Introduction
Pure primary neuroendocrine (NE) prostate cancer
(NEPC) is a rare entity (<1%) with an incidence of 35 cases per
10,000 individuals each year (1). The
majority of NEPCs originate from conventional prostatic
adenocarcinoma, and mixed NE carcinoma-acinar adenocarcinomas of
the prostate are formed (2). Marcus
et al (3) identified 502 cases
of NE carcinoma and this study showed that the 5-year overall
survival of NE carcinoma was 12.6% while the 5-year overall
survival for non-variant prostate adenocarcinoma was 76.5%. More
than one-half of patients presented with metastatic disease at
diagnosis and the prognosis of NEPCs was poor. A combination of
platinum plus etoposide may be helpful. However, the duration of
response was transient (1). Fléchon
et al (4) studied carboplatin
and etoposide administration in patients with anaplastic
progressive metastatic castration-resistant prostate cancer with NE
differentiation. The treatment combined carboplatin, with a target
area under the plasma concentration versus time curve of 4,
administered over 1 h on day 1, and etoposide at a dose of 100
mg/m2 per day administered intravenously over 2 h for 3
consecutive days. This regimen was repeated every 21 days, with a
maximum of 6 cycles. Of 41 patients treated, 13 (33%) obtained a
serum NE marker response (>50% decrease of neuron specific
enolase or chromogranin A). This study demonstrates that the
association of etoposide and either cisplatin or carboplatin is
active in poorly differentiated NE cancers (4). Another study performed by Fjällskog
et al (5) also demonstrates
that the combination of cisplatin and etoposide can produce
significant responses in patients with heavily pretreated and
poorly differentiated/rapidly progressing NE tumors. The present
study reports a typical case of mixed NE carcinoma-acinar
adenocarcinoma of the prostate. Patients with mixed NE
carcinoma-acinar adenocarcinoma of the prostate have rarely been
reported. The progression of this tumor is associated with
aggressive disease, frequent visceral metastases and a poor
prognosis (6). In the current study,
the case of a 78-year-old male is presented. Mixed NE
carcinoma-acinar adenocarcinoma of the prostate was confirmed by
transrectal prostate biopsy due to a 12-month history of urinary
frequency and urgency. The patient underwent transurethral
resection of the prostate after 6 months of androgen-deprivation
therapy for extraordinarily salient difficulty in urination.
Invasion of the rectum appeared several months later. The neoplasm
progressed extremely fast within a short time. The patient provided
written informed consent for publication of this case study.
Case report
In October 2013, a 78-year-old male presented to the
Department of Urology, The Third Xiangya Hospital of Central South
University (Changsha, China) with a 12-month history of urinary
frequency and urgency. The patient denied any difficulty of
urination, gross hematuria, bone pain or significant weight loss.
