR,
Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, et al:
Vascular endothelial growth factor and angiopoietin-1 stimulate
postnatal hematopoiesis by recruitment of vasculogenic and
hematopoietic stem cells. J Exp Med. 193:1005–1014. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kopp HG, Ramos CA and Rafii S:
Contribution of endothelial progenitors and proangiogenic
hematopoietic cells to vascularization of tumor and ischemic
tissue. Curr Opin Hematol. 13:175–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kopp HG, Avecilla ST, Hooper AT and Rafii
S: The bone marrow vascular niche: Home of HSC differentiation and
mobilization. Physiology (Bethesda). 20:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Heissig B, Hattori K, Dias S, Friedrich M,
Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et
al: Recruitment of stem and progenitor cells from the bone marrow
niche requires MMP-9 mediated release of kit-ligand. Cell.
109:625–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Powell TM, Paul JD, Hill JM, Thompson M,
Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, et
al: Granulocyte colony-stimulating factor mobilizes functional
endothelial progenitor cells in patients with coronary artery
disease. Arterioscler Thromb Vasc Biol. 25:296–301. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lévesque JP, Takamatsu Y, Nilsson SK,
Haylock DN and Simmons PJ: Vascular cell adhesion molecule-1
(CD106) is cleaved by neutrophil proteases in the bone marrow
following hematopoietic progenitor cell mobilization by granulocyte
colony-stimulating factor. Blood. 98:1289–1297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chang EI, Chang EI, Thangarajah H, Hamou C
and Gurtner GC: Hypoxia, hormones, and endothelial progenitor cells
in hemangioma. Lymphat Res Biol. 5:237–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ling CC, Ng KT, Shao Y, Geng W, Xiao JW,
Liu H, Li CX, Liu XB, Ma YY, Yeung WH, et al: Post-transplant
endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling
promotes liver tumor growth. J Hepatol. 60:103–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Spring H, Schüler T, Arnold B, Hämmerling
GJ and Ganss R: Chemokines direct endothelial progenitors into
tumor neovessels. Proc Natl Acad Sci USA. 102:18111–18116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shibata R, Skurk C, Ouchi N, Galasso G,
Kondo K, Ohashi T, Shimano M, Kihara S, Murohara T and Walsh K:
Adiponectin promotes endothelial progenitor cell number and
function. FEBS Lett. 582:1607–1612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nakamura N, Naruse K, Matsuki T, Hamada Y,
Nakashima E, Kamiya H, Matsubara T, Enomoto A, Takahashi M, Oiso Y
and Nakamura J: Adiponectin promotes migration activities of
endothelial progenitor cells via Cdc42/Rac1. FEBS Lett.
583:2457–2463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Landskroner-Eiger S, Qian B, Muise ES,
Nawrocki AR, Berger JP, Fine EJ, Koba W, Deng Y, Pollard JW and
Scherer PE: Proangiogenic contribution of adiponectin toward
mammary tumor growth in vivo. Clin Cancer Res. 15:3265–3276. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kucia M, Reca R, Miekus K, Wanzeck J,
Wojakowski W, Janowska-Wieczorek A, Ratajczak J and Ratajczak MZ:
Trafficking of normal stem cells and metastasis of cancer stem
cells involve similar mechanisms: Pivotal role of the SDF-1-CXCR4
axis. Stem Cells. 23:879–894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ceradini DJ, Kulkarni AR, Callaghan MJ,
Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP
and Gurtner GC: Progenitor cell trafficking is regulated by hypoxic
gradients through HIF-1 induction of SDF-1. Nat Med. 10:858–864.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bhakta S, Hong P and Koc O: The surface
adhesion molecule CXCR4 stimulates mesenchymal stem cell migration
to stromal cell-derived factor-1 in vitro but does not decrease
apoptosis under serum deprivation. Cardiovasc Revasc Med. 7:19–24.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kryczek I, Lange A, Mottram P, Alvarez X,
Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, et al: CXCL12
and vascular endothelial growth factor synergistically induce
neoangiogenesis in human ovarian cancers. Cancer Res. 65:465–472.
2005.PubMed/NCBI
|
|
69
|
Darash-Yahana M, Pikarsky E, Abramovitch
R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E
and Peled A: Role of high expression levels of CXCR4 in tumor
growth, vascularization and metastasis. FASEB J. 18:1240–1242.
2004.PubMed/NCBI
|
|
70
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kollet O, Shivtiel S, Chen YQ, Suriawinata
J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, et al:
HGF, SDF-1, and MMP-9 are involved in stress-induced human
CD34+ stem cell recruitment to the liver. J Clin Invest.
112:160–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Askari AT, Unzek S, Popovic ZB, Goldman
CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD,
DiCorleto PE, et al: Effect of stromal-cell-derived factor 1 on
stem-cell homing and tissue regeneration in ischaemic
cardiomyopathy. Lancet. 362:697–703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Arbab AS, Janic B, Knight RA, Anderson SA,
Pawelczyk E, Rad AM, Read EJ, Pandit SD and Frank JA: Detection of
migration of locally implanted AC133+ stem cells by
cellular magnetic resonance imaging with histological findings.