Digital rectal examination demonstrated an enlarged, firm prostate,
with nodules. The remainder of the physical examination was
unremarkable. The patient had no significant medical history other
than type II diabetes mellitus. The serum prostate-specific antigen
(PSA) level was 33 ng/ml, while the commonly used reference range
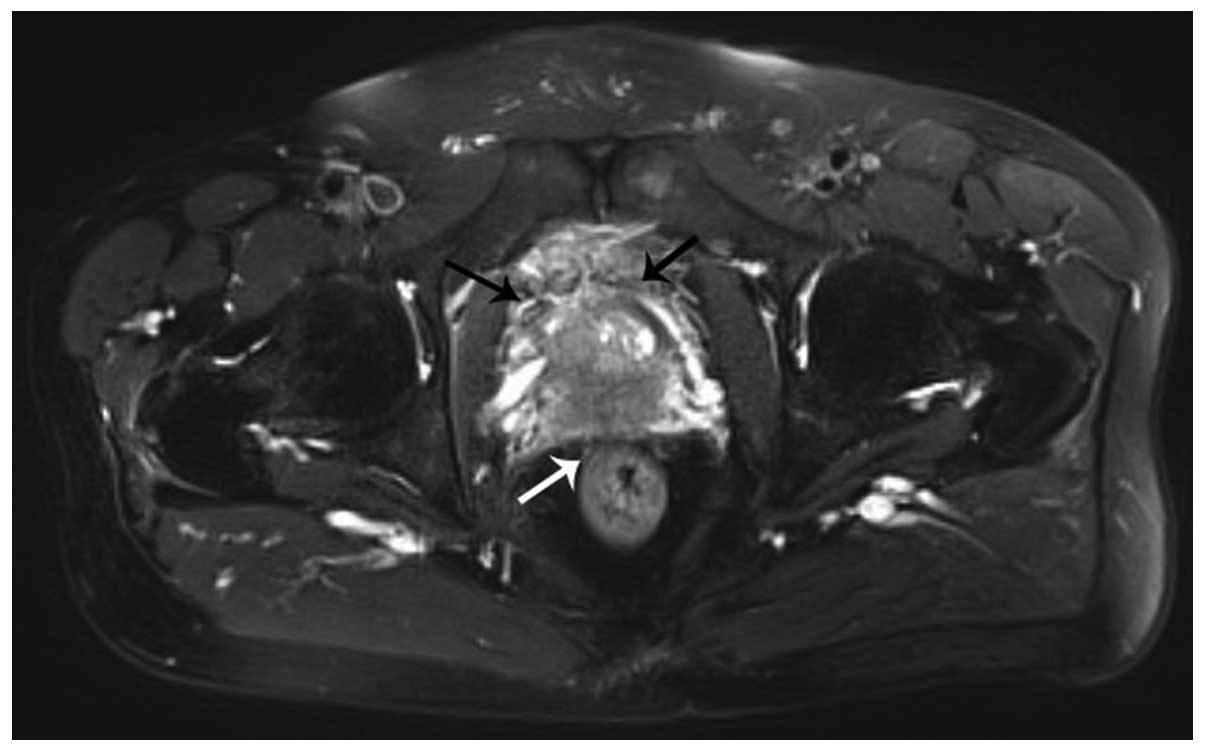
of PSA is <4 ng/ml.. Magnetic resonance imaging of the pelvis
disclosed the serious possibility of prostate cancer involving the
bilateral seminal vesicle glands and urinary bladder, with no lymph
node metastasis (Fig. 1). Bone scans
via single photon emission computed tomography disclosed metabolic
abnormalities on the upper segment of the left femoral bone. A
12-core transrectal ultrasound-guided prostate biopsy was
performed.
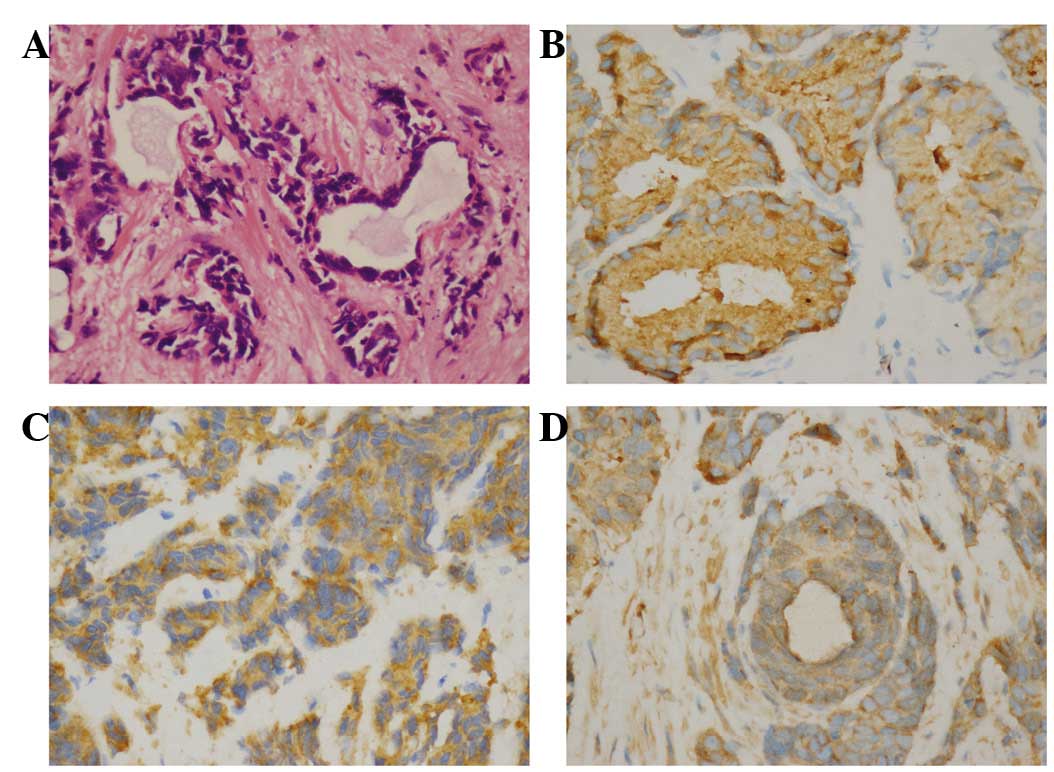
Histopathology revealed mixed NE carcinoma-acinar
adenocarcinoma (Fig. 2). Tissue
samples were fixed in 4% paraformaldehyde, dehydrated in a graded
ethanol series (70, 80, 95, 95, 100 and 100%) and
paraffin-embedded. Tissue paraffin blocks were sliced into 2.5-µm
sections after being frozen. Prostate cancer-associated protein
markers were detected by hematoxylin-eosin staining and
immunohistochemical staining. The antibodies used were as follows:
Anti-prostatic acid phosphatase antibody (rabbit monoclonal;
dilution, 1:250; catalog no., ab108984; Abcam, Cambridge, UK);
anti-synaptophysin antibody (rabbit monoclonal; dilution, 1:400;
catalog no., ab32127; Abcam); anti-chromogranin A antibody (rabbit
polyclonal; dilution, 1:400; catalog no., ab15160; Abcam); and IgG
secondary antibody (goat polyclonal; dilution, 1:100; catalog no.
ab175471; Abcam). The hematoxylin-eosin staining revealed poorly
differentiated acinar adenocarcinoma in certain areas of the
prostate, while the immunohistochemical examination revealed that
the tissue sections were positive for prostatic acid phosphatase,
synaptophysin and chromogranin A, leading to the diagnosis of mixed
NE carcinoma-acinar adenocarcinoma.
The patient was referred to a medical oncologist for
therapy of clinical stage T4N0M1b prostate cancer, according to the
prostate tumor-node-metastasis staging system of American Joint
Committee on Cancer (7th edition) (7). Combined androgen blockade with
bicalutamide and triptorelin was initiated (bicalutamide 50 mg
daily and triptorelin 15 mg every 3 months, with a total course of
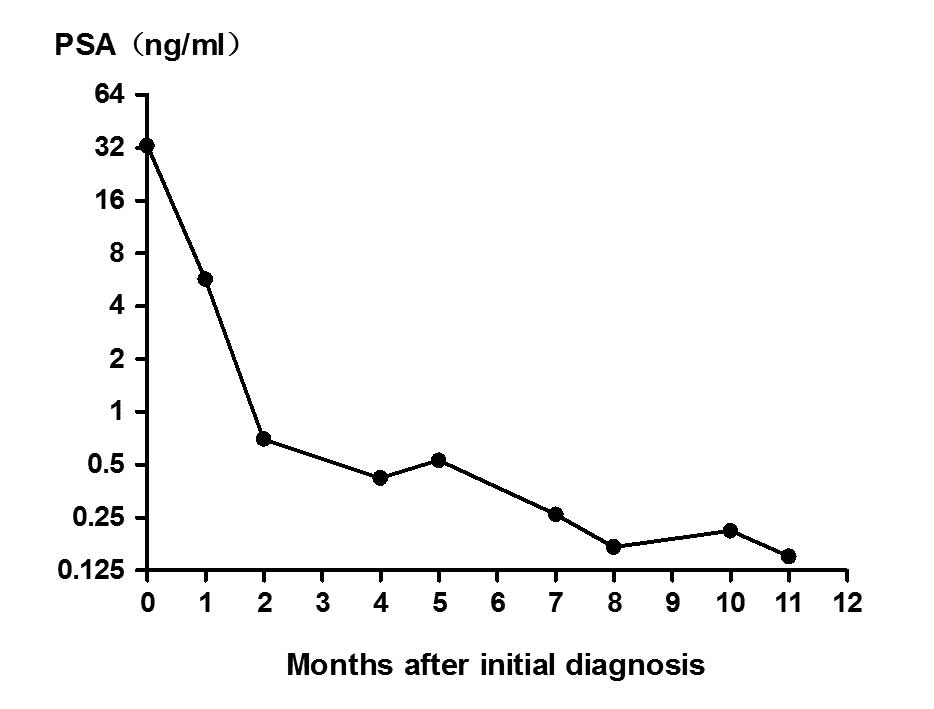
12 months). The patient's PSA level responded well, decreasing to
0.7 and 0.42 ng/ml after 2 and 5 months of treatment, respectively.
Approximately 1 month later, and 6 months after the initial
treatment, the patient presented with difficulty in urination and
urinary frequency. The PSA level was rechecked and found to be 0.53
ng/ml (Fig. 3). Transrectal
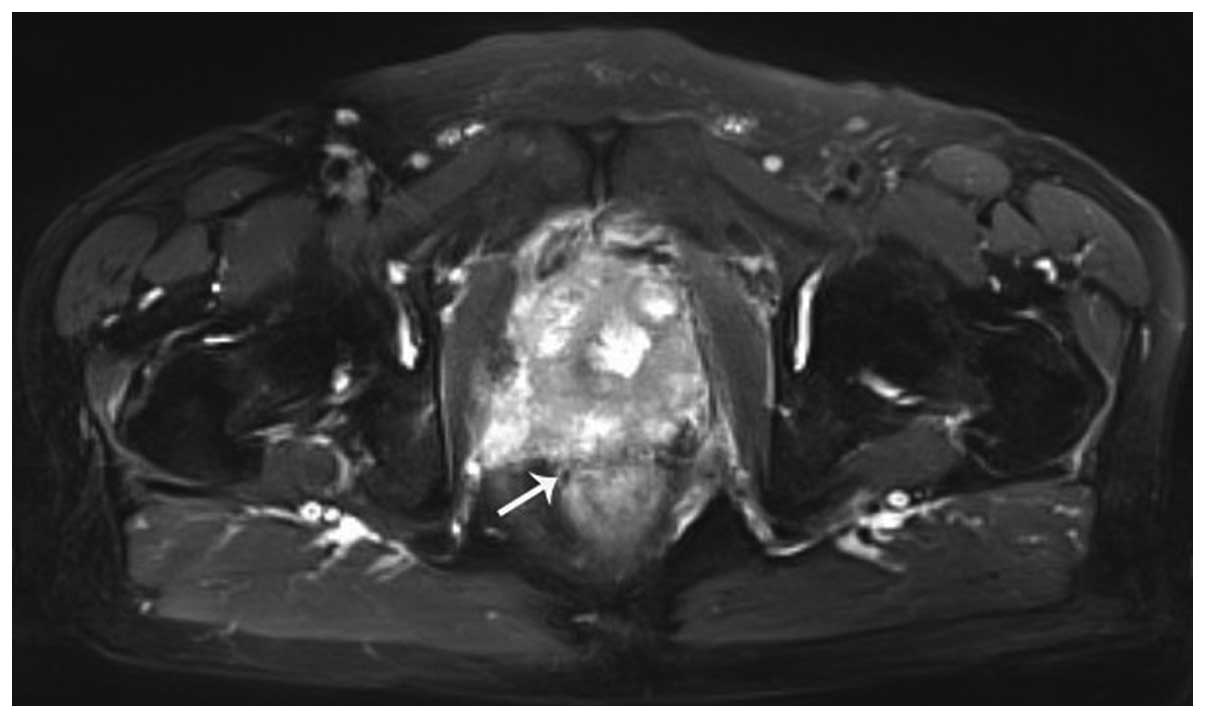
ultrasonography estimated the volume of the prostate to be larger
than previously. A plasmakinetic resection of the prostate was
performed. Combined chemotherapy based on etoposide and carboplatin