FASEB J. 22:3234–3246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gallagher KA, Liu ZJ, Xiao M, Chen H,
Goldstein LJ, Buerk DG, Nedeau A, Thom SR and Velazquez OC:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1 alpha.
J Clin Invest. 117:1249–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Abbott JD, Huang Y, Liu D, Hickey R,
Krause DS and Giordano FJ: Stromal cell-derived factor-1alpha plays
a critical role in stem cell recruitment to the heart after
myocardial infarction but is not sufficient to induce homing in the
absence of injury. Circulation. 110:3300–3305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Walter DH, Haendeler J, Reinhold J,
Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R,
Arenzana-Seisdesdos F, et al: Impaired CXCR4 signaling contributes
to the reduced neovascularization capacity of endothelial
progenitor cells from patients with coronary artery disease. Circ
Res. 97:1142–1151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Vajkoczy P, Blum S, Lamparter M,
Mailhammer R, Erber R, Engelhardt B, Vestweber D and Hatzopoulos
AK: Multistep nature of microvascular recruitment of ex
vivo-expanded embryonic endothelial progenitor cells during tumor
angiogenesis. J Exp Med. 197:1755–1765. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Di Santo S, Diehm N, Ortmann J, Völzmann
J, Yang Z, Keo HH, Baumgartner I and Kalka C: Oxidized low density
lipoprotein impairs endothelial progenitor cell function by
downregulation of E-selectin and integrin alpha(v)beta5. Biochem
Biophys Res Commun. 373:528–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lapidot T: Mechanism of human stem cell
migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice.
The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 938:83–95.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chavakis E, Aicher A, Heeschen C, Sasaki
K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek
K, Zeiher AM, et al: Role of beta2-integrins for homing and
neovascularization capacity of endothelial progenitor cells. J Exp
Med. 201:63–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Carmona G, Chavakis E, Koehl U, Zeiher AM
and Dimmeler S: Activation of Epac stimulates integrin-dependent
homing of progenitor cells. Blood. 111:2640–2646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bauters C, Marotte F, Hamon M, Oliviéro P,
Farhadian F, Robert V, Samuel JL and Rappaport L: Accumulation of
fetal fibronectin mRNAs after balloon denudation of rabbit
arteries. Circulation. 92:904–911. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chavakis E, Hain A, Vinci M, Carmona G,
Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T and Dimmeler S:
High-mobility group box 1 activates integrin-dependent homing of
endothelial progenitor cells. Circ Res. 100:204–212. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Caiado F and Dias S: Endothelial
progenitor cells and integrins: Adhesive needs. Fibrogenesis Tissue
Repair. 5:42012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hanjaya-Putra D, Yee J, Ceci D, Truitt R,
Yee D and Gerecht S: Vascular endothelial growth factor and
substrate mechanics regulate in vitro tubulogenesis of endothelial
progenitor cells. J Cell Mol Med. 14:2436–2447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jin H, Aiyer A, Su J, Borgstrom P, Stupack
D, Friedlander M and Varner J: A homing mechanism for bone
marrow-derived progenitor cell recruitment to the neovasculature. J
Clin Invest. 116:652–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qin G, Ii M, Silver M, Wecker A, Bord E,
Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, et al:
Functional disruption of alpha4 integrin mobilizes bone
marrow-derived endothelial progenitors and augments ischemic
neovascularization. J Exp Med. 203:153–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hall K and Ran S: Regulation of tumor
angiogenesis by the local environment. Front Biosci (Landmark Ed).
15:195–212. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
90
|
McKeage MJ and Baguley BC: Disrupting
established tumor blood vessels: An emerging therapeutic strategy
for cancer. Cancer. 116:1859–1871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ribatti D, Vacca A and Dammacco F: The
role of the vascular phase in solid tumor growth: A historical
review. Neoplasia. 1:293–302. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tosetti F, Ferrari N, De Flora S and
Albini A: Angioprevention': Angiogenesis is a common and key target
for cancer chemopreventive agents. FASEB J. 16:2–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Albini A, Noonan DM and Ferrari N:
Molecular pathways for cancer angioprevention. Clin Cancer Res.
13:4320–4325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li WW, Li VW, Hutnik M and Chiou AS: Tumor
angiogenesis as a target for dietary cancer prevention. J Oncol.
2012:8796232012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhao YH, Yuan B, Chen J, Feng DH, Zhao B,
Qin C and Chen YF: Endothelial progenitor cells: Therapeutic
perspective for ischemic stroke. CNS Neurosci Ther. 19:67–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Peters BA, Diaz LA, Polyak K, Meszler L,
Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein
B, et al: Contribution of bone marrow-derived endothelial cells to
human tumor vasculature. Nat Med. 11:261–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Murakami J, Li TS, Ueda K, Tanaka T and
Hamano K: Inhibition of accelerated tumor growth by blocking the
recruitment of mobilized endothelial progenitor cells after
chemotherapy. Int J Cancer. 124:1685–1692. 2009. View Article : Google Scholar : PubMed/NCBI
|