was then initiated simultaneously with the endocrine therapy
(etoposide 100 mg daily for 3 consecutive days, repeated every 4
weeks, and carboplatin injection 400 mg once, repeated every 4
weeks, with a total course of 6 months). The serum PSA remained at
a low level (<0.5 ng/ml) from then on. Conversely, the patient's
clinical discomfort progressed markedly (Fig. 4).
Approximately 5 months after the combined
chemotherapy and endocrine therapy, the patient presented with
acute urinary retention and underwent urethral dilatation.
Subsequently, 12 months after the initial diagnosis, the patient's
condition deteriorated. In addition to serious lower urinary tract
symptoms, the patient presented with urinary tract infection and
kidney failure, although the serum PSA remained at a low level
(<0.7 ng/ml). Endocrine therapy was terminated and docetaxel
(120 mg injection, every 4 weeks for 3 months) was used to replace
etoposide and carboplatin. However, those measures had little
effect on the patient's serious lower urinary tract symptoms.
Finally, 18 months subsequent to the initial diagnosis, the patient
succumbed to cachexia.
Discussion
The majority of conventional prostate cancers are
prostatic adenocarcinomas, which are formed from tumor cells with
luminal differentiation that express androgen receptors (ARs) and
secrete PSA. Therefore, hormonal therapy based on AR-dependent
signaling that results from AR gene amplification, constitutive
activation of AR, intratumoral androgen production or other
mechanisms is used in patients with prostatic adenocarcinoma at the
time of initial diagnosis, and achieves a therapeutic effect in
nearly all cases (8). Even
castration-resistant prostate cancer shows progression via
AR-dependent signaling mechanism (9).
The patient in the present case experienced rapid progression
following the diagnosis of mixed NE carcinoma-acinar adenocarcinoma
of the prostate.
NE cells exist in normal prostate tissues, without
expressing AR or secreting PSA. Extensive focal NE differentiation
is present in 10% of all conventional adenocarcinomas (10). It has been proposed that
androgen-deprivation therapy may facilitate progression of
pre-existing prostatic adenocarcinoma to NE prostate cancer. A
consequence of a lack of ARs is that deprivation hormonal therapy
does not eliminate NE cells (11).
This phenotype composed of aggressive and highly proliferative NE
cells shows conspicuous clinical progression with a low serum PSA
level. Kinebuchi et al (12)
reported a case of relapsed prostate cancer with high serum levels
of carcinoembryonic antigen and pro-gastrin-releasing peptide,
without elevation of serum PSA after bicalutamide and leuprorelin
acetate combined hormonal therapy for 39 months. The patient
finally succumbed after a survival period of 11 months following
the clinical progression. The present study patient underwent
transurethral resection of the prostate due to difficulty in
urination after receiving endocrine therapy for 6 months, and the
pathological examination indicated mixed NE and prostatic
adenocarcinoma once again.
The international classification standard for NE
prostate cancer is not yet clear. According to the Prostate Cancer
Foundation, the pathological classification system for prostatic
cancer NE differentiation consists of the following: i) Usual
prostate adenocarcinoma with NE differentiation; ii) adenocarcinoma
with Paneth cell NE differentiation; iii) carcinoid tumors; iv)
small cell carcinoma; v) large cell NE carcinoma; and vi) mixed NE
carcinoma-acinar adenocarcinoma (13). In the present case, the patient was
diagnosed with mixed NE carcinoma-acinar adenocarcinoma. Tanaka
et al (14) reported the
following characteristics for the clinical course of NE prostate
cancer: i) Survival time is short subsequent to relapse; ii) PSA
levels do not increase subsequent to relapse; and iii) the
metastatic sites are similar to those of common adenocarcinomas.
Marcus et al (15) reported a
median survival time of 10 months for patients with NE tumors and a
5-year overall survival rate of 12.6%. Given that NE prostate
cancer is independent of AR signaling, combination chemotherapy
must be added to the current therapeutic regimen. Yashi et
al (16) reported that
combination chemotherapy based on cisplatin and etoposide was
effective for controlling small cell NE carcinoma occurring after
use of a luteinizing hormone-releasing hormone analogue and
bicalutamide for 10 months in a 69-year-old patient who had
experienced progression for 7 months (16). In the present case, the patient
underwent a transurethral resection of the prostate for progressive
dysuria after 6 months of androgen-deprivation therapy. The patient
then received combined chemotherapy and endocrine therapy 2 months
later. After another 5 months, the patient experienced urinary
retention and underwent urethral dilatation.
In the present case, endocrine therapy of mixed NE
carcinoma-acinar adenocarcinoma of the prostate had a propensity to
facilitate progression of the tumor. Since patients who have
undergone failed hormonal therapy often present with multiple
metastases and a poor prognosis (17), biopsies or resections have rarely been
performed in such patients unless the entity has progressed to
severe dysuria. Therefore, the assessment of the incidence of
androgen-deprivation therapy-induced NE prostate cancer has been
neglected, making such cases underdiagnosed (18). In consequence of the widespread use of
novel androgen axis-targeting drugs, such as abiraterone acetate
and enzalutamide (19), the incidence
of NE prostate cancer may increase to a great extent.
In summary, the present study reported a case of
rapid progression in a mixed NE carcinoma-acinar adenocarcinoma of
the prostate. In patients diagnosed with mixed NE carcinoma-acinar
adenocarcinoma of the prostate, endocrine therapy shows a poor
effect, even if the serum PSA level has remained low, and a
combination chemotherapy may be useful to control the progression.
Close follow-up of symptoms and periodic digital rectal examination
are also important.
Glossary
Abbreviations
Abbreviations:
|
PSA
|
prostate-specific antigen
|
|
AR
|
androgen receptor
|
|
NE
|
neuroendocrine
|
References
|
1
|
Aggarwal R, Zhang T, Small EJ and
Armstrong AJ: Neuroendocrine prostate cancer: Subtypes, biology,
and clinical outcomes. J Natl Compr Canc Netw. 12:719–726.
2014.PubMed/NCBI
|
|
2
|
Wang HT, Yao YH, Li BG, Tang Y, Chang JW
and Zhang J: Neuroendocrine Prostate Cancer (NEPC) progressing from
conventional prostatic adenocarcinoma: Factors associated with time
to development of NEPC and survival from NEPC diagnosis - a
systematic review and pooled analysis. J Clin Oncol. 32:3383–3390.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marcus DM, Goodman M, Jani AB, Osunkoya AO
and Rossi PJ: A comprehensive review of incidence and survival in
patients with rare histological variants of prostate cancer in the
United States from 1973 to 2008. Prostate Cancer Prostatic Dis.
15:283–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fléchon A, Pouessel D, Ferlay C, Perol D,
Beuzeboc P, Gravis G, Joly F, Oudard S, Deplanque G, Zanetta S, et
al: Phase II study of carboplatin and etoposide in patients with
anaplastic progressive metastatic castration-resistant prostate
cancer (mCRPC) with or without neuroendocrine differentiation:
Results of the French Genito-Urinary Tumor Group (GETUG) P01 trial.
Ann Oncol. 22:2476–2481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fjällskog ML, Granberg DP, Welin SL,
Eriksson C, Oberg KE, Janson ET and Eriksson BK: Treatment with
cisplatin and etoposide in patients with neuroendocrine tumors.
Cancer. 92:1101–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmgren JS, Karavadia SS and Wakefield
MR: Unusual and underappreciated: Small cell carcinoma of the
prostate. Semin Oncol. 34:22–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung MS, Lee SH, Lee DH and Chung BH:
Evaluation of the 7th American Joint Committee on cancer TNM
staging system for prostate cancer in point of classification of
bladder neck invasion. Jpn J Clin Oncol. 43:184–188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lipianskaya J, Cohen A, Chen CJ, Hsia E,
Squires J, Li Z, Zhang Y, Li W, Chen X, Xu H and Huang J:
Androgen-deprivation therapy-induced aggressive prostate cancer
with neuroendocrine differentiation. Asian J Androl. 16:541–544.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beltran H, Tagawa ST, Park K, MacDonald T,
Milowsky MI, Mosquera JM, Rubin MA and Nanus DM: Challenges in
recognizing treatment-related neuroendocrine prostate cancer. J
Clin Oncol. 30:e386–e389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Sant'Agnese PA and Cockett AT: The
prostatic endocrine-paracrine (neuroendocrine) regulatory system
and neuroendocrine differentiation in prostatic carcinoma: A review
and future directions in basic research. J Urol. 152:1927–1931.
1994.PubMed/NCBI
|
|
11
|
Ramírez-Balderrama L, López-Briones S,
Daza-Benítez L, Macías MH, López-Gaytán T and Pérez-Vázquez V:
Neuroendocrine differentiation in prostate adenocarcinoma. Gac Med
Mex. 149:639–645. 2013.(In Spanish). PubMed/NCBI
|
|
12
|
Kinebuchi Y, Noguchi W, Irie K, Nakayama
T, Kato H and Nishizawa O: Relapsed prostate cancer with
neuroendocrine differentiation and high serum levels of
carcinoembryonic antigen without elevation of prostrate-specific
antigen: A case report. Int J Urol. 14:147–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Epstein JI, Amin MB, Beltran H, Lotan TL,
Mosquera JM, Reuter VE, Robinson BD, Troncoso P and Rubin MA:
Proposed morphologic classification of prostate cancer with
neuroendocrine differentiation. Am J Surg Pathol. 38:756–767. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka M, Suzuki Y, Takaoka K, Suzuki N,
Murakami S, Matsuzaki O and Shimazaki J: Progression of prostate
cancer to neuroendocrine cell tumor. Int J Urol. 8:431–437. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marcus DM, Goodman M, Jani AB, Osunkoya AO
and Rossi PJ: A comprehensive review of incidence and survival in
patients with rare histological variants of prostate cancer in the
United States from 1973 to 2008. Prostate Cancer Prostatic Dis.
15:283–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yashi M, Terauchi F, Nukui A, Ochi M,
Yuzawa M, Hara Y and Morita T: Small-cell neuroendocrine carcinoma
as a variant form of prostate cancer recurrence: A case report and
short literature review. Urol Oncol. 24:313–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reichardt P: Systematic hormone- and
chemotherapy in the management of skeletal metastases. Orthopade.
27:240–244. 1998.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lipianskaya J, Cohen A, Chen CJ, Hsia E,
Squires J, Li Z, Zhang Y, Li W, Chen X, Xu H and Huang J:
Androgen-deprivation therapy-induced aggressive prostate cancer
with neuroendocrine differentiation. Asian J Androl. 16:541–544.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aragon-Ching JB: The evolution of prostate
cancer therapy: Targeting the androgen receptor. Front Oncol.
4:2952014. View Article : Google Scholar : PubMed/NCBI
|